Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7281
Peer-review started: January 7, 2015
First decision: February 10, 2015
Revised: March 13, 2015
Accepted: April 17, 2015
Article in press: April 17, 2015
Published online: June 21, 2015
Processing time: 164 Days and 21.8 Hours
AIM: To confirm the efficacy and safety of bevacizumab/XELOX combination for the treatment of locally advanced or metastatic colorectal cancer (CRC) in Italy.
METHODS: This multicentric, prospective, open-label study included patients with CRC previously untreated with chemotherapy. Patients were administered bevacizumab in combination with XELOX. The primary efficacy end-point was progression-free survival (PFS). Secondary end-points included time to overall response (TOR), duration of response (DOR), time to treatment failure (TTF) and overall survival (OS). The incidence and type of adverse events AEs and severe AEs were evaluated. Also, the mutational status of BRAF and KRAS was assessed by high resolution melting and direct sequencing, and quality of life (QoL) was measured by the EuroQoL EQ-5D questionnaire at baseline and at the last visit.
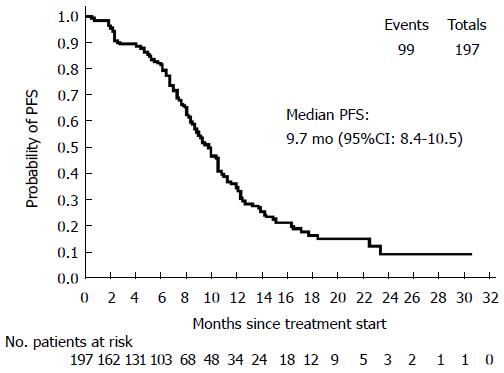
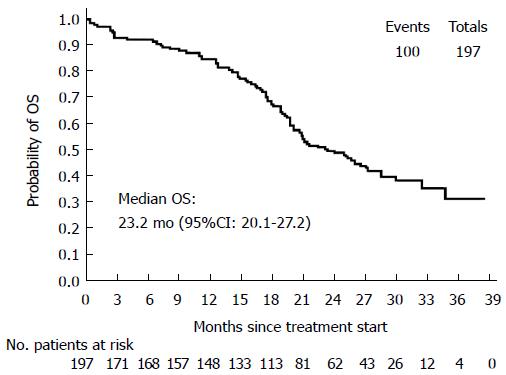
RESULTS: The intention-to-treat population included 197 patients (mean age: 62.3 ± 9.9 years, 56.4% males). At baseline, 16/34 evaluable subjects (47.1%) harbored a KRAS and/or a BRAF mutation; the mean QoL index was 80.2 ± 14.3. First-line therapy was given for 223.7 ± 175.9 d, and after a mean follow-up of 387.7 ± 238.8 d all patients discontinued from the study mainly for disease progression (PD, 45.4%) and AEs (25.4%). Median PFS was 9.7 mo (95%CI: 8.4-10.5) and the median values for secondary end-points were: TOR = 3.9 mo (95%CI: 2.6-4.7), DOR = 8.5 mo (95%CI: 7.3-10.3), TTF = 6.7 mo (95%CI: 6.0-7.7) and OS = 23.2 mo (95%CI: 20.1-27.2). Patients carrying at least one lesion had a lower overall response rate (66.7% vs 88.9%) and a lower probability of achieving complete or partial response than those without mutations, but the difference in relative risk was not statistically significant (P = 0.2). Mean EQ-5D-3L raw index score significantly decreased to 74.9 ± 19.1 at the last visit (signed-rank test, P = 0.0076), but in general the evaluation on QoL perceived by patients was good.
CONCLUSION: The efficacy of bevacizumab in combination with XELOX in terms of PFS in patients with aCRC or mCRC in Italy was confirmed, with acceptable toxicity.
Core tip: combined with fluorouracil-based chemotherapy, bevacizumab significantly improved survival, compared to placebo, in previously untreated metastatic colorectal cancer (mCRC) patients and in second-line treatment. In this perspective, the aim of this multicentric, prospective, open-label, single arm, non comparative study (the OBELIX study) was to confirm previous results on the positive outcome of bevacizumab/XELOX treatment in locally advanced CRC (aCRC) or mCRC patients in Italy. Our findings confirm the clinical benefit provided by bevacizumab plus XELOX in terms of proliferation-free survival, without the appearance of new areas of toxicity and an overall acceptable safety profile.
- Citation: Antonuzzo L, Giommoni E, Pastorelli D, Latiano T, Pavese I, Azzarello D, Aieta M, Pastina I, Di Fabio F, Bertolini A, Corsi DC, Mogavero S, Angelini V, Pazzagli M, Di Costanzo F. Bevacizumab plus XELOX as first-line treatment of metastatic colorectal cancer: The OBELIX study. World J Gastroenterol 2015; 21(23): 7281-7288
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7281
Vascular endothelial growth factor (VEGF) is a common target for the inhibition of tumor growth and metastasis, as it plays a central role in angiogenesis within the tumor[1]. Bevacizumab, a humanized anti-VEGF antibody, in combination with different chemotherapy regimens, has been tested in several studies for the treatment of colorectal cancer (CRC). Combined with fluorouracil-based chemotherapy, bevacizumab significantly improved survival, compared to placebo, in previously untreated metastatic CRC (mCRC) patients and in second-line treatment[2,3]. The efficacy of XELOX (capecitabine and oxaliplatin) in combination with bevacizumab was studied in a factorial-design study, where patients with mCRC were randomized to XELOX vs FOLFOX-4, and subsequently to bevacizumab vs placebo[4]. The clinical benefit reported in this study did not fully satisfy expectations, likely due to the early discontinuation of bevacizumab[4]. The BEAT and BriTE trials confirmed the safety profile of bevacizumab in first-line mCRC patients receiving various chemotherapy regimens, namely FOLFOX, XELOX, FOLFIRI or capecitabine[5,6].
In this perspective, the aim of this multicentric, prospective, open-label, single arm, non comparative study (the OBELIX study) was to confirm previous results on the positive outcome of bevacizumab/XELOX treatment in locally advanced CRC (aCRC) or mCRC patients in Italy.
This study is registered at ClinicalTrials.gov. The registration identification number is NCT00577031.
Patients were included in this single-arm, open-label, multicentre, phase IIIb, prospective study if they were ≥ 18 years old, had histologically/cytologically proven diagnosis of CRC, chemotherapy-naïve metastatic disease and ECOG (Eastern Cooperative Oncology Group) performance status (PS) between 0 and 1. Patients had a life expectancy of > 12 wk and > 1 measurable lesion according to “Response Evaluation Criteria In Solid Tumors”[7]. Patients provided written informed consent. This study was approved by the Independent Ethics Committee of each site.
Main exclusion criteria consisted of: radiotherapy to any site within 4 wk before the study, untreated brain metastases, history of central nervous system disease, non-healing wounds and evidence of bleeding diathesis or coagulopathy. Moreover, patients with uncontrolled hypertension, clinically significant cardiovascular disease, current or recent ongoing treatment with anticoagulants for therapeutic purposes, chronic treatment with high-dose aspirin (> 325 mg/d), treatment with any investigational drug within 30 d prior to enrolment, known allergy to any of the components of the study medications, other co-existing malignancies or malignancies diagnosed within the last 5 years, lack of physical integrity of the upper gastrointestinal tract, were excluded, as well as pregnant or lactating women, or of childbearing potential with either a positive or no pregnancy test at baseline and patients unwilling to practice contraception during the study.
The planned treatment schedule was the oral 5-fluorouracil pro-drug capecitabine in combination with oxaliplatin plus the humanized anti-VEGF antibody bevacizumab. After signing the informed consent, eligible patients received 21-d cycles according to the following scheme: bevacizumab 7.5 mg/kg and oxaliplatin 130 mg/m2 (both intravenously) every 21 d on the first day and capecitabine 1000 mg/m2 twice daily for 14 consecutive days and 7 d of rest. XELOX therapy was administered for no more than 8 cycles (6 mo), while bevacizumab until progression of disease (PD). Second-line chemotherapy was at the investigator’s discretion.
Baseline data included medical history, vital signs, results from physical examination, concomitant diseases, concomitant treatments, hematology and blood chemistry parameters, proteinuria, tumor evaluation and ECOG-PS.
The primary efficacy variable was progression-free survival (PFS). Patients without an event were censored at the time of the last contact where the patient was known to be progression-free or alive. The secondary efficacy parameters were represented by: overall response rate (ORR), time to overall response (TOR) such as complete response (CR) or partial response (PR), duration of response (DOR), time to treatment failure (TTF), overall survival (OS), radical (R0) resection rate, KRAS and BRAF mutation status (high resolution melting and direct sequencing) and Quality of Life (QoL, measured by the EuroQoL EQ-5D questionnaire)[8]. Adverse events (AEs) were recorded and graded according to the “National Cancer Institute Common Toxicity Criteria” version 3.0. AEs related to treatment were recorded for up to 6 mo after the last dose of study drug (serious events indefinitely) and were followed up until they stabilized or returned to baseline status. AEs unrelated to treatment were recorded and followed up for 28 d after last study dose.
Since comparison with historic control seems not appropriate in a single-arm, open-label, multicentre, non comparative phase III study.
The planned study enrollment of 200 patients was considered an appropriate sample size to allow the evaluation of 190 patients (considering a dropout rate of 5%), which would be sufficient to provide evidence for improved PFS compared to historic controls[9].
Both primary and secondary efficacy analyses were based on the intention-to-treat (ITT) population (at least one dose of study medication taken and at least one efficacy measurement at baseline available), and analyses on per-protocol population were considered as supportive. All “time-to-event” variables were presented in Kaplan-Meier survival plots and summarized by median and 95%CI. Descriptive statistics of the overall lesion response were provided by visit/tumor assessment. Absolute and relative frequencies of patients with KRAS and BRAF mutations were reported. QoL results were summarized by descriptive summary tables at baseline and over time, and changes from baseline were verified by an ANCOVA model. Descriptive statistics were applied to safety analysis, together with absolute and relative frequency of subjects with events. Statistical analysis was performed using SAS System for Windows version 9.2 (SAS Institute Inc., Cary, NC, United States). All statistical tests were performed with a significance level α = 0.05.
This study enrolled 205 patients from 34 centers across Italy between February 2008 and November 2009. Baseline demographic and clinical characteristics are shown in Table 1. The ITT population consisted of 197 patients. All patients discontinued from the study and the main reasons were PD (45.4%) and the presence of AEs (25.4%). The vast majority of patients had a baseline ECOG-PS of grade 0 (80.7%). Hypertension and diabetes were among the most frequent medical conditions accounting for 41.1% and 10.2% respectively. Surgical resection of primary tumor occurred in 152 patients (77.2%), on average at 61.2 ± 10.2 years. The mean baseline EQ-5D-3L QoL raw index was 78.9 ± 15.4 (range: 32-98).
| Characteristic | n (%)1 |
| Total recruitment | 205 (100) |
| ITT population | 197 (96.1) |
| Age (yr) | |
| Median (range) | 63 (34-80) |
| mean ± SD | 62.3 ± 9.9 |
| Gender | |
| Males | 111 (56.4) |
| Females | 86 (43.6) |
| ECOG-PS | |
| 0 | 159 (80.7) |
| 1 | 38 (19.3) |
| > 1 | 0 (0) |
| Site of primary tumor | |
| Colon (any region) | 141 (71.6) |
| Rectum | 46 (23.4) |
| Rectum + colon (any region) | 8 (4.1) |
| Unknown | 2 (1.0) |
| Prior treatments (adjuvant or neo-adjuvant) | |
| Chemotherapy | 49 (24.9) |
| Radiotherapy | 21 (10.7) |
| None | 148 (75.1) |
| N of metastases for each patient | |
| 1 | 105 (53.3) |
| 2 | 72 (36.5) |
| > 3 | 20 (10.2) |
| Site of metastases | |
| Liver | 150 (76.1) |
| Lung | 70 (35.5) |
| Lymph nodes | 27 (13.7) |
| Peritoneum | 14 (7.1) |
| Other | 98 (48) |
| Stage at first diagnosis | |
| Local regional | 75 (38.1) |
| Metastatic | 122 (61.9) |
| KRAS and BRAF mutation status (34 evaluated) | |
| Wild Type (KRAS/BRAF) | 18 (52.9) |
| Mutated KRAS | 13 (38.2) |
| Mutated BRAF | 2 (5.9) |
| Mutated KRAS and BRAF | 1 (2.9) |
Patients underwent 9.2 ± 7.3 cycles of bevacizumab, 5.9 ± 2.7 cycles of capecitabine and 5.9 ± 2.6 cycles of oxaliplatin. The number of patients without any dose modification or interruption was 44 (22.3%), 68 (34.5%) and 58 (29.4%) for bevacizumab, capecitabine and oxaliplatin, respectively.
Primary efficacy parameter: In the ITT population, median PFS was 9.7 mo (95%CI: 8.4-10.5) (Figure 1). Treatment discontinuation, for reasons other than PD or missing tumor assessment after baseline, occurred in 98 cases, therefore those patients were censored. The remaining 99 patients experienced the event of interest (PD or death) during the treatment period.
Secondary efficacy parameters: A summary of ORRs is reported in Table 2. Median OS was 23.2 mo (95%CI: 20.1-27.2) (Figure 2). Median values for the other secondary end-points were: TOR = 3.9 mo (95%CI: 2.6-4.7); DOR = 8.5 mo (95%CI: 7.3-10.3); TTF = 6.7 mo (95%CI: 6.0-7.7). During the study, 47 patients underwent R0 surgery of metastases and 5 patients had 2 surgical procedures, for a total of 52 ITT patients (26.4%). Summary of R0 resection rates are shown in Table 3.
| n (%)1 | |
| CR (best overall response) | 10 (5.1) |
| PR | 87 (44.2) |
| SD | 55 (27.9) |
| CB (CR + PR + SD) | 152 (77.1) |
| PD | 13 (6.6) |
| No tumor assessment | 32 (16.2) |
| Reason for surgery | Residual disease | n (%)1 |
| Curative | ||
| No (radical surgery) | 26 (55.8) | |
| Yes | 7 (13.5) | |
| Unknown | 2 (3.9) | |
| Not applicable | 4 (7.7) | |
| Palliative | ||
| No (radical surgery) | 2 (3.9) | |
| Yes | 4 (7.7) | |
| Unknown | 2 (3.9) | |
| Not applicable | 1 (1.9) | |
| Biopsy | ||
| Yes | 1 (1.9) | |
| Not applicable | 1 (1.9) | |
| Unknown | ||
| Unknown | 1 (1.9) | |
| Not applicable | 2 (3.9) | |
| Other | ||
| Yes | 1 (1.9) | |
| Not applicable | 2 (3.9) |
Analysis of KRAS and BRAF mutation status revealed that 16 out of 34 patients (47.1%) whose tumor was evaluated had at least one gene alteration (Table 1): 13 patients (38.2%) carried a KRAS mutation, 2 patients (5.9%) had a BRAF mutation and 1 patient (2.9%) harbored both mutations. Compared to wild type (wt) patients, subjects with KRAS or BRAF mutations had a lower ORR: 66.7% vs 88.9%. Indeed, the estimate relative risk (RR = 0.75) suggested that the probability of achieving a CR or PR as best overall response is lower for patients with gene alterations. However, RR was not found to be statistically significant (95%CI: 0.51-1.11, P = 0.2). Considering that all patients had ECOG-PS 0-1, two potential prognostic factors have been evaluated: number of metastatic sites (1 site vs > 1 site) and alkaline phosphatase levels (≥ 300 UI/mL vs < 300 UI/mL). Three groups were obtained: group 1 (n = 105, 53.3%) with only one site of metastasis, group 2 (n = 70, 35.5%) with more than one site of metastasis and alkaline phosphatase < 300 UI/L and group 3 (n = 14, 7.1%) with more than one site of metastasis and alkaline phosphatase ≥ 300 UI/L. For 8 patients (4.1%) this data was missing. Median PFS was 9.9 mo (95%CI: 8.4-12.7), 8.5 mo (95%CI: 7.2-11.2) and 10.4 mo (95%CI: 7.9-13.2) in group 1, 2 and 3 respectively (log-rank test P value = 0.527), whereas median OS was 25.9 mo (95%CI: 20.8-32.3), 19.9 mo (95%CI: 17.4-28.4) and 17.9 mo (95%CI: 12.4-26.5, log-rank test P value = 0.149), respectively. No statistically significant difference among the three groups was detected in terms of PFS or OS, possibly due to the different number of patients included in each group.
QoL was assessed at baseline and during the last visit: using the prevalence approach, 114 patients were evaluable and the mean EQ-5D-3L raw index score changed from 80.2 ± 14.3 at baseline to 74.9 ± 19.1 at the last visit; using the “Last Observation Carried Forward” (LOCF) approach, 142 patients were evaluable and the mean EQ-5D-3L raw index score changed from 80.2 ± 14.4 at baseline to 76.3 ± 18.6 at last visit. The decreasing evaluation of overall health during the study was statistically significant (signed-rank test performed on difference values gave a P = 0.0076 with prevalence approach and P = 0.0131 with LOCF approach).
Patients were exposed to first-line treatment for an average of 223.7 ± 175.9 d and were observed during follow-up for 387.7 ± 238.8 d.
Adverse events and deaths: The vast majority (94.4%) of the safety population experienced at least one AE. The majority of AEs were classified as “gastrointestinal disorders” (74.6%), “general disorders and administration site conditions” (58.9%), “nervous system disorders” (56.9%) and “vascular disorders” (32.0%). Serious AEs (SAEs) occurred in 56 patients (28.43%). Most AEs were drug-related (90.9%). Overall, 87.3% of patients had at least one AE suspected as related to chemotherapy. Concerning AEs of grade 4 suspected to be chemotherapy-related, 2 patients had “diarrhea”, 1 patient had “gastrointestinal disorders”, 1 patient experienced “vomiting” and 2 patients reported “asthenia”. Sixty-one patients (31.0%) experienced at least one AE suspected to be related to bevacizumab. Concerning AEs of grade 4 related to bevacizumab, 1 patient had “intestinal perforation”, 2 patients “pulmonary embolism”, 1 patient had “hypertension” and 1 patient had “hypertensive crisis”. Sixteen patients (8.1%) experienced at least one AE suspected to be related to the combination of chemotherapy and bevacizumab: “nausea” in 1.5% of patients, vomiting, fatigue, asthenia and/or hypertension in 1.0% each. One patient had “cardio-respiratory arrest” with toxicity grade 4 suspected to be related to the combination of chemotherapy and bevacizumab.
One-hundred patients (50.8%) died during the course of the study. Six subjects (3.1%) prematurely discontinued the study due to death and 94 (47.7%) died during follow-up mainly due to progression of their colorectal cancer (86 patients). Three cases were considered as related to bevacizumab, whereas no relationship with study drugs was reported in the remaining 9 patients who died due to a SAE.
In patients with mCRC, chemotherapy is recommended to reduce symptoms and prolong survival. Various combinations of chemotherapy and biological drugs are available for first-line treatment depending on patients’ features, disease characteristics and aim of therapy. The combination of chemotherapy with targeted agents as bevacizumab in molecularly unselected populations, and anti-EGFR cetuximab and panitumumab in RAS wt patients significantly prolonged survival in phase III randomized trials[2,10,11].
Results from the present study confirm the efficacy of bevacizumab in combination with XELOX in terms of PFS of metastatic or locally advanced CRC patients. All secondary end-points of this trial confirmed previous results, although OS was slightly higher in our study compared to results presented by Saltz and coworkers (23.2 mo vs 21.3 mo)[4]. This particular finding was not unexpected, since one limitation of the study by Saltz was that patients rarely received bevacizumab until PD, but, rather, treatment in the presence of toxicities was discontinued as a whole, instead of selectively, as the protocol permitted. A recently published phase II trial performed on elderly patients showed an even higher PFS (11.5 mo, 95%CI: 10.0-12.9)[12]. In contrast, in our study, bevacizumab was only discontinued after PD, which could explain the longer OS achieved.
We have no data on single patients treated with bevacizumab monotherapy until PD, after bevacizumab/XELOX. OS is affected by treatment after first progression, but unfortunately no data are available about the later lines of chemotherapy, as in this study second-line therapies were at the investigator’s discretion according to local guidelines, and thus were not recorded in the study’s CRF. In Italy, during the study period (Februry 2008-November 2009), patients treated in first-line with fluoropyrimidine and oxaliplatin plus bevacizumab received mostly second-line therapy with fluoropyrimidine plus irinotecan, whereas the use of bevacizumab beyond progression was not allowed, nor the use of FOLFIRI plus aflibercept. Therefore, we can assume that patients enrolled in the present study did not receive anti-VEGF therapy beyond PD (TML strategy)[13]. In the same period, patients with KRAS wt (exon 2, codon 12 and 13) tumors were treated with FOLFIRI plus cetuximab in second line or with cetuximab or panitumumab monotherapy in third line. Unfortunately, the total number of patients with KRAS wt tumor is unknown and consequently also the number of patients hypothetically treated with anti-EGFR antibody.
Main limitations of the present study were the lack of randomization and blinding, but since this was intended to be a confirmatory study, randomization and blinding would have been beyond the requirements of the study. Moreover, although the bevacizumab/XELOX combination has proven its efficacy in treating CRC patients, treatment prognostic markers are still needed. Further investigation is also warranted for selecting the optimal treatment duration and the role of bevacizumab in selected patients groups.
The exploratory analysis indicated a lower proportion of CR and PR as best overall response during first line treatment (66.7%) in patients with KRAS or BRAF mutation compared to wt patients (88.9%), even though the difference was not statistically significant. In patients with gene alterations, PFS and the incidence of PD or death were not significantly different compared to wt. This is in line with previous findings of an Australian group which evaluated the impact of KRAS and BRAF mutational status on outcomes following treatment with capecitabine alone or in combination with bevacizumab and mitomycin in aCRC. It was shown that KRAS status was neither prognostic for OS nor predictive of bevacizumab outcome in this set of patients[14]. Nevertheless, in our study, the lack of statistical significance could also be explained by the low number of patients.
The decrease on the judgment on overall health between last visit and baseline was highly expected, due to the nature of the disease, but in general the evaluation on QoL perceived by patients was good. Concerning safety, the incidence of bevacizumab-related AEs in this study was as expected for this kind of patients and study treatment, and comparable to that of previous studies[2-6,15].
The number of metastatic sites involved, PS, WBC count and alkaline phosphatase were evaluated in multivariate analysis as factors that influenced both response rate and OS in aCRC patients treated with 5-FU-based combinations[16]. Subsequently, Giacchetti et al[17] found that the number of sites, PS and alkaline phosphatase were independent prognostic factors. Saltz et al[18] reported that a normal lactate dehydrogenase level and a PS = 0 were predictive factors of improved PFS and OS. Also, hemoglobin levels of at least 11 g/dL and a normal WBC count were predictive for PFS and OS. Unexpectedly, an age of > 65 years was also associated with a longer PFS. In this study, however, the analysis of clinical prognostic factors did not find statistical differences among the three groups of patients identified on the basis of the number of metastatic sites and the values of alkaline phosphatase.
In conclusion, the present study achieved its primary objective of confirming previous positive PFS outcomes of bevacizumab/XELOX combination treatment in CRC patients in an Italian setting. Secondary safety and efficacy objectives were also achieved, confirming the usefulness of this treatment and its good overall tolerability profile.
The authors would like to thank Biagio Agostara, Livio Blasi, Andrea Bonetti, Corrado Boni, Sergio Bretti, Paola Buscaglia, Massimo Cirillo, Alessandro Comandone, Salvatore Del Prete, Sergio Fava, Francesco Ferraù, Vittorio Gebbia, Giovanni Ianniello, Aldo Iop, Basem Kildani, Anna Maria Lanzillo, Vito Lorusso, Bruno Massidda, Rodolfo Mattioli, Stefania Miraglia, Lorenzo Pavesi, Clementina Savastano, Cora N. Sternberg and Marco Tampellini for assisting in the enrolment of patients.
In metastatic colorectal cancer (CRC) different options are available as first-line therapy and beyond, that provided benefit in terms of clinical outcomes. In particular, the combination of different fluorouracil-based chemotherapy regimens with the humanized anti-Vascular endothelial growth factor (VEGF) antibody bevacizumab led to prolongation of patient survival, likely due to the pivotal role played by angiogenesis in CRC.
In Italy, at the time of this study, few options were available for patients with untreated locally advanced or metastatic CRC. Evidence pointed towards the use of the bevacizumab/XELOX combination as an effective and safe strategy, but data were lacking in this setting.
This multicentric, prospective, open-label, phase IIIb trial was designed as a confirmatory study of the efficacy and safety of front-line bevacizumab/XELOX in Italian patients with locally advanced or metastatic CRC. Indeed, the results confirm that this combination may provide a benefit in terms of progression-free survival in these patients, with an overall good safety profile.
Patients with untreated CRC may benefit from administration of bevacizumab plus XELOX, without suffering of particular toxicity.
CRC is a malignancy of colon and rectum and it is often diagnosed in already advanced stage. As to date it remains the second leading cause of cancer death worldwide, it is crucial to develop therapeutic strategies able to cure these patients. Bevacizumab was the first biologic drug approved by Food and Drug Administration in combination with chemotherapy for the treatment of adults with CRC. The rationale relies on the importance of physiological VEGF production, known to sustain angiogenesis, which in turn favors tumor growth.
This paper is interesting and confirms the results of progression-free survival objectified in the Phase III studies. The authors confirm previous results on the positive outcome of bevacizumab/XELOX combination treatment in patients with locally advanced or metastatic colorectal cancer in Italy. This is a meaningful research.
P- Reviewer: Samalin E, Sun YM S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Bronte G, Cicero G, Cusenza S, Galvano A, Musso E, Rizzo S, Sortino G, Roselli M, Bazan V, Fiorentino E. Monoclonal antibodies in gastrointestinal cancers. Expert Opin Biol Ther. 2013;13:889-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7724] [Article Influence: 367.8] [Reference Citation Analysis (1)] |
| 3. | Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1725] [Article Influence: 95.8] [Reference Citation Analysis (1)] |
| 4. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2267] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 5. | Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 6. | Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326-5334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 509] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 7. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21569] [Article Influence: 1348.1] [Reference Citation Analysis (1)] |
| 8. | EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199-208. [PubMed] |
| 9. | Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 634] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 10. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1392] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 11. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3121] [Article Influence: 195.1] [Reference Citation Analysis (1)] |
| 12. | Rosati G, Avallone A, Aprile G, Butera A, Reggiardo G, Bilancia D. XELOX and bevacizumab followed by single-agent bevacizumab as maintenance therapy as first-line treatment in elderly patients with advanced colorectal cancer: the boxe study. Cancer Chemother Pharmacol. 2013;71:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 895] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 14. | Price TJ, Hardingham JE, Lee CK, Weickhardt A, Townsend AR, Wrin JW, Chua A, Shivasami A, Cummins MM, Murone C. Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer. J Clin Oncol. 2011;29:2675-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523-3529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 453] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 16. | Köhne CH, Cunningham D, Di Costanzo F, Glimelius B, Blijham G, Aranda E, Scheithauer W, Rougier P, Palmer M, Wils J. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308-317. [PubMed] |
| 17. | Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136-147. [PubMed] |
| 18. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2221] [Article Influence: 88.8] [Reference Citation Analysis (0)] |