Published online Jun 28, 2015. doi: 10.3748/wjg.v21.i24.7522
Peer-review started: September 29, 2014
First decision: December 2, 2014
Revised: November 14, 2014
Accepted: March 30, 2015
Article in press: March 31, 2015
Published online: June 28, 2015
Processing time: 276 Days and 7.3 Hours
AIM: To investigate the value of magnetic resonance elastography (MRE) with regard to assessing liver functional reserve.
METHODS: Data from inpatients diagnosed with a liver tumor at an interventional radiology department from July 2013 to June 2014 were analyzed. A 3.0 Tesla magnetic resonance unit was used to scan 32 patients with confirmed diagnoses of hepatocellular carcinoma (HCC); an MRE sequence was added to the protocol, and the data were reconstructed and analyzed by two attending radiologists. Regions of interest were identified in different slices of the non-tumor liver parenchyma to measure average stiffness. In addition, the indocyanine green (ICG) test was performed no more than 1 wk before or after the magnetic resonance examination for all 32 patients; the ICG retention rate at 15 min (ICGR-15) and the ICG plasma clearance rate (ICG-K) were recorded. Correlational analyses were performed between the liver stiffness values and the ICGR-15 as well as between the liver stiffness values and the ICG-K.
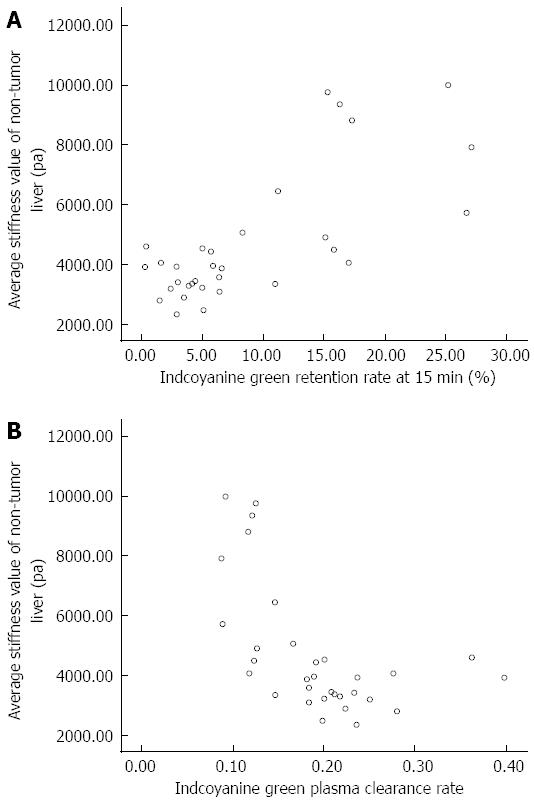
RESULTS: Magnetic resonance imaging, including an MRE sequence and the ICG test, was performed successfully in all 32 enrolled patients. None of the patients developed complications. The mean ± SD of the elasticity values measured by the two attending radiologists were 4.7 ± 2.2 kPa and 4.7 ± 2.1 kPa, respectively. The average liver stiffness value of the non-tumor parenchyma measured using MRE in HCC patients was 4.7 ± 2.2 kPa. The average ICGR-15 was 0.089 ± 0.077, and the average ICG-K was 0.19 ± 0.07. We found that the liver stiffness value of the non-tumor parenchyma was significantly and positively related to the ICGR-15 (r = 0.746, P < 0.01) as well as significantly and negatively related to the ICG-K (r = -0.599, P < 0.01). The ICGR-15 was significantly and negatively related to the ICG-K (r = -0.852, P < 0.01).
CONCLUSION: MRE is accurate and non-invasive; furthermore, it can be used to effectively assess the liver functional reserve of HCC patients.
Core tip: Liver functional determination is important for the preoperative evaluation of patients with hepatic carcinoma. We used magnetic resonance elastography to measure liver stiffness and then analyzed its correlation with the liver functional reserve test. The results showed that magnetic resonance elastography can be effectively used to assess liver functional reserve.
- Citation: Li B, Min J, Liang WR, Zhang GQ, Wu JJ, Jin K, Huang W, Ying CY, Chao M. Use of magnetic resonance elastography for assessing liver functional reserve: A clinical study. World J Gastroenterol 2015; 21(24): 7522-7528
- URL: https://www.wjgnet.com/1007-9327/full/v21/i24/7522.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i24.7522
Primary liver cancer is one of the most common malignant tumors in the world[1], and liver functional reserve is an important factor in developing a treatment strategy and determining a prognosis, especially given that the majority of patients with this disease have concurrent liver fibrosis of various degrees and may even have hepatic cirrhosis[2,3]. Therefore, accurate assessment of liver functional reserve is important for the safety of liver cancer treatment[4]. There were various kinds of methods for testing liver function, however, indocyanine green (ICG) clearance test has been recognized as the most effective method for assessing functional reserve of a liver in all kinds of liver function tests. The relationship between the degree of liver fibrosis and functional reserve has not been well studied at present, although we know that fibrosis can result in liver function loss. Magnetic resonance elastography (MRE) can be used to assess the elasticity characteristics of soft tissue and showed great advantages in assessing liver fibrosis; its advantages include being non-invasive, accurate, and repeatable[5,6]. To explore the value of MRE in the assessment of liver functional reserve, the current study used MRE to measure non-tumor stiffness of the liver parenchyma in patients with hepatocellular carcinoma (HCC). We also sought to determine the correlation between the liver stiffness (LS) values and liver functional reserve as measured by the ICG clearance test.
The ethics committee for human experiments at our hospital approved all procedures, and all enrolled patients signed consent documents after they were recruited for the study. The study enrolled 32 HCC patients who had been admitted to the Department of Interventional Radiology at the Second Affiliated Hospital of Zhejiang University School of Medicine for interventional treatment from July 2013 to June 2014. These participants included 29 men and 3 women, ranging in age from 36 to 74 years (mean: 51 years) (Table 1). The average body mass index (BMI) was 22.66 ± 2.70 kg/m2 (range, 17.15 kg/m2 to 28.98 kg/m2). All patients had hepatitis B. They underwent routine blood tests, routine liver enhanced magnetic resonance (MR) imaging, liver MRE and ICG clearance tests. All patients met the HCC clinical diagnostic criteria of the European Association for the Study of the Liver (EASL)[7]. In addition, the patients did not have the following conditions: (1) obstructive jaundice; (2) tumor thrombus in the main portal vein; (3) severe fatty liver (LS increases when fatty liver progresses to inflammation)[8], the inphase-outphase sequence showed significant fatty signal; and (4) intrahepatic iron overload (a previous study showed that iron overload decreases the MRI signal in liver examinations)[9], the T2 sequence showed a significant signal decrease.
| Age (yr) | |
| mean ± SD | 51 ± 9.3 |
| Range | 36-74 |
| Gender | |
| Male | 29 |
| Female | 3 |
| Total bilirubin (mg/dL) | |
| mean ± SD | 12.98 ± 6.15 |
| Range | 5-31.2 |
| Albumin (g/dL) | |
| mean ± SD | 39.4 ± 4.0 |
| Range | 32.3-47.7 |
| Alanine aminotransferase (U/L) | |
| mean ± SD | 42.1 ± 44.3 |
| Range | 10-225 |
| Aspartate transaminase (U/L) | |
| mean ± SD | 41.5 ± 28.8 |
| Range | 17-139 |
| HBV(+) | 32 |
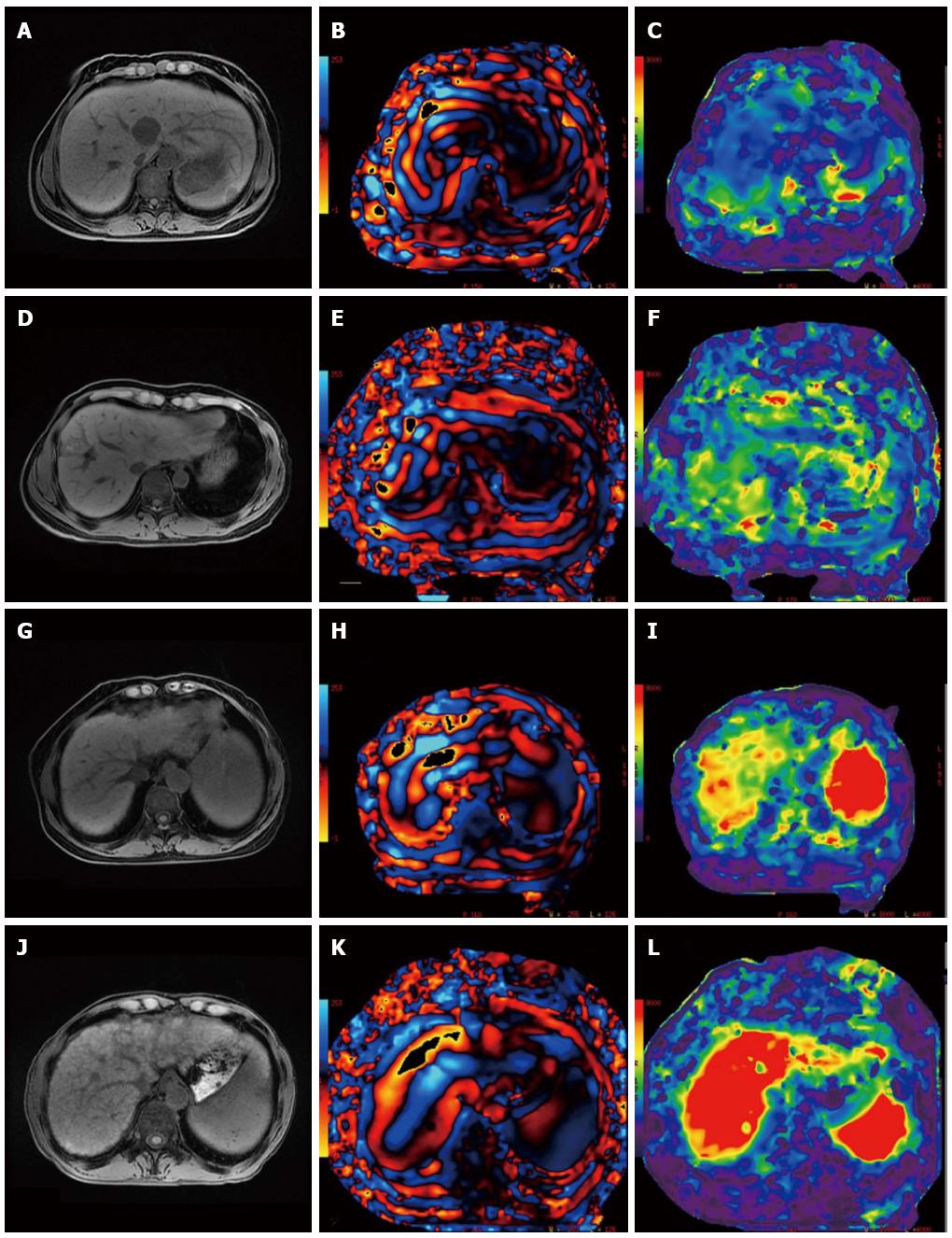
All the patients received routine serum tests. Child-Pugh scoring was performed by collecting the data of total bilirubin, albumin, and prothrombin time. Then the imaging data to detect seroperitoneum were analyzed to evaluate whether hepatic encephalopathy existed. MR scanning was performed under a fasting condition in the morning or more than 4 h after fasting and water deprivation (increased splanchnic blood flow after a meal may affect hepatic stiffness)[10]. The MR scan was performed using a 3.0 Tesla (T) MR unit (Discovery 750; GE Medical Systems) with a 32-channel body coil and an acoustic vibration device. The patient was placed in a supine position with his or her hands above the head. Respiratory gating was added to the abdomen to monitor the patient’s breathing and to train the patient to hold his or her breath. The acoustic vibration device conducted a vibration wave to a round non-metallic driver via a plastic tube; the driver was placed on the right costal arch of the patient and fixed with an elastic cummerbund. The patient was asked to hold his or her breath in the end-expiratory phase for 21 s. Meanwhile, 60 Hz of continuous mechanical vibration was introduced into the body by the driver, and a 2-D echo-planar imaging (EPI) MR elastography sequence was used to obtain transverse-section MR signals (TR = 600.0 ms, TE = 58.2 ms, FOV = 38 cm × 38 cm). MRE (GE Healthcare) post-processing software and a GE ADW4.6 post-processing station were used to process the MR signals to obtain the wave and elastography maps. Two attending radiologists independently analyzed the data. On the elastography map, circular regions of interest (ROIs) were drawn from 3 slices within the liver parenchyma. The ROIs were drawn from sites more than 3 cm from the tumor tissue, avoiding the hepatic edge region and large blood vessels[11]. The areas of the ROIs were 600-1000 mm2; 3 ROI stiffness values were measured for the different slices, and the mean of the 3 values was used as the non-tumor LS value.
The ICG clearance test was performed under fasting conditions on the morning or more than 6 h after fasting and water deprivation. Sterile water for the injection was used to dilute ICG to 5 mg/mL and a 0.5 mg/kg dose for rapid intra-elbow vein injection; the injection was completed within 10 s. A pulsed pigment concentration image analyzer DDG-3300K (made in Japan) was used to monitor blood ICG concentrations via a finger probe, record the ICG plasma clearance rate (ICG-K) values and assess the ICG retention rate at 15 min (ICGR-15).
SPSS 16.0 was used to analyze the correlations between the non-tumor LS values and the ICGR-15 as well as between the LS values and ICG-K. If the data exhibited a normal distribution, Pearson’s bivariate correlation analysis was used; if the data did not exhibit a normal distribution, Spearman’s bivariate rank correlation analysis was used. P values < 0.05 were considered significant.
All 32 patients successfully completed the MRE examination, liver functional reserve tests and routine serum liver function tests; the detailed results are shown in Table 1. No patient developed any complications. The means ± SD of the elasticity values measured by the two attending radiologists were 4.7 ± 2.2 kPa and 4.7 ± 2.1 kPa, respectively. The intraclass correlation coefficient was 0.991, indicating an almost perfect correlation between the LS values measured by the two doctors. The average LS of the non-tumor parenchyma measured by the MRE scan in HCC patients was 4.7 ± 2.2 kPa. The average ICGR-15 was 0.089 ± 0.077. The average ICG-K was 0.19 ± 0.07. The average total bilirubin was 12.98 ± 6.15 mg/dL. The average albumin was 39.4 ± 4.0 g/L. The average prothrombin time was 12.7 ± 9.3 S; 2 patients had mild seroperitoneum, and the rest had no seroperitoneum. None of the patients had hepatic encephalopathy. Based on the Child-Pugh scoring system, all 32 patients were classified as grade A; 27 had a score of 5 and 5 had a score of 6. The correlation analysis between the LS and ICG tests in the patients showed that LS was significantly and positively related to ICGR-15 (r = 0.746, P < 0.01) and significantly and negatively related to ICG-K (r = - 0.599, P < 0.01) (Figures 1 and 2; Table 2).
| Stiffness value of the non-tumor liver tissue | |
| mean ± SD | 4.7 ± 2.2 KPa |
| Range | 2.3-10 KPa |
| ICGR-15 | |
| mean ± SD | 0.089 ± 0.077 |
| Range | 0.003-0.271 |
| ICG-K | |
| mean ± SD | 0.19 ± 0.07 |
| Range | 0.087-0.398 |
| Correlation between LS and ICGR-15 | |
| r | 0.736 |
| P value | < 0.010 |
| Correlation between LS and ICG-K | |
| r | -0.562 |
| P value | < 0.010 |
The liver plays a role in metabolism, synthesis, secretion, immunity and many other functions[12]. Liver fibrosis can cause liver lobule structural changes and hemodynamic changes that, directly or indirectly, disrupt the above liver functions and decrease liver functional reserve[13,14]. Lau et al[15] reported that liver functional reserve is closely associated with postoperative HCC mortality. Therefore, the status of liver functional reserve can be used to determine a treatment strategy for HCC patients, as well as their prognosis[3]. However, because these patients often develop liver fibrosis of varying degrees of severity, non-tumor liver tissue volume measurements alone cannot be used to accurately assess liver functional reserve. At present, the methods most commonly used for clinical assessment of liver functional reserve include static liver functional assessment systems (e.g., the Child-Pugh scoring system) and dynamic liver function assessment systems (e.g., the ICG test)[12,16]. These systems assess liver synthetic function and metabolic function, respectively.
The Child-Pugh scoring system assesses liver function by detecting relevant serum or plasma indicators, primarily reflecting hepatocellular synthesis capacity. This system effectively assesses patients with decompensated hepatocirrhosis[17]; according to the literature, patients with a Child-Pugh liver function classification of grade A commonly develop postoperative hepatic failure[2,15]. Imamura et al[18] reported that the Child-Pugh score cannot accurately assess liver functional reserve. In the present study, all patients had grade A liver function according to the Child-Pugh score, but the average non-tumor LS values and the ICGR-15 and ICG-K were distributed in large range and no clear corresponding relationships were detected. Therefore, we agree that Child-Pugh scoring does not accurately or effectively assess liver functional reserve in compensated stages.
The ICG test is the most common method for assessing hepatic functional reserve in Asia[19,20]. This test assesses liver excretion function primarily by measuring ICG clearance in the liver cells and can relatively accurately reflect the functional reserve of liver tissue[21]. Sakka[19] reported that the ICG test is currently the most effective clinical method for assessing liver function. In this study, we observed that the ICGR-15 progressively increased and the ICG-K progressively decreased as non-tumor LS increased. However, the ICG test has limitations in clinical application. Because hepatic blood flow significantly influences ICG clearance rate, any factor that affects liver blood flow (e.g., portal vein cancer embolus, post-portal vein embolization, and local hepatic blood flow variation) will influence the test results. In addition, hyperbilirubinemia, vasodilators and other factors have a significant influence, and obstructive jaundice might reduce ICG excretion rates[4,22]. Therefore, although the ICG test can accurately assess liver functional reserve, it has some limitations, especially among patients with extensive vascular tumor invasion. Given that bile duct invasion is relatively common, use of this test in these patients is inappropriate.
MRE can noninvasively and quantitatively assess the elasticity characteristics of soft tissue, and it can be used to ascertain the stage of liver fibrosis and even replace liver biopsy[23,24]. Bonekamp et al[25] compared the current methods for non-invasive diagnosis of liver fibrosis (e.g., ultrasound elastography, MRE, PET/SPECT, and CT), and the results showed that MRE is the most effective technique for non-invasive diagnosis and grading of liver fibrosis. MRE has the following additional advantages for diagnosing liver fibrosis: (1) the procedure is simple, easily repeated and not substantially affected by subjective factors[26]; (2) its accuracy in assessing liver fibrosis is high because it can capture the quantitative indicators of stiffness in the entire liver and different hepatic regions[27]; (3) it is more comprehensive than liver puncture biopsies and ultrasound elastography; and (4) it is not affected by obesity, ascites, portal vein cancer embolus, bile ducts and other conditions[28,29].
Most patients with primary liver cancer also exhibit liver fibrosis of various degrees of severity. The degree of liver fibrosis is closely related to liver functional reserve[2,4,30]. Lao et al[31] reported that ICGR-15 increased as the degrees of liver fibrosis increased, and this result was related to postoperative liver failure. Kusaka et al[30] reported that LS in surgical specimens measured using a homemade detector was closely related to the ICGR-15. In our study, MRE was used in lieu of liver biopsy to accurately assess the degree of liver fibrosis in non-tumor tissues in HCC patients. We then explored the correlation between the average non-tumor LS value and liver functional reserve. The results showed that the average non-tumor LS value in HCC patients was significantly and positively related to ICGR-15 (r = 0.746, P < 0.01) and significantly and negatively related to ICG-K (r = -0.599, P < 0.01), indicating a close correlation between the degree of liver fibrosis and liver functional reserve. In addition, accurate assessments of functional reserve for partial or total non-tumor liver tissue can be achieved using MRE in HCC patients. Thus, the influence of blood vessels and bile ducts on the assessment of liver functional reserve can be avoided.
Although our study determined the correlation between LS (measured using MRE) and liver functional reserve (measured using the ICG test), it has several limitations: (1) Liver fibrosis leads to changes in liver volume, and the current study did not consider the influence of liver volume on hepatic functional reserve; (2) The sample size was small, and numerous clinical applications are needed for further validation; and (3) MRE examination requires patient cooperation (i.e., patients must hold their breath for a relatively long time); furthermore, MRE employs a continuous vibration to the liver, which might cause physical discomfort. Future studies should quantitatively assess liver functional reserve based on liver volume to establish an accurate linear correlation between liver functional reserve and degree of liver fibrosis.
In summary, MRE examination can be used to achieve accurate, noninvasive assessment of liver functional reserve in the non-tumor tissue of HCC patients. Thus, this technique can provide useful information for clinical treatment strategies and prognostic evaluations.
Liver function, which is closely related to liver fibrosis, is an important parameter in patients with chronic liver disease or hepatic carcinoma. Magnetic resonance elastography (MRE) is the most effective non-invasive method for diagnosing and staging liver fibrosis. The indocyanine green (ICG) test is the most effective method for evaluating liver functional reserve in patients with chronic liver disease or hepatic carcinoma.
MRE is a rapidly developed technology for assessing the elasticity characteristics of soft tissue. The advantages of the technique include being non-invasive, accurate, and reliable. It has been established as the only non-invasive method for the accurate diagnosis and staging of liver fibrosis, but its value in assessing liver function has not yet been studied.
MRE assesses liver stiffness (LS) to measure liver functional reserve; this method introduces a new procedure into clinical practice that will simplify liver function assessment in the future.
The positive relationship between LS (measured by MRE) and liver functional reserve showed that the authors can introduce MRE into clinical practice to assess liver function in the future.
MRE assesses the elasticity characteristics of soft tissue; its advantages include being non-invasive, accurate, and reliable.
The ICG test is the most common method for assessing liver functional reserve in Asia; this method assesses liver excretion function primarily by measuring the ICG clearance in liver cells, and it can relatively accurately indicate the functional reserve of liver tissue.
This paper describes a new method for assessing liver function using MRE; it provides information regarding the clinical treatment of patients with liver disease. Although the value of MRE in assessment of liver fibrosis has been extensively researched, its value in assessing liver function has not yet been studied. If the idea that MRE can accurately assess liver functional reserve is proved in further study, then it is likely that MRE will replace the indocyanine green test, resulting in more efficient liver function assessment procedures in the future.
P- Reviewer: Sijens PE S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Llovet JM, Beaugrand M. Hepatocellular carcinoma: present status and future prospects. J Hepatol. 2003;38 Suppl 1:S136-S149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005;12:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Mizuguchi T, Kawamoto M, Meguro M, Hui TT, Hirata K. Preoperative liver function assessments to estimate the prognosis and safety of liver resections. Surg Today. 2014;44:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Chen X, Zhang HB, Li ZQ, Yu XF, Yang MF, Wang HH, Teng LS. Indocyanine green clearance in evaluating the recovery of liver reserve function after superselective transarterial chemoembolization. Hepatobiliary Pancreat Dis Int. 2013;12:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Motosugi U, Ichikawa T, Sano K, Sou H, Muhi A, Koshiishi T, Ehman RL, Araki T. Magnetic resonance elastography of the liver: preliminary results and estimation of inter-rater reliability. Jpn J Radiol. 2010;28:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Rouvière O, Yin M, Dresner MA, Rossman PJ, Burgart LJ, Fidler JL, Ehman RL. MR elastography of the liver: preliminary results. Radiology. 2006;240:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 7. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 8. | Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 9. | Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009;193:14-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Hines CD, Bley TA, Lindstrom MJ, Reeder SB. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010;31:725-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 500] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 12. | Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 13. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1293] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 15. | Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255-1259. [PubMed] |
| 16. | Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 19. | Sakka SG. Assessing liver function. Curr Opin Crit Care. 2007;13:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Sci. 2010;17:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Nanashima A, Abo T, Tobinaga S, Nonaka T, Fukuoka H, Hidaka S, Takeshita H, Sawai T, Yasutake T, Nagayasu T. Prediction of indocyanine green retention rate at 15 minutes by correlated liver function parameters before hepatectomy. J Surg Res. 2011;169:e119-e125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Wagener G. Assessment of hepatic function, operative candidacy, and medical management after liver resection in the patient with underlying liver disease. Semin Liver Dis. 2013;33:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Godfrey EM, Mannelli L, Griffin N, Lomas DJ. Magnetic resonance elastography in the diagnosis of hepatic fibrosis. Semin Ultrasound CT MR. 2013;34:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Venkatesh SK, Wang G, Lim SG, Wee A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2014;24:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Bonekamp S, Kamel I, Solga S, Clark J. Can imaging modalities diagnose and stage hepatic fibrosis and cirrhosis accurately? J Hepatol. 2009;50:17-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Runge JH, Bohte AE, Verheij J, Terpstra V, Nederveen AJ, van Nieuwkerk KM, de Knegt RJ, Baak BC, Jansen PL, Sinkus R. Comparison of interobserver agreement of magnetic resonance elastography with histopathological staging of liver fibrosis. Abdom Imaging. 2014;39:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Yoon JH, Lee JM, Woo HS, Yu MH, Joo I, Lee ES, Sohn JY, Lee KB, Han JK, Choi BI. Staging of hepatic fibrosis: comparison of magnetic resonance elastography and shear wave elastography in the same individuals. Korean J Radiol. 2013;14:202-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Glaser KJ, Manduca A, Ehman RL. Review of MR elastography applications and recent developments. J Magn Reson Imaging. 2012;36:757-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Lee DH, Lee JM, Han JK, Choi BI. MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging. 2013;38:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Kusaka K, Harihara Y, Torzilli G, Kubota K, Takayama T, Makuuchi M, Mori M, Omata S. Objective evaluation of liver consistency to estimate hepatic fibrosis and functional reserve for hepatectomy. J Am Coll Surg. 2000;191:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |