Published online Jul 14, 2015. doi: 10.3748/wjg.v21.i26.8052
Peer-review started: December 27, 2014
First decision: January 22, 2015
Revised: April 1, 2015
Accepted: May 20, 2015
Article in press: May 21, 2015
Published online: July 14, 2015
Processing time: 199 Days and 23 Hours
AIM: To investigate the effects of our tumor vaccines on reversing immune tolerance and generating therapeutic response.
METHODS: Vaccines were synthesized by solid phase using an Fmoc strategy, where a small molecule toll-like receptor-7 agonist (T7) was conjugated to a monoclonal gastric cancer 7 antigen mono-epitope (T7-MG1) or tri-epitope (T7-MG3). Cytokines were measured in both mouse bone marrow dendritic cells and mouse spleen lymphocytes after exposed to the vaccines. BALB/c mice were intraperitoneally immunized with the vaccines every 2 wk for a total of three times, and then subcutaneously challenged with Ehrlich ascites carcinoma (EAC) cells. Three weeks later, the mice were killed, and the tumors were surgically removed and weighed. Serum samples were collected from the mice, and antibody titers were determined by ELISA using an alkaline phosphate-conjugated detection antibody for total IgG. Antibody-dependent cell-mediated cytotoxicity was detected by the lactate dehydrogenase method using natural killer cells as effectors and antibody-labeled EAC cells as targets. Cytotoxic T lymphocyte activities were also detected by the lactate dehydrogenase method using lymphocytes as effectors and EAC cells as targets.
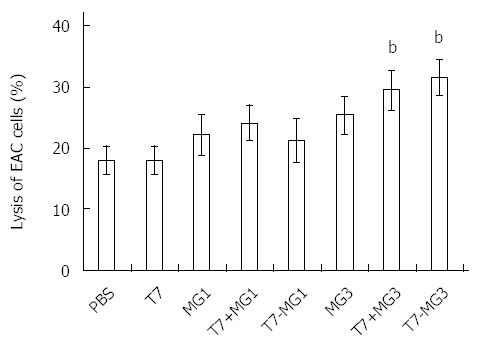
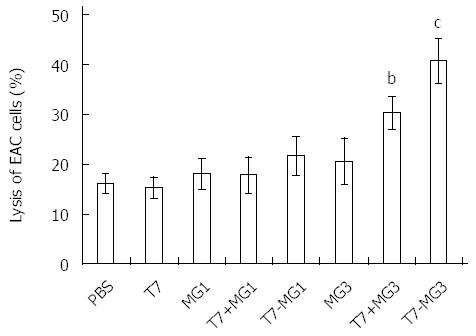
RESULTS: Vaccines were successfully synthesized and validated by analytical high performance liquid chromatography and electrospray mass spectrometry, including T7, T7-MG1, and T7-MG3. Rapid inductions of tumor necrosis factor-α and interleukin-12 in bone marrow dendritic cells and interferon γ and interleukin-12 in lymphocytes occurred in vitro after T7, T7-MG1, and T7-MG3 treatment. Immunization with T7-MG3 reduced the EAC tumor burden in BALB/c mice to 62.64% ± 5.55% compared with PBS control (P < 0.01). Six or nine weeks after the first immunization, the monoclonal gastric cancer 7 antigen antibody increased significantly in the T7-MG3 group compared with the PBS control (P < 0.01). As for antibody-dependent cell-mediated cytotoxicity, antisera obtained by immunization with T7-MG3 were able to markedly enhance cell lysis compared to PBS control (31.58% ± 2.94% vs 18.02% ± 2.26%; P < 0.01). As for cytotoxic T lymphocytes, T7-MG3 exhibited obviously greater cytotoxicity compared with PBS control (40.92% ± 4.38% vs 16.29% ± 1.90%; P < 0.01).
CONCLUSION: A successful method is confirmed for the design of gastric cancer vaccines by chemical conjugation of T7 and multi-repeat-epitope of monoclonal gastric cancer 7 antigen.
Core tip: Immunization with toll-like receptor-7 agonist conjugated with a monoclonal gastric cancer 7 antigen tri-epitope was efficacious in reversing tolerance and generating a therapeutic response in Ehrlich ascites carcinoma tumor-bearing mice. This occurred by enhancing the specific humoral and cellular immunity, which were displayed as higher antibody titers, antibody-dependent cell-mediated cytotoxicity, and cytotoxic T lymphocyte activity.
- Citation: Wang XD, Gao NN, Diao YW, Liu Y, Gao D, Li W, Wan YY, Zhong JJ, Jin GY. Conjugation of toll-like receptor-7 agonist to gastric cancer antigen MG7-Ag exerts antitumor effects. World J Gastroenterol 2015; 21(26): 8052-8060
- URL: https://www.wjgnet.com/1007-9327/full/v21/i26/8052.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i26.8052
Gastric cancer is one of the most common malignancies and the second leading cause of cancer-related death worldwide. Surgery, chemotherapy, and radiotherapy are frequently used in the treatment of gastric cancer. Immunotherapy, especially tumor vaccine, has now drawn more and more attention for its advantages, such as low toxicity and long-term effect. However, due to the lack of tumor-specific antigens, few effective gastric cancer vaccines have been investigated.
Monoclonal gastric cancer 7 antigen (MG7-Ag), discovered by Dr. Fan Dai-Ming (the Fourth Military Medical University, Xi’an, China), is a tumor-associated antigen with high specificity and selectivity. MG7-Ag expression in gastric mucosa is closely associated with high risk of gastric atypical hyperplasia and malignant change, which suggests that MG7-Ag plays an important role in gastric cancer progression[1,2]. Many studies have been carried out concerning tumor prevention and treatment based on MG7-Ag, including DNA vaccines and recombinant protein vaccines[3,4]. However, weak immunogenicity of tumor-associated antigens is a drawback of tumor vaccines, which requires the addition of adjuvants for optimal performance.
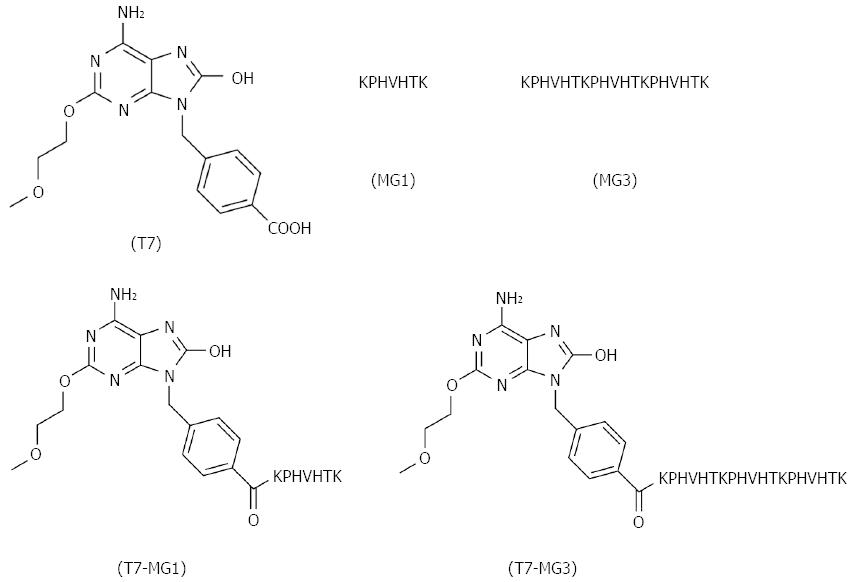
Toll-like receptors (TLRs) are fundamental elements of immune system, which facilitate our understanding of the innate and adaptive immunity. The application of TLR ligands as adjuvants in vaccine development is currently under intensive investigation, as they are not only capable of protecting against infectious diseases[5,6], but also serving as a promising method for cancer treatment[7,8]. Evidence has revealed that vaccine-mediated tumor inhibition was caused by specific immunity against MUC1 and nonspecific adjuvant effects of the TLR2 agonist[9]. Among all kinds of TLR ligands, the TLR7 agonist is the only choice of small molecule synthetic compound, which is more convenient to obtain than other biomacromolecules, such as TLR4 and TLR9 ligands. The TLR7 agonist has also been reported with immunostimulatory capacity, and applied in the research of vaccines against several types of tumors, such as leukemia and breast cancer[10,11]. Imiquimod, the classic TLR7 agonist, lacks the chemical group to couple with proteins or peptides. Therefore, we adopted another well-studied TLR7 agonist (T7; chemical structure is shown in Figure 1) where its free carboxyl group can link to the amino group of peptides[12].
Here, we constructed a novel gastric cancer vaccine by covalently coupling the small molecule T7 with MG7-Ag mono-epitope (MG1; sequence used widely in tumor vaccines), and examined its efficiency in eliciting humoral and cellular immune response, reversing tumor tolerance, and generating a protective effect. Furthermore, we designed an MG7-Ag tri-epitope (MG3; epitope sequence repeated three times) as a superantigen, and conjugated it with T7 to constitute a more potent vaccine. Our research revealed that conjugation of T7 and MG3 was critical for inducing optimal immune responses, displayed by cytokine detection, antibody titer determination, antibody-dependent cell-mediated cytotoxicity (ADCC), cytotoxic T lymphocyte (CTL) activity, and a tumor challenge assay.
T7 was synthesized as described before[12]. The peptides were synthesized by solid phase using an Fmoc strategy[13]. 2-Chlorotrityl chloride resin (GL BIO Company, Shanghai, China) was the solid support loaded at 1.0 mmol/g. The following N-protected Fmoc amino acids were applied as the functionalized amino acids: Fmoc-Lys(Boc)-OH, Fmoc-Thr(tBu)-OH, Fmoc-His(Trt)-OH, Fmoc-Val-OH, Fmoc-Pro-OH (GL BIO Company, Shanghai, China) and T7. For the synthesis, 2-(1 H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate was used as the coupling reagent, while each amino acid was used at eight equal molar quantities. Fmoc deprotection was carried out in a mixture of piperidine and dimethylformamide at a ratio of 2:8 (v/v). Disaggregation of the peptide from the resin was performed for 3.5 h in a solution containing trifluoroacetic acid, phenol, water, and triisprpylsilane at a ratio of 88:5:5:2 (v/v). Following addition of cold diethyl ether for precipitation, peptides were dissolved with 0.1% trifluoroacetic acid in water/acetonitrile and lyophilized. Finally, a purity of at least 95% (UV detection at 214 nm and 254 nm) in all peptides was validated by analytical high performance liquid chromatography using a C18 column (5 μm, 300 Å, 10 mm × 200 mm). Electrospray mass spectrometry was also performed to further identify the peptides.
Cytokines were measured in both mouse bone marrow dendritic cells (BMDCs) and mouse spleen lymphocytes. BMDCs were generated as described previously[14]. Briefly, bone marrow cells from femurs and tibiae of BALB/c mice were cultured at 37 °C for 6 d in X-vivo 15 medium (Lonza Group, Basel, Switzerland) containing granulocyte-macrophage colony-stimulating factor (GM-CSF) (10 ng/mL) and interleukin (IL)-4 (10 ng/mL). Spleen lymphocytes were isolated from BALB/c mice using Mouse Lymphocyte Separation Medium (Dakewe, Beijing, China), according to the supplier’s manual. BMDCs and lymphocytes were seeded in 96-well plates at a density of 5 × 104 cells per well. Vaccines were added at a final concentration ranging from 0.01 μmol/L to 10 μmol/L and incubated for 24 h. Culture supernatants were collected and cytokine quantification of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-12 was performed using Mouse TNF-α, IFN-γ, and IL-12 p70 ELISA Ready-SET-Go reagent sets (eBioscience, San Diego, CA, United States), according to the supplier’s manual. Briefly, an ELISA plate was first coated with capture antibody overnight at 4 °C, and then filled successively with block solution, culture supernatant, and detection antibody for 1 h at room temperature. Finally, substrate and stop solution were added to each well, and the optical density was measured at 450 nm with a spectrophotometer (BioTek, Winooski, VT, United States).
This study was approved by the Laboratory Animal Ethics Committee of Shenzhen University. The animal protocol was designed to minimize pain or discomfort to the animals. Female 4-wk-old BALB/c mice weighing 15-20 g were purchased from the Medical Laboratory Animal Center (Guangzhou, Guangdong, China). All mice were housed in constant laboratory conditions of a 12 h light/dark cycle and specific pathogen-free conditions, and fed with water and food ad libitum. After being acclimated for 1 wk, BALB/c mice were used to evaluate the immunogenicity of the vaccines, by immunizations with T7, MG1, T7+MG1 (unconjugated), T7-MG1 (conjugated), MG3, T7+MG3 (unconjugated), T7-MG3 (conjugated), and PBS as control. For these experiments, 25 μg of T7-MG3 or equal molar quantities of other vaccines were administered i.p. to each mouse every 2 wk for a total of three times.
After 6 wk immunizations, each BALB/c mouse was subcutaneously challenged with 1 × 107 Ehrlich ascites carcinoma (EAC) cells, and the expression of MG7-Ag was assessed by Western blot using MG7-Ag antibody (a gift from Dr. Fan Dai-Ming and Dr. Nie Yong-Zhan). Three weeks after challenge, the mice were killed, and the tumors were surgically removed and weighed.
After each immunization, blood samples were collected from the mice and centrifuged at 3000 g for 15 min to obtain serum samples. Antibody titers in serum were determined by ELISA using an alkaline phosphate-conjugated detection antibody for total IgG (Millipore Corp., Billerica, MA, United States). Briefly, an ELISA plate was coated with BSA-MG1 (peptide sequence is BSA-KPHVHTK) overnight at 4 °C, then incubated successively with block solution for 2 h, serum samples (1:50 diluted), and detection antibody for 1 h at room temperature. Finally, p-NPP substrate (Millipore Corp.) and stop solution were added to each well, and the optical density was measured at 405 nm with a spectrophotometer (BioTek).
At the time of sacrifice, serum samples from the mice were diluted 1:25 and incubated with EAC tumor cells for 30 min at 37 °C. Natural killer (NK) cells, isolated from normal BALB/c mice using a Mouse NK Cell Separation Kit (Hao Yang, Tianjin, China), were used as effectors and seeded with the antibody-labeled EAC cells for 4 h at an effector-to-target cell ratio of 50:1. Cytotoxicity was measured by the lactate dehydrogenase (LDH) method using Non-Radioactive Cytotoxicity Assay (Promega Corp., Madison, WI, United States), according to the supplier’s manual. Briefly, after incubation, culture supernatant was transferred to an ELISA plate, followed by the addition of substrate solution for 30 min at room temperature. Finally, stop solution was filled, and the optical density was measured at 490 nm with a spectrophotometer (BioTek).
At the time of sacrifice, lymphocytes, separated from the spleen of each mouse by Mouse Lymphocyte Separation Medium (Dakewe, Beijing, China), were used as effectors. EAC tumor cells were used as target cells and incubated with lymphocytes for 4 h at an effector-to-target cell ratio of 100:1. Cytotoxicity was also measured by the LDH method as described above.
Data are expressed as mean ± SE for the indicated number of independently performed experiments. Student’s t test was used for the determination of statistical significance. The difference was considered to be statistically significant at P < 0.05. The statistical methods of this study were reviewed by Dr. Gao Kai-Ping from the School of Medicine, Shenzhen University.
T7 was synthesized as described above and used in the preparation of other vaccines (Figure 1). The following four peptides were synthesized by solid phase method: MG1 (peptide sequence is KPHVHTK), MG3 (peptide sequence is KPHVHTKPHVHTKPHVHTK), T7-MG1, and T7-MG3. T7 and MG7-Ag were also used in combination without chemical conjugation by mixing equal molar quantities of T7 and MG1 (T7+MG1) or MG3 (T7+MG3). All of above-mentioned compounds were confirmed by mass spectrometry and high performance liquid chromatography.
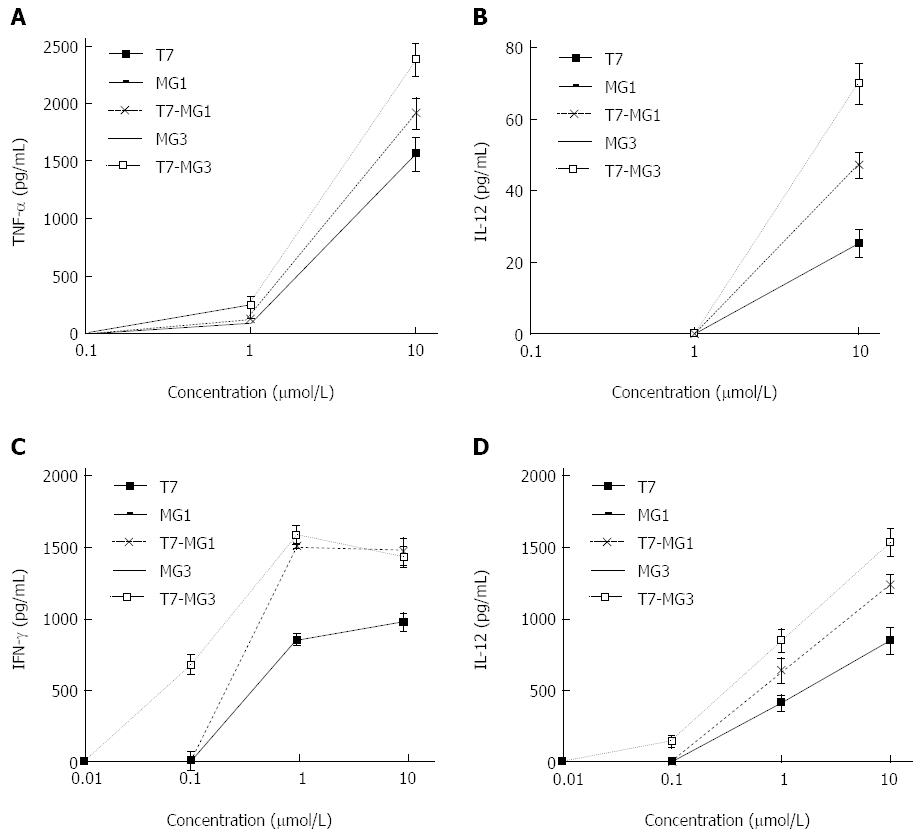
To examine the activity on initiating the production of necessary cytokines, mouse BMDCs and spleen lymphocytes were exposed to vaccines at indicated concentrations. As shown in Figure 2A and B, the levels of two cytokines (TNF-α and IL-12) remained unchanged when the BMDCs were incubated with MG1 or MG3 alone. However, T7 displayed dose-dependent increases of TNF-α release in the range of 0.1 μmol/L to 10 μmol/L, and IL-12 release in the range of 1 μmol/L to 10 μmol/L. Significantly higher levels of cytokines were detected when the MG7-Ag epitope was conjugated to T7 (T7-MG1 or T7-MG3) compared to T7 alone (P < 0.05). Moreover, 10 μmol/L T7-MG3 was more potent in raising cytokine release levels of TNF-α and IL-12 than T7-MG1. Similar results were demonstrated for cytokines released in lymphocytes (Figure 2C and D). Furthermore, 0.1 μmol/L T7-MG3, rather than 0.1 µmol/L T7-MG1, increased the levels of IFN-γ and IL-12, indicating that T7-MG3 had a stronger stimulating effect on mouse lymphocytes than T7-MG1.
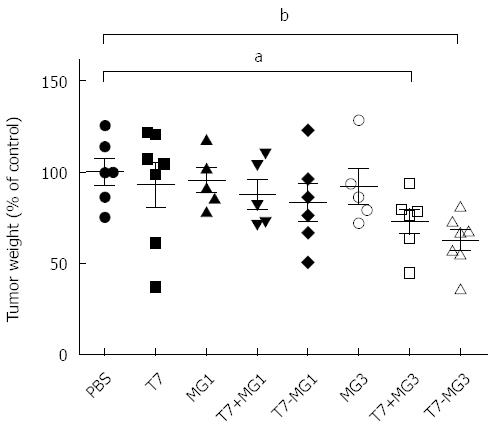
EAC cells, which have been verified for the presence of MG7-Ag[3], were used to challenge the BALB/c mice to investigate the protective ability of tumor vaccines. We also confirmed the expression of MG7-Ag in EAC cells by Western blot (data not shown). As shown in Figure 3, immunization with T7, MG1, or MG3 alone did not exhibit much improvement of antitumor properties as compared with PBS control. Although T7+MG1 and T7-MG1 led to somewhat smaller tumors, their differences were not significant compared with PBS control. Obvious reduction of tumor weight was observed when T7 was used together with the MG3, whether as a commixture (T7+MG3) or chemical conjugation (T7-MG3); the difference between T7+MG3 and T7-MG3 was not significant. Meanwhile, mouse breast cancer 4T1 cells (absence of MG7-Ag expression ascertained by Western blot) were included as a negative control. Neither T7+MG3 nor T7-MG3 could reduce the 4T1 tumor burden (data not shown), indicating the MG7-Ag specificity of the observed effects in EAC cells.
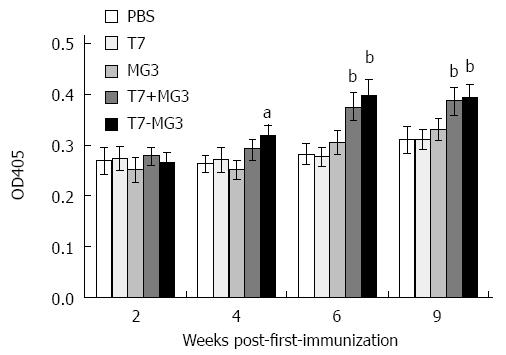
Serum antibody titers against MG7-Ag were determined by ELISA assay. Six or nine weeks after first immunization, MG7-Ag antibody increased significantly in T7+MG3 and T7-MG3 groups compared to PBS control, while the difference of immune effect was negligible between chemical conjugation (T7-MG3) and simple commixture (T7+MG3). On the other hand, T7, MG1, or MG3 alone exerted little impact on MG7-Ag antibody (Figure 4). Vaccines constructed with MG1 (T7+MG1 and T7-MG1) also could not elicit antibody response, independent of the times of immunization (data not shown).
ADCC was determined by addition of serum samples and NK cells (cytotoxic effector cells) to EAC tumor cells (target cells), and measurement of released LDH activity (Figure 5). Antisera collected from the mice immunized with T7 and MG1, whether used alone or in combination (T7+MG1 or T7-MG1), did not induce significant cancer cell lysis compared with PBS control. Antibodies derived from MG3 displayed stronger cytotoxicity to a certain degree, yet still not significantly different from the PBS control. However, antisera obtained by immunization with T7+MG3 and T7-MG3 were able to markedly increase cell lysis compared to PBS or MG3 alone. No remarkable ADCC effect of vaccines was found when mouse breast cancer 4T1 cells were used as target cells for negative control (data not shown).
To assess the ability of vaccines to activate cellular immunity, CTL activity was determined by LDH assay, which is identically sensitive to the 51Cr release assay when measuring cytotoxicity. As shown in Figure 6, immunization with T7, MG1, or MG3 alone did not induce more CTL in BALB/c mice than in PBS control. As expected, CTL activated by T7+MG3 and T7-MG3 exhibited obviously greater cytotoxicity compared with control, being significantly higher with T7-MG3 compared to T7+MG3 (P < 0.05). However, vaccines constructed with MG1, both T7+MG1 and T7-MG1, did not display similar high lytic activity as with T7+MG3 and T7-MG3. Similar with ADCC, no CTL effect of vaccines was detected with 4T1 cells as negative control (data not shown).
Gastric cancer is the second leading cause of cancer-related death worldwide, and therapeutic effect was poor by traditional treatment (surgery, chemotherapy, and radiation) with a low five-year survival rate. Therefore, immunotherapy offers another direction in prevention and treatment of gastric cancer. Nowadays, adoptive cell therapy, gene therapy, monoclonal antibody therapy, and cancer vaccines are frequently used with some success[15]. Clinical studies of adoptive immunotherapies have shown that longer survival was achieved in gastric cancer patients treated with chemotherapy in combination with tumor-associated lymphocytes or cytokine-induced killer cells than with chemotherapy alone[16,17]. As for cancer vaccines, nanoparticles with the melanoma-associated antigen 3 peptide show the ability to stimulate immune responses in vivo and kill mouse fore-stomach carcinoma cells[18]. Vaccination with human vascular endothelial growth factor receptors 1 and 2 combined with chemotherapy is well tolerated and highly effective in advanced or recurrent gastric cancer[19]. Occurrence of novel tumor antigen is one of the most important immunologic characteristics of tumor cells. So far, many tumor-associated antigens have been explored and applied in the research of tumor vaccines in order to elicit specific immune responses in the host, either by themselves or after loading onto antigen presenting cells. Sipuleucel-T, containing prostate acid phosphatase antigen, is the first cell-based immunotherapeutic vaccine approved by the Food and Drug Administration for patients with metastatic castration-resistant prostate cancer[20]. Clinical data have also shown that therapeutic cancer vaccine targeting the MUC1 antigen is effective in non-small-cell lung cancer patients[21].
MG7-Ag, first discovered in the KATO III cell line, is one kind of gastric cancer-associated antigen and is expressed at a high level in the serum and tissue of gastric cancer patients[22]. Recently, it has been investigated as a biomarker with high sensitivity and specificity for gastric cancer diagnosis[23], and many methods for MG7-Ag detection are being developed, such as immunohistochemical stain and ELISA[24,25]. It is worth noting that MG7-Ag has also been applied in the development of tumor vaccines. Heterologous prime-boost immunization with oral MG7-Ag DNA vaccine induces significant immune response to gastric cancer[3]. A fusion protein containing MG7-Ag antibody and superantigen staphylococcal enterotoxin B has been constructed with the ability to kill SGC7901 human gastric cancer cells[4]. Despite the potency of the MG7-Ag against cancer shown by previous studies, MG7-Ag epitope peptides are much more widely used in current gastric cancer vaccines, circumventing the difficulty of isolating and purifying MG7-Ag directly from tumor tissue. Moreover, a multi-repeat-epitope is a well-defined strategy of vaccine construction for enhancing the effect of immunization, which has also been adopted in MG7-Ag epitope-based research[26]. Therefore, in this study, MG7-Ag mono-epitope and tri-epitope peptides were used as antigens and expected to achieve specific antitumor effect. However, neither MG1 nor MG3 alone had much impact on immunity enhancement of BALB/c mice, displayed as a minimal change of antibody titers, ADCC, CTL (in vivo), and cytokine induction (in vitro) after antigen immunization. MG1 and MG3 alone also could not efficiently inhibit the growth of EAC tumor cells in vivo. These results confirm the fact that tumor-related antigens, especially epitopes, are often poorly immunogenic and do not sufficiently activate immune cells for recognizing and invading the tumor cells.
Several studies have demonstrated that conjugations of TLR7 ligands and antigens trigger better immunogenicity compared to noncoupled antigens alone[27]. Oh et al[28] showed that a TLR7 agonist-Ag conjugate elicited CD8+ T cell responses based on the engagement of DC cross-presentation pathways. Similarly, we used a promising small molecule compound (T7) for the attachment to MG7-Ag in the study of tumor immunotherapy. Chemical conjugation of this agonist and mouse serum albumin resulted in 10-100-fold potent cytokine production in vitro, and significant delay in mortality in BALB/c mice inoculated with influenza virus[29]. When the compound was conjugated to phospholipid and administered systemically in mice, prolonged increases occurred in the levels of proinflammatory cytokines. The phospholipid-TLR7 agonist conjugate could be further used as adjuvant during vaccination, which resulted in the boost of both Th1 and Th2 antigen-specific immune responses[12].
The data presented here showed that T7 improved the innate immunity by rapid induction of inflammatory mediators TNF-α and IL-12 in BMDCs and IFN-γ and IL-12 in lymphocytes in vitro, while the conjugation (T7-MG1 or T7-MG3) yielded more potent effects. Cytokines produced following the activation of TLR7 can stimulate the expression of costimulatory proteins for optimum interactions between helper T, B, and antigen-presenting cells[9]. Therefore, covalent attachment of T7 and MG7-Ag is critical for the induction of adaptive immunity, including CTLs and antibodies. Indeed, vaccination with T7-MG3 reduced the EAC tumor burden in vivo, via eliciting cytotoxicity of T lymphocytes and IgG antibodies that could specifically lyse cancer cells. The results of the present study also showed that the differences between T7-MG3 and PBS control in CTL activities were more noticeable than in antibody titers and ADCC activities, implying that cellular immunity made a greater contribution than humoral immunity to the vaccination of T7-MG3.
Over the past decade, numerous studies have been carried out concerning how to promote antigen presentation for improving immune responses, such as with heat shock proteins or bacterial toxins linked to antigens[30]. These strategies for DC uptake have the disadvantage that immune suppression might occur due to the antigenicity of the targeting device, which could be avoided by the low intrinsic immunity of small molecule T7. Meanwhile, no remarkable effect of T7+MG1 or T7-MG1 was observed on either the promotion of immunity or the reduction of tumor, revealing the importance of multi-repeat-epitope used for vaccine development. As for MG3, chemical conjugation with T7 was more effective than simple commixture when for vaccination of BALB/c mice, especially on the induction of CTLs. Thus, based on the existing reports and the results present here, it is reasonable to suppose that covalent attachment is a better method for the investigation of vaccines than simple mixture, considering that distribution and metabolism of T7 and antigen could be more coincident systemically.
In conclusion, an effective tumor vaccine targeting MG7-Ag has been constructed by chemical conjugation of T7 with MG3. Its capacity was ascertained to generate CTLs and ADCC-mediating antibodies recognizing MG7-Ag and provide a therapeutic benefit in EAC tumor-bearing mice. T7 could elicit the nonspecific antitumor responses and strengthen the specific humoral and cellular immune responses, which is imperative in tumor vaccines due to the fact that tumor antigens rarely possess immunogenicity. This concept of vaccine construction can be further applied in other kinds of tumors, either as single immunotherapy or combined with other therapies.
We thank Dr. Dai-Ming Fan and Dr. Yong-Zhan Nie from the Fourth Military Medical University for their guidance and assistance in this study.
Gastric cancer is one of the most common malignant tumors, in which tumor vaccines play increasingly important roles for their advantages, such as low toxicity and long-term effect. Weak immunogenicity of tumor-associated antigens and lack of immune recognition are main reasons that tumor cell escape surveillance and eradication by the immune system, thus requiring the addition of adjuvant for optimal performance of tumor-associated antigens.
Recently, monoclonal gastric cancer 7 antigen (MG7-Ag) was investigated as a biomarker with high sensitivity and specificity for gastric cancer diagnosis, and many detecting methods are being developed, such as immunohistochemical stain and ELISA. It is worth noting that MG7-Ag has also been applied in the development of tumor vaccines, such as DNA and recombinant protein vaccines. On the other hand, the applications of toll-like receptor (TLR) ligand as an adjuvant in vaccine development are under intensive investigation, against not only infectious diseases, but also malignant tumors.
In this study, the authors constructed tumor vaccines by covalent attachment of MG7-Ag and a TLR7 agonist (T7). Conjugation of T7 with an MG7-Ag tri-epitope (T7-MG3) significantly increased the release of tumor necrosis factor-α and interleukin (IL)-12 in bone marrow dendritic cells and interferon-γ and IL-12 in mouse lymphocytes in vitro. Immunization with T7-MG3 was efficacious in reversing tolerance and generating therapeutic response in Ehrlich ascites carcinoma-bearing mice, through enhancing specific humoral and cellular immunity, which were displayed as higher antibody titers, antibody-dependent cell-mediated cytotoxicity, cytotoxic T lymphocyte activity. Although a simple commixture of T7 and MG3 was able to inhibit tumor growth in tumor-bearing mice because of the immune enhancement, the effects of T7-MG3 were greater, especially on the induction of cytotoxic T lymphocytes. However, vaccines with an MG7-Ag mono-epitope showed no significant effect on either the promotion of immunity or the reduction of tumor size.
This study demonstrated a successful way for the design of gastric cancer vaccines by combined use of T7 and MG7-Ag, and the importance of multi-repeat-epitopes and chemical conjugation. This concept of vaccine construction can be further applied in other kinds of tumors, either as single immunotherapy or combined with other therapies.
MG7-Ag is a tumor-associated antigen with high specificity and selectivity, whose expression in gastric mucosa is closely associated with high risk of gastric atypical hyperplasia and malignant change. Among all kinds of TLR ligands, the TLR7 agonist is the only choice of small molecule synthetic compounds, which is more convenient to obtain than other ligands.
This study aims to develop a vaccine against gastric cancer by conjugating multi-repeat epitopes of gastric cancer antigen MG7-Ag and TLR7 agonist. The authors demonstrate that this conjugate elicits specific humoral, natural killer, and cytotoxic T lymphocyte responses in mice and can reduce the tumor burden. This paper is clearly presented and is of potential interest and clinical relevance.
P- Reviewer: Line A S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Guo DL, Dong M, Wang L, Sun LP, Yuan Y. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H. pylori -associated gastric diseases. World J Gastroenterol. 2002;8:1009-1013. [PubMed] |
| 2. | Liu J, Hu JL, Zhang XY, Qiao TD, Chen XT, Wu KC, Ding J, Fan DM. The value of MG7 antigen in predicting cancerous change in dysplastic gastric mucosa. Int J Clin Pract. 2002;56:169-172. [PubMed] |
| 3. | Lin T, Liang S, Meng F, Han Q, Guo C, Sun L, Chen Y, Liu Z, Yu Z, Xie H. Enhanced immunogenicity and antitumour effects with heterologous prime-boost regime using vaccines based on MG7-Ag mimotope of gastric cancer. Clin Exp Immunol. 2006;144:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Tong Q, Liu K, Lu XM, Shu XG, Wang GB. Construction and characterization of a novel fusion protein MG7-scFv/SEB against gastric cancer. J Biomed Biotechnol. 2010;2010:121094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN. A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93. PLoS One. 2014;9:e83884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Winckelmann AA, Munk-Petersen LV, Rasmussen TA, Melchjorsen J, Hjelholt TJ, Montefiori D, Østergaard L, Søgaard OS, Tolstrup M. Administration of a Toll-like receptor 9 agonist decreases the proviral reservoir in virologically suppressed HIV-infected patients. PLoS One. 2013;8:e62074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Fujita Y, Taguchi H. Overview and outlook of Toll-like receptor ligand-antigen conjugate vaccines. Ther Deliv. 2012;3:749-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Baxevanis CN, Voutsas IF, Tsitsilonis OE. Toll-like receptor agonists: current status and future perspective on their utility as adjuvants in improving anticancer vaccination strategies. Immunotherapy. 2013;5:497-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA, Gendler SJ, Boons GJ. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci USA. 2012;109:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 445] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 10. | Smits EL, Cools N, Lion E, Van Camp K, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The Toll-like receptor 7/8 agonist resiquimod greatly increases the immunostimulatory capacity of human acute myeloid leukemia cells. Cancer Immunol Immunother. 2010;59:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Demaria S, Vanpouille-Box C, Formenti SC, Adams S. The TLR7 agonist imiquimod as an adjuvant for radiotherapy-elicited in situ vaccination against breast cancer. Oncoimmunology. 2013;2:e25997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Chan M, Hayashi T, Kuy CS, Gray CS, Wu CC, Corr M, Wrasidlo W, Cottam HB, Carson DA. Synthesis and immunological characterization of toll-like receptor 7 agonistic conjugates. Bioconjug Chem. 2009;20:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Huang J, Cao Y, Bu X, Wu C. Residue analysis of a CTL epitope of SARS-CoV spike protein by IFN-gamma production and bioinformatics prediction. BMC Immunol. 2012;13:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, Liu L, Wang C, Cai L, Ma Y. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J Control Release. 2013;168:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20:1657-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Kono K, Takahashi A, Ichihara F, Amemiya H, Iizuka H, Fujii H, Sekikawa T, Matsumoto Y. Prognostic significance of adoptive immunotherapy with tumor-associated lymphocytes in patients with advanced gastric cancer: a randomized trial. Clin Cancer Res. 2002;8:1767-1771. [PubMed] |
| 17. | Jiang JT, Shen YP, Wu CP, Zhu YB, Wei WX, Chen LJ, Zheng X, Sun J, Lu BF, Zhang XG. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol. 2010;16:6155-6162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Yang J, Li ZH, Zhou JJ, Chen RF, Cheng LZ, Zhou QB, Yang LQ. Preparation and antitumor effects of nanovaccines with MAGE-3 peptides in transplanted gastric cancer in mice. Chin J Cancer. 2010;29:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Masuzawa T, Fujiwara Y, Okada K, Nakamura A, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Osawa R. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol. 2012;41:1297-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Westdorp H, Sköld AE, Snijer BA, Franik S, Mulder SF, Major PP, Foley R, Gerritsen WR, de Vries IJ. Immunotherapy for prostate cancer: lessons from responses to tumor-associated antigens. Front Immunol. 2014;5:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Butts C, Maksymiuk A, Goss G, Soulières D, Marshall E, Cormier Y, Ellis PM, Price A, Sawhney R, Beier F. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol. 2011;137:1337-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Ren J, Chen Z, Juan SJ, Yong XY, Pan BR, Fan DM. Detection of circulating gastric carcinoma-associated antigen MG7-Ag in human sera using an established single determinant immuno-polymerase chain reaction technique. Cancer. 2000;88:280-285. [PubMed] |
| 23. | Zhang L, Ren J, Pan K, Ma J, Li J, Shen L, Zhang X, Li J, Fan D, Gail M. Detection of gastric carcinoma-associated MG7-Ag by serum immuno-PCR assay in a high-risk Chinese population, with implication for screening. Int J Cancer. 2010;126:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Fang X, Tie J, Xie Y, Li Q, Zhao Q, Fan D. Detection of gastric carcinoma-associated antigen MG7-Ag in human sera using surface plasmon resonance sensor. Cancer Epidemiol. 2010;34:648-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Jin B, Wang X, Jin Y, Xia W, Chen B, Liu L, Chen Z, Hong L, Du W, Yan K. Detection of serum gastric cancer-associated MG7-Ag from gastric cancer patients using a sensitive and convenient ELISA method. Cancer Invest. 2009;27:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Chen Y, Wu K, Guo C, Liu C, Han S, Lin T, Ning X, Shi R, Shi Y, Fan D. A novel DNA vaccine containing four mimicry epitopes for gastric cancer. Cancer Biol Ther. 2005;4:308-312. [PubMed] |
| 27. | Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1553] [Cited by in RCA: 1410] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 28. | Oh JZ, Kedl RM. The capacity to induce cross-presentation dictates the success of a TLR7 agonist-conjugate vaccine for eliciting cellular immunity. J Immunol. 2010;185:4602-4608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Wu CC, Hayashi T, Takabayashi K, Sabet M, Smee DF, Guiney DD, Cottam HB, Carson DA. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc Natl Acad Sci USA. 2007;104:3990-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Tacken PJ, Torensma R, Figdor CG. Targeting antigens to dendritic cells in vivo. Immunobiology. 2006;211:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |