Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8195
Peer-review started: December 16, 2014
First decision: January 22, 2015
Revised: February 13, 2015
Accepted: March 18, 2015
Article in press: March 19, 2015
Published online: July 14, 2015
Processing time: 214 Days and 9.9 Hours
AIM: To compare the safety and efficacy of carbon dioxide (CO2) and air insufflation during gastric endoscopic submucosal dissection (ESD).
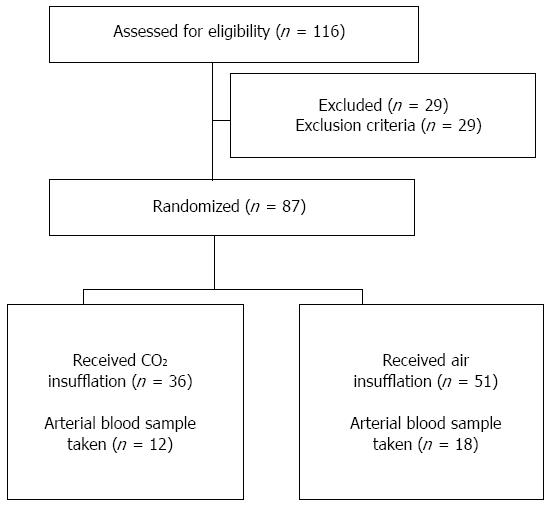
METHODS: This study involved 116 patients who underwent gastric ESD between January and December 2009. After eliminating 29 patients who fit the exclusion criteria, 87 patients, without known pulmonary dysfunction, were randomized into the CO2 insufflation (n = 36) or air insufflation (n = 51) groups. Standard ESD was performed with a CO2 regulation unit (constant rate of 1.4 L/min) used for patients undergoing CO2 insufflation. Patients received diazepam for conscious sedation and pentazocine for analgesia. Transcutaneous CO2 tension (PtcCO2) was recorded 15 min before, during, and after ESD with insufflation. PtcCO2, the correlation between PtcCO2 and procedure time, and ESD-related complications were compared between the two groups. Arterial blood gases were analyzed after ESD in the first 30 patients (12 with CO2 and 18 with air insufflation) to assess the correlation between arterial blood CO2 partial pressure (PaCO2) and PtcCO2.
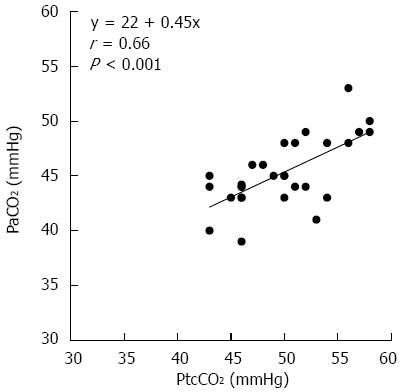
RESULTS: There were no differences in respiratory functions, median sedative doses, or median procedure times between the groups. Similarly, there was no significant difference in post-ESD blood gas parameters, including PaCO2, between the CO2 and air groups (44.6 mmHg vs 45 mmHg). Both groups demonstrated median pH values of 7.36, and none of the patients exhibited acidemia. No significant differences were observed between the CO2 and air groups with respect to baseline PtcCO2 (39 mmHg vs 40 mmHg), peak PtcCO2 during ESD (52 mmHg vs 51 mmHg), or median PtcCO2 after ESD (50 mmHg vs 50 mmHg). There was a strong correlation between PaCO2 and PtcCO2 (r = 0.66; P < 0.001). The incidence of Mallory-Weiss tears was significantly lower with CO2 insufflation than with air insufflation (0% vs 15.6%, P = 0.013). CO2 insufflation did not cause any adverse events, such as CO2 narcosis or gas embolisms.
CONCLUSION: CO2 insufflation during gastric ESD results in similar blood gas levels as air insufflation, and also reduces the incidence of Mallory-Weiss tears.
Core tip: The safety and efficacy of carbon dioxide (CO2) and air insufflation during gastric endoscopic submucosal dissection (ESD) were compared in a randomized controlled trial. The transcutaneous CO2 tension and the partial pressure of CO2 in the arterial blood were measured to directly evaluate CO2 retention or acidemia. The findings strongly suggest that CO2 insufflation is as safe as air insufflation with regard to blood gas levels. The present study is the first randomized controlled trial to demonstrate the benefit of CO2 insufflation in reducing the risk of Mallory-Weiss tears during ESD.
- Citation: Takada J, Araki H, Onogi F, Nakanishi T, Kubota M, Ibuka T, Shimizu M, Moriwaki H. Safety and efficacy of carbon dioxide insufflation during gastric endoscopic submucosal dissection. World J Gastroenterol 2015; 21(26): 8195-8202
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8195.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8195
Endoscopic submucosal dissection (ESD) for gastric neoplasms enables en bloc resection of even an extensive superficial lesion[1-8]. However, gastric ESD is technically difficult and time consuming, and therefore, extensive gas insufflation is required to maintain adequate visualization during the procedure. Although air is commonly used for insufflation, it results in the retention of a large amount of residual gas after ESD. Residual gas in the gastrointestinal tract can induce post-ESD pain or discomfort, and in rare cases can give rise to life-threatening complications such as air embolism and tension pneumothorax[9-17].
It is well known that carbon dioxide (CO2) is absorbed faster in the body than air and is also rapidly excreted through the lungs, except in cases of pulmonary dysfunction. Therefore, CO2 insufflation is expected to reduce the pain and abdominal discomfort associated with endoscopic examination and therapy[18-25].
Perforation and major bleeding are severe complications of ESD. The reported incidence of perforation in ESD ranges from 1% to 6.1%[1-5], and subsequent peritonitis or mediastinitis could be fatal. CO2 insufflation reportedly minimizes these ESD-related complications[26]. The safety and efficacy of CO2 insufflation during ESD for lesions of the esophagus, stomach, and colorectum have been demonstrated in randomized controlled trials (RCTs)[27,28] and prospective studies[29-31]. However, these RCTs measured only transcutaneous CO2 tension (PtcCO2) or end-tidal CO2 pressure, not partial pressure of CO2 in the arterial blood (PaCO2).
The aim of the present prospective RCT was to assess the safety and efficacy of CO2 insufflation during ESD for gastric neoplasms in patients under conscious sedation. Both PtcCO2 and PaCO2 were measured in order to directly evaluate CO2 retention or acidemia. Furthermore, a continuous PtcCO2 measuring system to monitor patient safety during CO2 insufflation was validated.
This study was designed as a single-center RCT. Between January 2009 and December 2009, all consecutive patients undergoing ESD for gastric neoplasms at Gifu University Hospital in Japan were screened for this study. Gastric ESD was indicated for differentiated adenocarcinoma that was confined to the mucosa with no risk of lymph node metastasis, and for adenoma, regardless of its size or the presence of ulceration.
Patients were excluded if: (1) they had chronic pulmonary dysfunction defined as a forced expiratory volume in 1.0 second/forced vital capacity (FEV1.0%) of < 70% or a vital capacity (%VC) of < 80%; (2) they were unable to understand the consent information required for participation; or (3) they declined participation. The study design was approved by the ethics committee for clinical research at Gifu University Hospital. All eligible individuals provided written informed consent prior to study enrollment. Randomization was conducted using sealed envelopes and patients were divided into two groups: the CO2 insufflation group (CO2 group) and the air insufflation group (Air group).
ESD was conducted during the first afternoon after hospital admission. On the second day in the hospital, blood tests, esophagogastroduodenoscopy, and CT of the chest and abdomen were performed. Blood tests for leukocyte count and C-reactive protein levels were repeated on the third hospital day. Axillary temperature was assessed 1 h after ESD and daily thereafter, at 06:00, 14:00, and 20:00 h.
The standard ESD procedure was performed using a gastroscope with a single working channel and water jet function (GIF-Q260J; Olympus Optical Co., Tokyo, Japan) and a cap attachment (D-201-11804; Olympus). The gastric lesion was resected using either the DualKnife (KD-630L; Olympus) or the ITKnife2 (KD-611L; Olympus), depending on its location. A 0.4% high-molecular-weight hyaluronic acid solution containing epinephrine was injected into the submucosal layer to raise the lesion. Incision of the mucosal layer around the circumferential markings and subsequent direct dissection of the submucosal layer were performed with the DualKnife and ITKnife2.
Patients received diazepam for conscious sedation and pentazocine for analgesia. At the start of the ESD procedure, 5-10 mg of diazepam and 7.5-15.0 mg of pentazocine were injected intravenously for induction of anesthesia and analgesia, with an additional 5 mg of diazepam or 7.5 mg of pentazocine administered repeatedly as necessary. When the combination of diazepam and pentazocine did not achieve conscious sedation, intravenous midazolam was administered. Oxygen was administered nasally at 2.0 L/min during ESD, and the flow volume was adjusted by monitoring transcutaneous oxygen saturation (SpO2). Arterial blood samples were immediately analyzed using a blood gas analyzer (ABL700; Radiometer Medical, Copenhagen, Denmark) after the ESD procedure, for the first 30 consecutive patients.
CO2 was delivered using a CO2 regulation unit (Olympus UCR; Olympus). The TOSCA measurement system and TOSCA 500 monitor (Linde Medical Sensors, Basel, Switzerland) were used to measure the PtcCO2 noninvasively and continuously with an earlobe sensor attached by a low-pressure clip. We used a default temperature setting of 42 °C for the sensor and recalibrated the system before each ESD. The low-flow gas tube (MAJ-1742; Olympus) of the Olympus UCR was set at a constant rate of 1.4 L/min for CO2 insufflation in all patients.
Operation time was measured from the start of circumferential marking to the completion of resection. A diagnosis of perforation was made by direct endoscopic observation of visceral organs during ESD or by the presence of free air on follow-up plain chest radiography. Evidence of aspiration pneumonia was determined by the appearance of an obvious pneumonia shadow on a plain chest CT one day after ESD. Bleeding was defined as clinical evidence of bleeding after ESD, such as hematemesis or melena that required endoscopic treatment. A Mallory-Weiss tear (MWT) was defined as a mucosal tear or laceration adjacent to the esophagogastric junction with active bleeding, either spurting or oozing, during ESD.
Values are expressed as the number and percentage of patients or median (range). Differences in distribution of categorical variables between the two groups were analyzed by χ2 or by Fisher’s exact tests when required. The nonparametric Mann-Whitney U test was used for comparing continuous variables. A P < 0.05 was considered significant. All statistical analyses were conducted with JMP version 10 (SAS Institute, Cary, NC, United States).
Of the 116 candidate patients for gastric ESD, 87 were enrolled in the trial and randomized. Among them, 36 received CO2 insufflation and 51 received air insufflation. Twenty-nine patients were excluded due to impaired respiratory function (n = 24), severe chronic obstructive pulmonary disease requiring oxygen (n = 3), and inability to understand the consent information required for participation (n = 2). The first 30 participants (12 from the CO2 group and 18 from the Air group) underwent arterial blood gas analysis (Figure 1).
Baseline characteristics for each treatment group are shown in Table 1. The location (P = 0.012) and histopathology (P = 0.020) of the gastric lesions were significantly different between the groups. The median procedure time was 46 min in the CO2 group and 48 min in the Air group (not significant). There were no differences in respiratory function (FEV1.0% and %VC) between the groups. No significant differences were observed in the median dose of sedative drugs administered to the patients in each group.
| Characteristic | CO2 group (n = 36) | Air group (n = 51) | P value |
| Age, yr | 74 (52-87) | 70 (45-93) | NS |
| Sex, male/female | 22/14 | 36/15 | NS |
| FEV1.0% | 72 (70-89) | 73 (70-93) | NS |
| %VC | 103 (80-102) | 109 (80-152) | NS |
| Location of lesion, n: upper/middle/lower | 5/22/9 | 12/15/24 | 0.012 |
| En bloc resection, n (%) | 36 (100) | 51 (100) | NS |
| Histopathologic type, n: tub1/tub2/por/sig/adenoma | 18/3/0/0/15 | 36/6/2/1/6 | 0.020 |
| Histologic depth, n: M/SM1/SM2 | 32/2/2 | 42/1/8 | NS |
| Histopathologically curative resection, n (%) | 31 (86.1) | 43 (84.3) | NS |
| Tumor size, mm | 18 (4-75) | 17 (3-47) | NS |
| Resection size, mm | 35 (22-110) | 37 (23-95) | NS |
| Procedure time, min | 46 (18-194) | 48 (15-145) | NS |
| Dose of diazepam, mg | 20 (5-30) | 20 (5-30) | NS |
| Dose of pentazocine, mg | 18.8 (7.5-45) | 22.5 (7.5-45) | NS |
| Patients receiving midazolam, n (%) | 3 (8.3) | 5 (9.8) | NS |
| Dose of midazolam, mg | 10.0 (2.5-20) | 7.5 (2.5-10) | NS |
No significant differences were observed between the two groups that received blood gas analysis after ESD with respect to the median procedure time and the median dose of sedative drugs (Table 2). There was no significant difference between the CO2 group and the Air group in any blood gas parameters, including PaCO2 (44.6 mmHg vs 45 mmHg). The median pH values were 7.36 in both groups, and there were no patients with acidemia. As shown in Figure 2, PtcCO2 was significantly correlated with PaCO2 (r = 0.66; P < 0.001). The median difference between PaCO2 and PtcCO2 was 4.8 mmHg.
| Characteristic | CO2 group (n = 12) | Air group (n = 18) | P value |
| Age, yr | 73 (63-82) | 70 (45-87) | NS |
| Procedure time, min | 66 (26-156) | 56 (23-107) | NS |
| Dose of diazepam, mg | 17.5 (10.0-22.5) | 20.0 (5.0-30.0) | NS |
| Dose of pentazocine, mg | 15.0 (15.0-30.0) | 22.5 (15.0-37.5) | NS |
| Patients receiving midazolam, n (%) | 1 (8.3) | 1 (5.6) | NS |
| Dose of midazolam, mg | 2.5 | 5 | |
| pH value | 7.36 (7.34-7.39) | 7.36 (7.33-7.40) | NS |
| PaCO2, mmHg | 44.6 (39-53) | 45 (40-50) | NS |
| PaO2, mmHg | 168 (68-203) | 143 (78-259) | NS |
| HCO3-, mEq/L | 25.1 (23.0-30.0) | 25.5 (22.0-27.0) | NS |
| Base excess, mEq/L | -0.05 (-2-4) | 0.3 (-3.1-2.6) | NS |
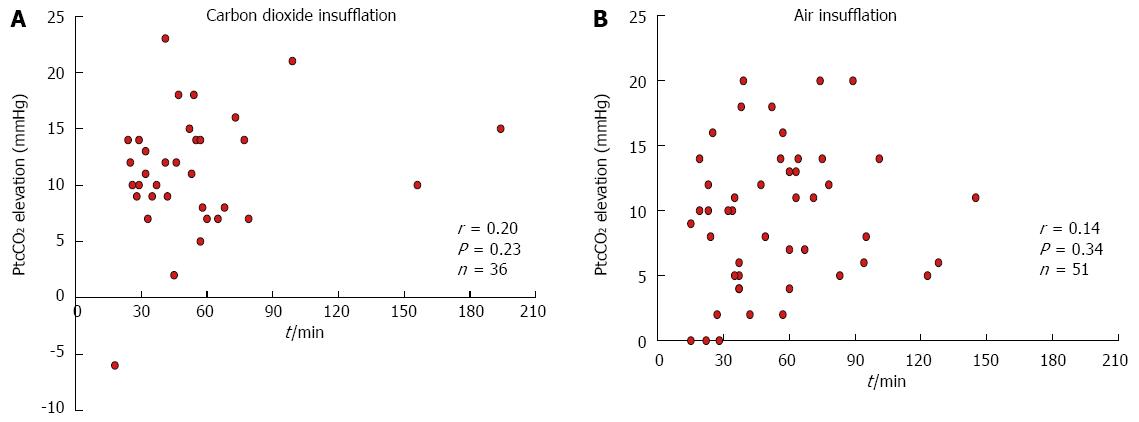
The median PtcCO2 before (baseline) and after ESD was 39 mmHg (28-52 mmHg) and 50 mmHg (41-68 mmHg), respectively, in the CO2 group, and 40 mmHg (22-51 mmHg) and 50 mmHg (40-64 mmHg), respectively, in the Air group. The PtcCO2 increased significantly (P < 0.001) after the procedure in both groups, though there was no significant difference between the groups. The median peak PtcCO2 during the procedure was 52 mmHg (43-68 mmHg) in the CO2 group and 51 mmHg (40-64 mmHg) in the Air group (not significant, Table 3). There was no correlation between the procedure time and PtcCO2 elevation in either the CO2 group or the Air group (Figure 3). The median minimum SpO2 level and oxygen flow rate were similar between the groups (98% and 2.0 L/min, respectively).
| Variable | CO2 group (n = 36) | Air group (n = 51) | P value |
| Baseline PtcCO2, mmHg | 39 (28-52)1 | 40 (22-51)1 | NS |
| PtcCO2 after ESD, mmHg | 50 (41-68)1 | 50 (40-64)1 | NS |
| Peak PtcCO2, mmHg | 52 (43-68) | 51 (40-64) | NS |
| PtcCO2 > 60 mmHg during ESD | 3 (8.3) | 2 (3.9) | NS |
| Minimum SpO2, % | 98 (90-100) | 98 (89-100) | NS |
| Oxygen flow rate, L/min | 2 (1-5) | 2 (2-4) | NS |
ESD-related complications and the duration of the hospital stay are listed in Table 4. CO2 insufflation did not cause any adverse events such as CO2 narcosis or gas embolism. No significant difference was observed between the two groups with respect to the incidence of fever (body temperature > 37.5 °C), pneumonia, perforation, or post-ESD hemorrhage. The incidence of MWTs was significantly lower in the CO2 group than in the Air group (P = 0.013). Serum C-reactive protein levels and white blood cell counts on days 1 and 3 after ESD were not significantly different between the groups, and the median hospital stay was equivalent at 7 d for each group.
| Variable | CO2 group (n = 36) | Air group (n = 51) | P value |
| Fever (body temperature > 37.5 °C) | 9 (25.0) | 9 (17.6) | NS |
| Pneumonia | 3 (8.3) | 5 (9.8) | NS |
| Perforation | 1 (2.7) | 1 (1.9) | NS |
| Post-procedure hemorrhage | 0 | 4 (7.8) | NS |
| Mallory-Weiss tears | 0 | 8 (9.8) | 0.013 |
| CRP on day 1 after ESD, mg/dL | 0.30 (0.09-6.19) | 0.40 (0.04-3.62) | NS |
| CRP on day 3 after ESD, mg/dL | 2.00 (0.18-7.83) | 2.00 (0.08-14.20) | NS |
| WBC on day 1 after ESD, n/μL | 9020 (3730-15680) | 8090 (4510-13450) | NS |
| WBC on day 3 after ESD, n/μL | 6310 (2560-11200) | 6260 (3100-10660) | NS |
| Hospital stay, d | 7 (7-16) | 7 (7-20) | NS |
The safety and efficacy of insufflation using CO2 as an alternative to air has been demonstrated in several RCTs for various kinds of endoscopic procedures[18-20,22-24,27,28]. In gastric ESD, Maeda et al[28] reported that CO2 insufflation significantly reduced the volume of residual gas in the digestive tract compared with air insufflation. In the present study, under similar ESD conditions with regard to procedure time, respiratory function, sedative drug doses, and minimum SpO2, neither the post-procedure PaCO2 nor the median PtcCO2 differed between the CO2 group and the Air group. The peak PtcCO2 during ESD also did not differ between the two groups. Furthermore, we confirmed a strong correlation between PaCO2 and PtcCO2. Therefore, the PtcCO2 value can be used as a surrogate marker of CO2 retention in patients who received CO2 insufflation during ESD.
In this study, the maximum PtcCO2 and PaCO2 reached 68 mmHg and 53 mmHg in the CO2 group, and 64 mmHg and 50 mmHg in the Air group, respectively. However, no adverse events such as acidemia, CO2 narcosis, or SpO2 depression were reported in either group. The elevated PtcCO2 in both groups after the procedures is likely due to respiratory depression associated with conscious sedation. Several studies have demonstrated that such respiratory depression is involved in the elevation of PaCO2 or PtcCO2 in patients undergoing endoscopic treatment[27,29-31]. There was no correlation between procedure time and PtcCO2 elevation in the present study. These results indicate that CO2 insufflation is as safe as air insufflation for gastric ESD when the CO2 insufflation rate is 1.4 L/min and the median procedure time is 48 min.
With regard to complications, the incidence of MWTs was significantly lower in the CO2 group than in the Air group. This may be due to the rapid absorption of CO2 by the body compared to air. Indeed, CO2 insufflation in esophagogastroduodenoscopy efficiently reduces MWTs by lowering the tension of the gastric mucosa caused by residual gas in the stomach[32]. To our knowledge, the present study is the first RCT to demonstrate the benefit of CO2 insufflation in reducing the risk of MWTs during ESD.
Because respiratory depression due to conscious sedation may lead to CO2 retention, arterial CO2 monitoring during lengthy endoscopic procedures is important, even if the patient’s respiratory function is normal. However, arterial blood sampling is invasive and it is not practical to measure PaCO2 serially in all ESD patients. Instead, PtcCO2, which correlates well with PaCO2, can be measured noninvasively and continuously. PtcCO2 is usually greater than PaCO2 by 5-6 mmHg[33,34]. Indeed, in the present study, the median difference between these values was 4.8 mmHg. Because of the strong correlation between PtcCO2 and PaCO2, a PtcCO2 monitoring system is considered a reliable and efficient alternative to PaCO2 measuring. Thus, arterial blood analysis was not continued after the first 30 patients.
This study has some limitations. First, 27 patients (23.3%) who had chronic pulmonary dysfunction were excluded, as the safety of CO2 insufflation during gastric ESD has not been established for these patients. We recently reported the safety of CO2 insufflation during gastric ESD in patients with pulmonary dysfunction (FEV1.0% < 70% or %VC < 80%) under conscious sedation[35]. However, in patients with severe obstructive pulmonary disease, a longer procedure time may increase the risk of CO2 retention because there is a significant correlation between PtcCO2 elevation and ESD procedure time in patients with pulmonary dysfunction[35]. Therefore, PtcCO2 should be carefully monitored in these patients to avoid severe complications such as CO2 narcosis and acidemia. Second, the number of patients who underwent arterial blood gas analysis in the present study may be too small. The present study was also a single-center trial. Therefore, further larger prospective multicenter studies are required to confirm the safety and efficacy of CO2 insufflation for gastric ESD by evaluating both PtcCO2 and PaCO2 values.
In conclusion, this study strongly suggests that CO2 insufflation is safe and effective during gastric ESD under conscious sedation in patients without pulmonary dysfunction. Furthermore, CO2 insufflation reduces the incidence of MWTs compared to air insufflation. However, conscious sedation might increase the risk of CO2 retention and the PtcCO2 should be monitored carefully in these cases.
The safety and efficacy of insufflation using carbon dioxide (CO2) as an alternative to air has been demonstrated in several randomized controlled trials for various kinds of endoscopic procedure. However, there has been no report on the safety and efficacy of CO2 insufflation for gastric endoscopic submucosal dissection (ESD) based on the measurement of both the partial pressure of CO2 in the arterial blood and transcutaneous CO2 tension.
Based on the observation that CO2 is rapidly absorbed from the bowel, this study investigated the effect of CO2 insufflation on patients undergoing gastric ESD.
CO2 insufflation remarkably reduced the incidence of Mallory-Weiss tears without any adverse events.
The safety and efficacy of CO2 insufflation during gastric ESD in patients under conscious sedation were demonstrated in this study. Further investigation in patients with pulmonary dysfunction is necessary.
The present study is the first randomized controlled trial to demonstrate that CO2 insufflation can reduce the risk of Mallory-Weiss tears during ESD, and represents a major contribution to this field.
P- Reviewer: Dogan UB, Lankarani KB, Sinagra E S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 2. | Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 4. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 526] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Yamamoto H, Kita H. Endoscopic therapy of early gastric cancer. Best Pract Res Clin Gastroenterol. 2005;19:909-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J Gastroenterol. 2006;12:5108-5112. [PubMed] |
| 9. | Morley AP, Lau JY, Young RJ. Tension pneumothorax complicating a perforation of a duodenal ulcer during ERCP with endoscopic sphincterotomy. Endoscopy. 1997;29:332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Katzgraber F, Glenewinkel F, Fischler S, Rittner C. Mechanism of fatal air embolism after gastrointestinal endoscopy. Int J Legal Med. 1998;111:154-156. [PubMed] |
| 11. | Rai A, Iftikhar S. Tension pneumothorax complicating diagnostic upper endoscopy: a case report. Am J Gastroenterol. 1999;94:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Nayagam J, Ho KM, Liang J. Fatal systemic air embolism during endoscopic retrograde cholangio-pancreatography. Anaesth Intensive Care. 2004;32:260-264. [PubMed] |
| 13. | Green BT, Tendler DA. Cerebral air embolism during upper endoscopy: case report and review. Gastrointest Endosc. 2005;61:620-623. [PubMed] |
| 14. | Stabile L, Cigada M, Stillittano D, Morandi E, Zaffroni M, Rossi G, Lapichino G. Fatal cerebral air embolism after endoscopic retrograde cholangiopancreatography. Acta Anaesthesiol Scand. 2006;50:648-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Bisceglia M, Simeone A, Forlano R, Andriulli A, Pilotto A. Fatal systemic venous air embolism during endoscopic retrograde cholangiopancreatography. Adv Anat Pathol. 2009;16:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Finsterer J, Stöllberger C, Bastovansky A. Cardiac and cerebral air embolism from endoscopic retrograde cholangio-pancreatography. Eur J Gastroenterol Hepatol. 2010;22:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | van Boxel GI, Hommers CE, Dash I, Goodman AJ, Green J, Orme RM. Myocardial and cerebral infarction due to massive air embolism following endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy. 2010;42 Suppl 2:E80-E81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E, Hoff G. NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut. 2002;50:604-607. [PubMed] |
| 19. | Sumanac K, Zealley I, Fox BM, Rawlinson J, Salena B, Marshall JK, Stevenson GW, Hunt RH. Minimizing postcolonoscopy abdominal pain by using CO(2) insufflation: a prospective, randomized, double blind, controlled trial evaluating a new commercially available CO(2) delivery system. Gastrointest Endosc. 2002;56:190-194. [PubMed] |
| 20. | Church J, Delaney C. Randomized, controlled trial of carbon dioxide insufflation during colonoscopy. Dis Colon Rectum. 2003;46:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc. 2009;69:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Dellon ES, Velayudham A, Clarke BW, Isaacs KL, Gangarosa LM, Galanko JA, Grimm IS. A randomized, controlled, double-blind trial of air insufflation versus carbon dioxide insufflation during ERCP. Gastrointest Endosc. 2010;72:68-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Hirai F, Beppu T, Nishimura T, Takatsu N, Ashizuka S, Seki T, Hisabe T, Nagahama T, Yao K, Matsui T. Carbon dioxide insufflation compared with air insufflation in double-balloon enteroscopy: a prospective, randomized, double-blind trial. Gastrointest Endosc. 2011;73:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Lenz P, Meister T, Manno M, Pennazio M, Conigliaro R, Lebkücher S, Ullerich H, Schmedt A, Floer M, Beyna T. CO2 insufflation during single-balloon enteroscopy: a multicenter randomized controlled trial. Endoscopy. 2014;46:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Bretthauer M, Hoff G, Thiis-Evensen E, Grotmol T, Holmsen ST, Moritz V, Skovlund E. Carbon dioxide insufflation reduces discomfort due to flexible sigmoidoscopy in colorectal cancer screening. Scand J Gastroenterol. 2002;37:1103-1107. [PubMed] |
| 26. | Maeda Y, Hirasawa D, Fujita N, Obana T, Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y. A pilot study to assess mediastinal emphysema after esophageal endoscopic submucosal dissection with carbon dioxide insufflation. Endoscopy. 2012;44:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Maeda Y, Hirasawa D, Fujita N, Obana T, Sugawara T, Ohira T, Harada Y, Yamagata T, Suzuki K, Koike Y. A prospective, randomized, double-blind, controlled trial on the efficacy of carbon dioxide insufflation in gastric endoscopic submucosal dissection. Endoscopy. 2013;45:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Kikuchi T, Fu KI, Saito Y, Uraoka T, Fukuzawa M, Fukunaga S, Sakamoto T, Nakajima T, Matsuda T. Transcutaneous monitoring of partial pressure of carbon dioxide during endoscopic submucosal dissection of early colorectal neoplasia with carbon dioxide insufflation: a prospective study. Surg Endosc. 2010;24:2231-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Suzuki T, Minami H, Komatsu T, Masusda R, Kobayashi Y, Sakamoto A, Sato Y, Inoue H, Serada K. Prolonged carbon dioxide insufflation under general anesthesia for endoscopic submucosal dissection. Endoscopy. 2010;42:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Wang WL, Wu ZH, Sun Q, Wei JF, Chen XF, Zhou DK, Zhou L, Xie HY, Zheng SS. Meta-analysis: the use of carbon dioxide insufflation vs. room air insufflation for gastrointestinal endoscopy. Aliment Pharmacol Ther. 2012;35:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Epstein MF, Cohen AR, Feldman HA, Raemer DB. Estimation of PaCO2 by two noninvasive methods in the critically ill newborn infant. J Pediatr. 1985;106:282-286. [PubMed] |
| 34. | Bhavani-Shankar K, Steinbrook RA, Mushlin PS, Freiberger D. Transcutaneous PCO2 monitoring during laparoscopic cholecystectomy in pregnancy. Can J Anaesth. 1998;45:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Takada J, Araki H, Onogi F, Nakanishi T, Kubota M, Ibuka T, Shimizu M, Moriwaki H. Safety of carbon dioxide insufflation during gastric endoscopic submucosal dissection in patients with pulmonary dysfunction under conscious sedation. Surg Endosc. 2015;29:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |