Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7563
Peer-review started: January 7, 2015
First decision: January 22, 2015
Revised: February 20, 2015
Accepted: March 30, 2015
Article in press: March 31, 2015
Published online: June 28, 2015
Processing time: 173 Days and 16.6 Hours
AIM: To validate 4-sample lactose hydrogen breath testing (4SLHBT) compared to standard 13-sample LHBT in the clinical setting.
METHODS: Irritable bowel syndrome patients with diarrhea (IBS-D) and healthy volunteers (HVs) were enrolled and received a 10 g, 20 g, or 40 g dose lactose hydrogen breath test (LHBT) in a randomized, double-blinded, controlled trial. The lactase gene promoter region was sequenced. Breath samples and symptoms were acquired at baseline and every 15 min for 3 h (13 measurements). The detection rates of lactose malabsorption (LM) and lactose intolerance (LI) for a 4SLHBT that acquired four measurements at 0, 90, 120, and 180 min from the same data set were compared with the results of standard LHBT.
RESULTS: Sixty IBS-D patients and 60 HVs were studied. The genotype in all participants was C/C-13910. LM and LI detection rates increased with lactose dose from 10 g, 20 g to 40 g in both groups (P < 0.001). 4SLHBT showed excellent diagnostic concordance with standard LHBT (97%-100%, Kappa 0.815-0.942) with high sensitivity (90%-100%) and specificity (100%) at all three lactose doses in both groups.
CONCLUSION: Reducing the number of measurements from 13 to 4 samples did not significantly impact on the accuracy of LHBT in health and IBS-D. 4SLHBT is a valid test for assessment of LM and LI in clinical practice.
Core tip: Lactose hydrogen breath test (LHBT) is the preferred method for clinical diagnosis of lactose malabsorption (LM) and lactose intolerance (LI); however, repeated measurements are time consuming and require dedicated personnel. In our study, we found 4-sample LHBT has a high level of agreement with standard 13-breath sample for the clinical diagnosis of LM and LI. The 4SLHBT is a valid replacement for standard LHBT in clinical practice.
- Citation: Yang JF, Fox M, Chu H, Zheng X, Long YQ, Pohl D, Fried M, Dai N. Four-sample lactose hydrogen breath test for diagnosis of lactose malabsorption in irritable bowel syndrome patients with diarrhea. World J Gastroenterol 2015; 21(24): 7563-7570
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7563.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7563
Lactose malabsorption (LM) and lactose intolerance (LI) are common conditions affecting many of the world’s adult population[1]. The prevalence of these condition ranges from below 10% in Northern Europe to over 90% in China and Southeast Asia[2-4]. Lactose hydrogen breath test (LHBT) remains the preferred method for the clinical diagnosis of LM and LI[5,6]. In the presence of intestinal brush-border enzyme lactase, lactose is cleaved into the readily absorbable monosaccharides glucose and galactose. If lactase expression has reduced after weaning or if lactase activity is suppressed [i.e., primary or secondary lactase deficiency (LD)], unabsorbed lactose reaches the colon. Here it is fermented by the commensal flora to produce small chain fatty acids and, as a byproduct, hydrogen (H2). Hydrogen quickly diffuses through the intestinal mucosa into the venous bloodstream and is then exhaled through the lungs. Quantification of hydrogen in exhaled breath, as acquired in the hydrogen breath test, provides objective information regarding digestion and absorption of lactose.
Performing a LHBT is a time-consuming process that requires dedicated personnel. After baseline measurements and ingestion of lactose, breath hydrogen concentration and abdominal symptoms are measured every 15 min for 3-6 h. If the breath hydrogen concentration exceeds a given threshold (e.g., 20 ppm) from the baseline for two consecutive measurements, LM is diagnosed; in addition, if clinically relevant abdominal symptoms develop with or soon after an increase in breath hydrogen, then LI is diagnosed[1,7].
In 2002, Enattah et al[8] reported the single-nucleotide polymorphism C/T-13 910 above the structural gene coding for the enzyme lactose on the short arm of chromosome 2q.21-22. Many recent studies have shown that CC-13910 is a good predictor of LM and LI; there was an excellent agreement between genetic testing of lactose C/T-13 910 and LHBT[9,10]. With LHBT as the gold standard, the CC-13 910 genotype had a high sensitivity (97%) and specificity (95%) with high kα value (0.9) in diagnosing LM[11]. For the diagnosis of LM, compared to genetic testing, LHBT has been shown to range in sensitivity between 76%-94% and specificity of 77%-96%[12]. Additionally, LHBT could identify patients with LI who experience symptoms associated with LM. Disadvantages that impact the clinical application of LHBT are long testing time (typically 3-6 h), and tedious process (breath hydrogen concentration is measured every 15 min), which not only affect the patient’s compliance but also demand considerable medical resources. It has been reported that a simplified test procedure with hydrogen measurements at three time points (between 0 and 180 min) after a high dose (40-50 g) oral lactose challenge maintained high sensitivity and specificity for the diagnosis of LM and LI[13-15]. These high doses of lactose are appropriate for epidemiological studies; however, lower doses may be more relevant in clinical studies since 10-20 g lactose is more typical of the normal diet[16,17]. Further intolerance to these low- and intermediate-doses of lactose is characteristic in irritable bowel syndrome patients with diarrhea (IBS-D), a chronic functional bowel disorder that is characterized by visceral hypersensitivity to physiological stimuli[18,19]. These findings may help to identify patients with unexplained abdominal symptoms[20] and IBS-D patients[21,22] who could benefit from dietary managements.
In recent studies, we found that hydrogen breath concentration at all three doses (10 g, 20 g, 40 g) of lactose most often increased after 90 min of lactose intake and peaked between 150-180 min. Based on a sensitivity analysis of a large dataset and practical considerations, we hypothesized that reducing the number of LHBT within those time points, specifically 0, 90, 120, and 180 min (Four-Sample Lactose Hydrogen Breath Test, 4SLHBT), would not significantly impact the performance of LHBT at a low-dose (10 g, “control”), intermediate-dose (20 g, reflecting typical intake at a single meal) and high-dose (40 g, “reference standard”) lactose. The objective of this study was to test this hypothesis and validate the performance and clinical utility of 4SLHBT by comparing it with standard LHBT in the diagnosis of LM and LI among healthy volunteers (HVs) and IBS-D patients who received various doses of lactose.
IBS-D group: Consecutive IBS-D patients seen at the Gastroenterology Clinic of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine from September 2009 to August 2010 were enrolled into this trial. IBS-D was diagnosed with Rome III criteria after screening for alarm criteria[23] and excluding structural gastrointestinal pathology where clinically indicated. The control group consisted of HVs with no history of gastrointestinal disorders recruited at the same time from the urban community in Hangzhou.
Eligible participants completed genetic sequencing of the lactase gene regulatory sequence and a questionnaire about food containing lactose. A randomized, double-blinded, three-way cross-over trial was performed. All IBS-D patients and HVs underwent LHBT after an oral load of lactose at doses of 10 g, 20 g, and 40 g. The interval between each test was 7-14 d in randomized order using a random number table. The lactose meal was prepared by a researcher not involved in the examination. This researcher randomly assigned lactose dose and marked the order on disposable cups. Both the subjects and examiner were blinded from the lactose dose information.
White cells were isolated from a whole blood sample using a modified salting-out procedure, and DNA was extracted (Axygen, Union City, CA, United States). A 446-bp region within intron 13 of MCM6 (13807bp to 14253bp upstream of the lactase gene) was amplified by 35 PCR cycles [forward primer (5’-CGGATGCACTGCTGTGATGA-3’), reverse primer (5’-ACTGACCTATCCTCGTGGAATG-3’)]. Single Nucleotide Polymorphisms (SNPs) associated with lactase persistence in European (C/T-13910), African and Arabian (C/G-13907, T/G-13915, G/C-14010) populations were identified by bi-directional sequencing using Sequencher software (vs 4.0.5, GeneCodes)[8,24].
Laboratory equipment: Handheld hydrogen breath tester (Micro H2 Meter, Micro Medical Limited, England), reset before the test and calibrated with standard 100 ppm hydrogen (Micro Medical Limited, England).
Preparation before the test: Subjects were restricted from taking antibiotics and probiotics for one month and prokinetics at least 1 week before the test. Subjects refrained from eating dairy products, soy products, and other foods rich in fiber one day before the test.
Test procedure: Breath hydrogen concentration was measured twice for each subject in the fasted state before the test. The average value was taken as baseline. Then subjects drank lactose solution (10 g, 20 g, or 40 g lactose dissolved in 250 mL warm water), followed by breath hydrogen testing every 15 min for 3 h. Gastrointestinal symptoms, including nausea, bloating, borborygmi, abdominal pain and diarrhea, were also recorded during the trial. Subjects refrained from food/drink, smoking and strenuous activity during the trial.
Symptom score: The severity of gastrointestinal symptoms: nausea, bloating, borborygmus, abdominal pain, diarrhea, was self-assessed by the subjects using a 5-level visual rating scale: none, mild, moderate, severe, and very severe, scored from 0 to 4 points respectively. The highest rating score from each symptom was then added to calculate the total symptom score. Subjects were also interviewed for above gastrointestinal symptoms within 24 h after trial completion by telephone, and rated their maximum symptoms as per the visual scale.
Diagnostic criteria: LM was diagnosed if patients’ hydrogen breath concentration elevated more than 20 ppm from baseline twice consecutively within three hours of the testing period. LI was diagnosed if patients met LM diagnostic criteria, and had a one-point or more increase in the severity of above-mentioned gastrointestinal symptoms above baseline occurring more than twice during the 24 h follow-up period.
4SLHBT analysis values were derived from the same data set of hydrogen breath samples of standard LHBT at 0, 90, 120, and 180 min. Outcomes of interest included: (1) incidence of LM, LI with various lactose doses using LHBT; (2) H2 peak (P): the maximum hydrogen breath values (ppm), time to H2 peak (Tp): the time (min) taken from baseline to maximum hydrogen value; and (3) sensitivity of LM and LI diagnosis with the 4SLHBT method, using standard LHBT as reference (measuring every 15 min within 3 h of lactose intake). The primary analysis was the concordance rate between the two methods.
The Kolmogorov-Smirnov test was used for normal distribution test of continuous variables. Normally distributed data were summarized using mean ± SD, and the rates between two groups were compared using a χ2 test. All analyses were performed with SPSS 16.0 statistical software (SPSS, Chicago, IL, United States). P≤ 0.05 was regarded as significant.
The study enrolled 63 IBS-D patients and 64 healthy volunteers, who completed LHBT at all 3 lactose dose levels (10 g, 20 g, 40 g). Of them, 3 IBS-D patients and 4 HVs were excluded from the final analysis due to hydrogen breath value > 20 ppm at baseline. Thus, the final study population included 60 IBS-D patients and 60 HVs.
The genotype in all participants was C/C-13910 and no other SNP was identified on gene sequencing of the putative lactase gene regulatory sequence in any participant irrespective of group or phenotype defined by LHBT.
In IBS-D patients, with increasing dose of lactose intake (10 g, 20 g, 40 g), LM detection rates increased gradually from 41.6%, 86.6%, to 93.3%, respectively; LI detection rates increased from 18.3%, 46.7%, to 85%, respectively.
In the control group, with increasing dose of lactose intake (10 g, 20 g, 40 g), LM detection rates increased gradually from 35%, 80%, to 91.6%, respectively; and LI detection rates increased from 3.3% to 21.7% and 68.3%, respectively (Table 1).
| Lactose dose | ||||
| 10 g | 20 g | 40 g | ||
| IBS-D | LM | 25 (41.6) | 52 (86.6) | 56 (93.3) |
| LI | 11 (18.3) | 28 (46.7) | 51 (85) | |
| HVs | LM | 21 (35) | 48 (80) | 55 (91.6) |
| LI | 2 (3.3) | 13 (21.7) | 41 (68.3) | |
With increasing dose of lactose, H2 peaks were significantly higher (P < 0.01) in both the IBS-D and HV groups. Mean time to H2 peak in IBS-D group by lactose dose was: 153.5 ± 32.9 min (10 g), 151.5 ± 35.9 min (20 g), and 151.3 ± 29.6 min (40 g), respectively (P = 0.875). In the HV group the time to H2 peak by lactose dose was measured as 152.1 ± 25.2 min (10 g), 148.1 ± 30.7 min (20 g) and 139.3 ± 30.0 min (40 g) P = 0.357) (Table 2). In 96.1% (49/51) IBS-D patients and 92.7% (38/41) HVs, LI symptoms occurred between 90-180 min after lactose intake.
| Lactose dose | P value | |||||
| 10 g | 20 g | 40 g | 10 g vs 20 g | 20 g vs 40 g | ||
| IBS-D | H2 peak | 38.5 | 57 | 92 | 0.010 | 0.000 |
| (ppm) | (27.3-47.3) | (32.0-85.0) | (58.0-127.0) | |||
| Tp | 153.5 ± 32.9 | 151.5 ± 35.9 | 151.3 ± 29.6 | 0.702 | 0.613 | |
| (min) | ||||||
| HVs | H2 peak | 38 | 66 | 99 | 0.004 | 0.001 |
| (ppm) | (29.5-46) | (36.5-90.5) | (56.5-135.5) | |||
| Tp | 152.1 ± 25.2 | 148.1 ± 30.7 | 139.3 ± 30.0 | 0.602 | 0.127 | |
| (min) | ||||||
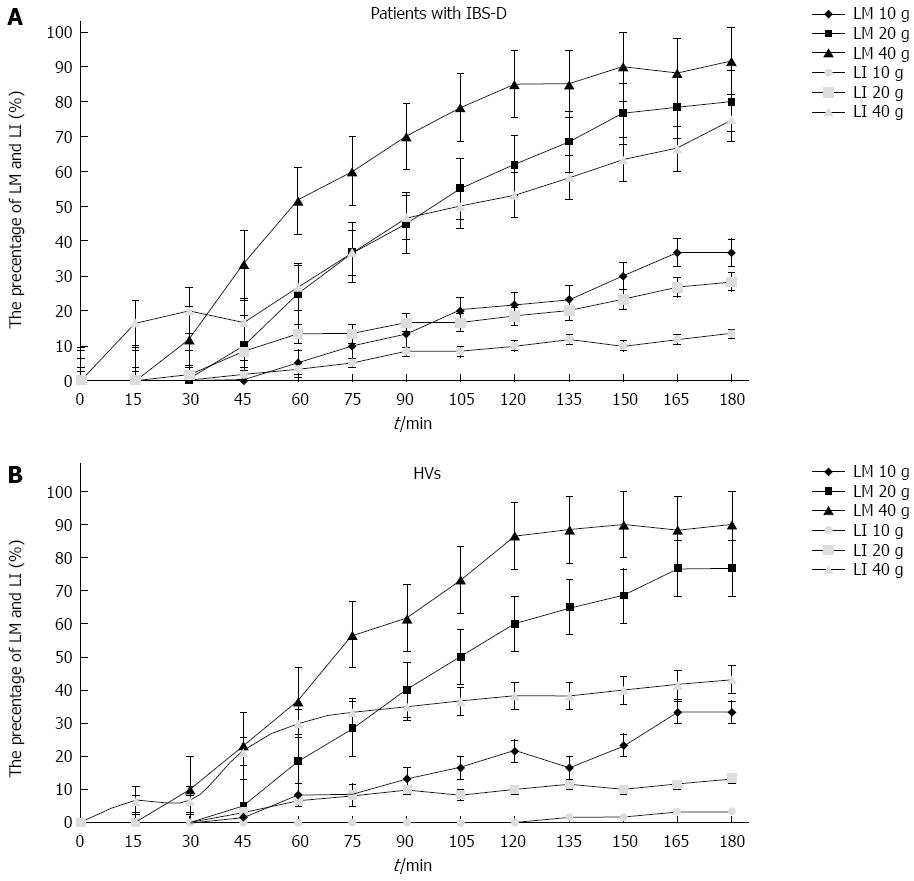
In both the IBS-D and HV groups, the diagnostic yield of LHBT increased over time for all lactose doses (10 g, 20 g, 40 g), reaching a peak at 150-180 min after lactose consumption (Figure 1A and B).
In the IBS-D group, 4SLHBT and standard LHBT showed high agreement for LM [Kappa = 0.93 (10 g), 0.87 (20 g), 0.88 (40 g)] and LI diagnosis (Kappa = 0.94 (10 g), 0.93 (20 g), 0.84 (40 g); compared to standard LHBT, 4SLHBT had high sensitivity (91%-98%) and specificity (100%) in detecting LM and LI in three-dose LHBT.
In the HV group, 4SLHBT and standard LHBT also showed high agreement for LM [Kappa = 0.93 (10 g), 0.90 (20 g), 0.82 (40 g)] and LI diagnosis (Kappa = 1.0 (10 g), 0.95 (20 g), 0.89 (40 g); compared to standard LHBT, 4SLHBT had high sensitivity (90%-100%) and specificity (100%) in detecting LM and LI in three-dose LHBT.
Agreement, sensitivity, specificity, positive predictive value, and negative predictive value of 4SLHBT are summarized in Tables 3 and 4.
| Standard LHBT | |||||||||
| 4SLHBT | LM | LI | |||||||
| IBS-D | (+) | (-) | Kappa | P value | (+) | (-) | Kappa | P value | |
| 10 g | (+) | 23 | 0 | 0.931 | < 0.001 | 10 | 0 | 0.942 | < 0.001 |
| (-) | 2 | 35 | 1 | 49 | |||||
| 20 g | (+) | 50 | 0 | 0.870 | < 0.001 | 26 | 0 | 0.933 | < 0.001 |
| (-) | 2 | 8 | 2 | 32 | |||||
| 40 g | (+) | 55 | 0 | 0.880 | < 0.001 | 47 | 0 | 0.839 | < 0.001 |
| (-) | 1 | 4 | 3 | 10 | |||||
| HVs | |||||||||
| 10 g | (+) | 19 | 0 | 0.925 | < 0.001 | 2 | 0 | 1.000 | < 0.001 |
| (-) | 2 | 39 | 0 | 58 | |||||
| 20 g | (+) | 46 | 0 | 0.902 | < 0.001 | 12 | 0 | 0.949 | < 0.001 |
| (-) | 2 | 12 | 1 | 47 | |||||
| 40 g | (+) | 53 | 0 | 0.815 | < 0.001 | 38 | 0 | 0.889 | < 0.001 |
| (-) | 2 | 5 | 3 | 19 | |||||
| LHBT | Sensitivity | Specificity | PPV | NPV | ||
| IBS-D | 10 g | LM | 92% | 100% | 100% | 94.6% |
| LI | 91% | 100% | 100% | 98% | ||
| 20 g | LM | 96% | 100% | 100% | 80% | |
| LI | 93% | 100% | 100% | 94.1% | ||
| 40 g | LM | 98% | 100% | 100% | 80% | |
| LI | 94% | 100% | 100% | 76.9% | ||
| HVs | 10 g | LM | 90% | 100% | 100% | 95.1% |
| LI | 100% | 100% | 100% | 100% | ||
| 20 g | LM | 96% | 100% | 100% | 85.7% | |
| LI | 92% | 100% | 100% | 97.9% | ||
| 40 g | LM | 96% | 100% | 100% | 71.4% | |
| LI | 93% | 100% | 100% | 86.4% |
This study determined the performance of a simplified four-sample LHBT (4SLHBT) method in a population with high prevalence of LD. LHBT is the “standard” test for the clinical diagnosis of LM and LI[16]. At high doses this test has a high sensitivity for the diagnosis of LM and can detect elevated breath hydrogen levels from bacterial degradation of as little as 2-6 g lactose in the colon[25]. In this study, the genotype in all participants was C/C-13910 and no other SNP associated with lactase persistence was present in the lactase gene regulatory sequence, which confirmed 100% of participants had LD. Compared to the genetic test, 20 g and 40 g LHBT provided an accurate diagnosis of LM with 87% and 93% sensitivity, respectively. Even this may be an underestimate because 4 patients (6.7%) reported typical LI symptoms without an increase in breath hydrogen, likely to be false negative findings because colonic fermentation did not produce hydrogen in these individuals[1,26]. This finding confirms that LHBT provides an accurate assessment of LM. The results of this study also showed that the H2 peak increased with the dose in a dose-dependent manner from 10 g to 20 g and 40 g. High H2 peak values above 100 ppm may be of clinical interest because these are associated with increased bloating and, especially, borborygmi, as found in recent clinical studies performed in the same population[18,19].
Conventional LHBT is time-consuming (usually takes 3-6 h) and requires multiple hydrogen breath tests (once every 15 min). This tedious process may have a negative impact on its clinical application and patient’s compliance. Several studies have investigated whether reducing the number of hydrogen breath samples in LHBT impacts on the sensitivity of LM diagnosis[13-15]. Compared to standard LHBT with samples taken every 15 min over 3 h, our Swiss study showed that three-sample tests had sensitivity and specificity of 83.4 and 99.7% (0-60-90 min), 95.0 and 99.2% (0-60-120 min), and 95.0 and 98.9% (0-90-120 min), respectively[15]. Similar results were reported by Di Camillo et al[14]; measurements at baseline, 120 min and 180 min had low false-negative results for LM (5.9%) compared to measurements taken every 30 min for 4 h. However, both these studies used high-dose lactose (50 g, equivalent of 1 liter of milk) and were conducted primarily to establish the presence of LM in a population with low prevalence of LD. In China, LD is universal and, therefore, the focus of study is to identify patients who experience abdominal symptoms at low doses of lactose found in the normal diet. The results of this study showed that with 3 lactose loading doses, 10g, 20 g, and 40 g, 4SLHBT achieved high sensitivity (LM > 92%, LI > 91%) and specificity (100%) for both LM and LI diagnosis compared to the standard method at all three doses. The primary analysis showed a high level of concordance between standard and 4SLHBT methods (97%-100%) with excellent agreement confirmed by the Kappa statistic (0.815-0.942).
As previous studies have shown[13-15], three samples (0, 120, 180) were also almost as equally helpful in diagnosing LM compared with 4 samples (0, 90, 120, 180) for LHBT in our study. Considering small intestinal bacterial overgrowths (SIBO) could bring about false positive results on LHBT, the H2 value of 90 min could help us estimate whether the participant had SIBO. When this point value is very high, even higher than that of 120 min or 180 min, SIBO should be considered since oro-caecal transit time in this study population is 60-90 min[27,28]. However, in a population with high prevalence of LD, SIBO would not alter the number of patients diagnosed with LM.
This study has some important strengths: (1) A total of 120 subjects was recruited, a large number compared to most other validation studies; (2) not only healthy subjects, but also IBS-D patients were recruited, a group that often present with self-reported food intolerance and are often referred for LHBT; (3) genetic testing was used as a gold-standard to confirm the presence of LD; and (4) diagnostic agreement was checked at multiple lactose doses confirming that the 4SLHBT can be applied at high doses for diagnosis of LM and also at low doses for diagnosis of LI at the levels found in the normal diet.
Limitations of the study include: (1) performance of studies in a Chinese population with primary LD could limit generalizability; however, previous studies have shown that a similar proportion of patients with LD in Western populations have lactose malabsorption and intolerance. Further, in the same cohort, reduced breath test sampling is adequate at high doses[14,15]; and (2) the duration of the standard LHBT was limited to 3 h which is shorter than that used by some other authors (up to 6 h). This decision was based on published experience with a combined LHBT scintigraphy test that showed oro-caecal transit time in the study population of 60-90 min[27,28]. Moreover, in the present study, breath hydrogen increased after a median 90 min and peaked at a median 150 min with the vast majority (93%) of participants reporting symptoms 90-180 min after the ingestion of lactose; only five individuals reported symptoms after the study at 24 h follow-up. Two IBS-D patients developed moderate diarrhea at 215 min, 245 min after lactose intake. One HV developed severe diarrhea at 230 min, two HVs developed severe bloating and borborygmi in 255, 300 min after lactose intake. The primary aim of this study was to validate 4SLHBT and not to improve the specificity of LM and LI diagnosis by LHBT.
In conclusion, these findings demonstrate a high level of agreement between standard 13-breath-sample and 4-sample LHBT for the clinical diagnosis of LM and LI. The 4SLHBT provided accurate results at high doses for formal diagnosis of LM and also at low doses for diagnosis of clinically relevant LI. Reducing the number of breath samples will free up personnel and allow more patients to take LHBT simultaneously, thus making best use of limited medical resources. The 4SLHBT is a valid replacement for standard LHBT in clinical practice.
We gratefully acknowledge the statistical support from Yun-Xian Yu in the Department of Epidemiology and Health Statistics, School of Public Health, Zhejiang University, Hangzhou, China.
Lactose hydrogen breath tests (LHBT) are widely used to diagnose lactose malabsorption (LM) and lactose intolerance (LI). The main time-consuming part of the test relates to the sampling frequency and number of breath samples.
There are many different protocols to simplify LHBT; however, most of these studies used high-dose lactose (50 g) and were conducted in a population with low prevalence of lactase deficiency.
Four-sample lactose hydrogen breath test (4SLHBT) showed high diagnostic agreement with standard LHBT (97%-100%, Kappa 0.815-0.942) with high sensitivity (90%-100%) and specificity (100%) at all three lactose doses in both study groups.
Reducing the number of measurements of breath hydrogen and symptoms from 13 to 4 samples did not impact on the LHBT results in health and IBS-D. 4SLHBT is a valid test for assessment of LM and LI in clinical practice.
This is a very interesting study. The authors assessed diagnostic accuracy of 4-sample LHBT compared to standard 13-sample LHBT and genetic tests of primary lactase deficiency in 60 irritable bowel syndrome patients with diarrhea and 60 healthy volunteers with a 10 g, 20 g, or 40 g lactose dose. The authors found 4-sample LHBT has excellent sensitivity and specificity for LM and LI. LHBT can be simplified to 4 samples (0, 90, 120, 180).
P- Reviewer: Gok F, Green J S- Editor: Qi Y L- Editor: Logan S E- Editor: Liu XM
| 1. | Levitt M, Wilt T, Shaukat A. Clinical implications of lactose malabsorption versus lactose intolerance. J Clin Gastroenterol. 2013;47:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Lomer MC, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice--myths and realities. Aliment Pharmacol Ther. 2008;27:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Wang YG, Yan YS, Xu JJ, Du RF, Flatz SD, Kühnau W, Flatz G. Prevalence of primary adult lactose malabsorption in three populations of northern China. Hum Genet. 1984;67:103-106. [PubMed] |
| 4. | Latorre G, Besa P, Parodi CG, Ferrer V, Azocar L, Quirola M, Villarroel L, Miquel JF, Agosin E, Chianale J. Prevalence of lactose intolerance in chile: a double-blind placebo study. Digestion. 2014;90:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Law D, Conklin J, Pimentel M. Lactose intolerance and the role of the lactose breath test. Am J Gastroenterol. 2010;105:1726-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Braden B. Methods and functions: Breath tests. Best Pract Res Clin Gastroenterol. 2009;23:337-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 675] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Kerber M, Oberkanins C, Kriegshäuser G, Kollerits B, Dossenbach-Glaninger A, Fuchs D, Ledochowski M. Hydrogen breath testing versus LCT genotyping for the diagnosis of lactose intolerance: a matter of age? Clin Chim Acta. 2007;383:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Krawczyk M, Wolska M, Schwartz S, Gruenhage F, Terjung B, Portincasa P, Sauerbruch T, Lammert F. Concordance of genetic and breath tests for lactose intolerance in a tertiary referral centre. J Gastrointestin Liver Dis. 2008;17:135-139. [PubMed] |
| 11. | Pohl D, Savarino E, Hersberger M, Behlis Z, Stutz B, Goetze O, Eckardstein AV, Fried M, Tutuian R. Excellent agreement between genetic and hydrogen breath tests for lactase deficiency and the role of extended symptom assessment. Br J Nutr. 2010;104:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Arola H. Diagnosis of hypolactasia and lactose malabsorption. Scand J Gastroenterol Suppl. 1994;202:26-35. [PubMed] |
| 13. | Casellas F, Malagelada JR. Applicability of short hydrogen breath test for screening of lactose malabsorption. Dig Dis Sci. 2003;48:1333-1338. [PubMed] |
| 14. | Di Camillo M, Marinaro V, Argnani F, Foglietta T, Vernia P. Hydrogen breath test for diagnosis of lactose malabsorption: the importance of timing and the number of breath samples. Can J Gastroenterol. 2006;20:265-268. [PubMed] |
| 15. | Oberacher M, Pohl D, Vavricka SR, Fried M, Tutuian R. Diagnosing lactase deficiency in three breaths. Eur J Clin Nutr. 2011;65:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J. 2013;1:151-159. [PubMed] |
| 17. | Ghoshal UC, Kumar S, Misra A, Mittal B. Lactose malabsorption diagnosed by 50-g dose is inferior to assess clinical intolerance and to predict response to milk withdrawal than 25-g dose in an endemic area. J Gastroenterol Hepatol. 2013;28:1462-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Yang J, Deng Y, Chu H, Cong Y, Zhao J, Pohl D, Misselwitz B, Fried M, Dai N, Fox M. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:262-268.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Zhu Y, Zheng X, Cong Y, Chu H, Fried M, Dai N, Fox M. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol. 2013;108:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Haberkorn BC, Ermens AA, Koeken A, Cobbaert CM, van Guldener C. Improving diagnosis of adult-type hypolactasia in patients with abdominal complaints. Clin Chem Lab Med. 2012;50:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Yang J, Fox M, Cong Y, Chu H, Zheng X, Long Y, Fried M, Dai N. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther. 2014;39:302-311. [PubMed] |
| 22. | Rana SV, Malik A. Breath tests and irritable bowel syndrome. World J Gastroenterol. 2014;20:7587-7601. [PubMed] [DOI] [Full Text] |
| 23. | Rome Foundation. Guidelines--Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J Gastrointestin Liver Dis. 2006;15:307-312. [PubMed] |
| 24. | Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS, Powell K, Mortensen HM, Hirbo JB, Osman M. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007;39:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1095] [Cited by in RCA: 855] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 25. | Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 347] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Knudsen CD, Di Palma JA. Carbohydrate challenge tests: do you need to measure methane? South Med J. 2012;105:251-253. [PubMed] |
| 27. | Zhao J, Fox M, Cong Y, Chu H, Shang Y, Fried M, Dai N. Lactose intolerance in patients with chronic functional diarrhoea: the role of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2010;31:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Zhao J, Zheng X, Chu H, Zhao J, Cong Y, Fried M, Fox M, Dai N. A study of the methodological and clinical validity of the combined lactulose hydrogen breath test with scintigraphic oro-cecal transit test for diagnosing small intestinal bacterial overgrowth in IBS patients. Neurogastroenterol Motil. 2014;26:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |