Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.7036
Peer-review started: November 27, 2014
First decision: December 26, 2014
Revised: January 8, 2015
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: June 14, 2015
Processing time: 203 Days and 20.5 Hours
AIM: We undertook this meta-analysis to investigate the relationship between revascularization and outcomes after liver transplantation.
METHODS: A literature search was performed using MeSH and key words. The quality of the included studies was assessed using the Jadad Score and the Newcastle-Ottawa Scale. Heterogeneity was evaluated by the χ2 and I2 tests. The risk of publication bias was assessed using a funnel plot and Egger’s test, and the risk of bias was assessed using a domain-based assessment tool. A sensitivity analysis was conducted by reanalyzing the data using different statistical approaches.
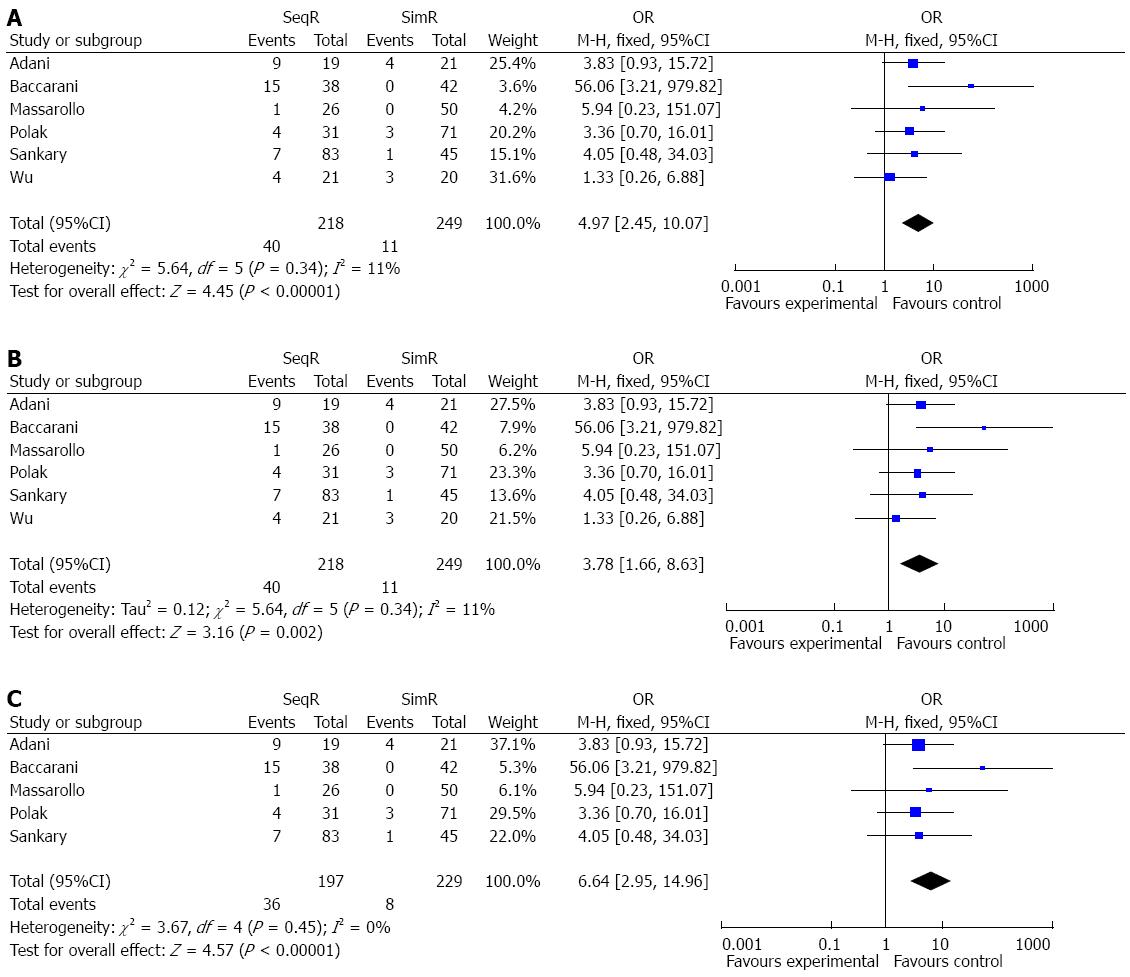
RESULTS: Six studies with a total of 467 patients were included. Ischemic-type biliary lesions were significantly reduced in the simultaneous revascularization group compared with the sequential revascularization group (OR = 4.97, 95%CI: 2.45-10.07; P < 0.00001), and intensive care unit (ICU) days were decreased (MD = 2.00, 95%CI: 0.55-3.45; P = 0.007) in the simultaneous revascularization group. Although warm ischemia time was prolonged in simultaneous revascularization group (MD = -25.84, 95%CI: -29.28-22.40; P < 0.00001), there were no significant differences in other outcomes between sequential and simultaneous revascularization groups. Assessment of the risk of bias showed that the methods of random sequence generation and blinding might have been a source of bias. The sensitivity analysis strengthened the reliability of the results of this meta-analysis.
CONCLUSION: The results of this study indicate that simultaneous revascularization in liver transplantation may reduce the incidence of ischemic-type biliary lesions and length of stay of patients in the ICU.
Core tip: The current methods of revascularization in liver transplantation can be divided into two main groups. We carried out this meta-analysis in order to study the relationship between revascularization and outcomes after liver transplantation. Ischemic-type biliary lesions were significantly reduced in the simultaneous revascularization group compared with the sequential revascularization group (P < 0.00001), and intensive care unit days were decreased (P = 0.007) in the simultaneous revascularization group. There were no significant differences in other outcomes between sequential and simultaneous revascularization groups, such as blood transfusions, hospital days, graft failure and mortality in one month and one year, operation time.
-
Citation: Wang JZ, Liu Y, Wang JL, Lu L, Zhang YF, Lu HW, Li YM. Sequential
vs simultaneous revascularization in patients undergoing liver transplantation: A meta-analysis. World J Gastroenterol 2015; 21(22): 7036-7046 - URL: https://www.wjgnet.com/1007-9327/full/v21/i22/7036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.7036
Sequential portal and arterial revascularization (SeqR) and simultaneous portal and arterial revascularization (SimR) have been advocated to improve outcomes after liver transplantation[1-5]. In SeqR, the liver graft is sequentially reperfused by portal and arterial reperfusion. By contrast, in SimR, the liver graft is simultaneously reperfused by the portal vein and the hepatic artery. Because the portal vein contributes approximately three fourths of the blood supply to the liver and is easily anastomosed to shorten the warm ischemia time (WIT) and the anhepatic phase during the operation, SeqR is the more widely performed sequence of revascularization[6]. However, the primary disadvantage of SeqR is the potential increased risk of arterial ischemic injury to the bile ducts, which depend solely on the arterial blood supply[7]. Therefore, some authors have recommended the use of SimR for its reduction of the risk of arterial ischemic damage to biliary epithelial cells, which are more susceptible to ischemia-reperfusion injury than hepatocytes[8,9]. However, the disadvantage of SimR is that it prolongs the WIT and the anhepatic phase, which can be detrimental to patient mortality and morbidity related to the graft[10]. The better method of revascularization in liver transplantation remains controversial.
Although some meta-analyses have been conducted to compare the incidence of total biliary complications between SimR and SeqR in liver transplantation[1,2], the method that results in a greater reduction in the incidence of ischemic-type biliary lesions (ITBLs) and other outcomes remains unclear. The primary purpose of this meta-analysis was to investigate the relationship between revascularization and ITBLs. In addition, we also evaluated other outcomes, such as blood transfusions, hospital days, graft failure and mortality in one month and one year, operation time.
This systematic review and meta-analysis were conducted according to the PRISMA statement[11].
To identify relevant studies, a search of the literature was performed in MEDLINE, the Cochrane database, the Science Citation Index (SCI), PLOS ONE, Wiley Online Library, Springer, and China National Knowledge Infrastructure (CNKI) without restrictions on the year or language. We performed a systematic search using both “MeSH” and “key words” protocols. More specifically, the following terms retrieved from the MeSH browser provided by PubMed were utilized: [“liver” (All Fields) OR “hepatic” (All Fields)] AND “transplantation” (All Fields) AND “revascularization” (All Fields) OR “reperfusion” (MeSH). A multiple “key words” search was performed with the terms “liver transplantation” AND “revascularization”.
Types of studies: Clinical studies conducted comparing SimR and SeqR in liver transplantation were included regardless of blinding, publication status, or sample size. Further, these clinical studies had to contain sufficient data about outcomes after liver transplantation. Literature on animal experiments, reviews, letters to the editor and clinical studies conducted without control groups were excluded.
Types of participants: Patients undergoing SeqR or SimR in liver transplantation were included regardless of age, gender, nationality, or reason for liver transplantation.
Types of interventions: Studies with comparisons between SeqR and SimR were included regardless of whether piggy-back or conventional orthotopic liver transplantation was performed.
Types of outcomes: (1) ITBLs; (2) blood transfusions (units of blood and plasma); (3) Hospital days [intensive care unit (ICU) and total hospital days]; (4) graft failure and mortality in one month and one year; and (5) Operation time (total operation and WIT).
Two independent reviewers (Wang JL, Lu L) evaluated each title, abstract, citation, and selected relevant studies according to the eligibility criteria. Disagreements were resolved by another reviewer (Lu HW). Data were extracted separately from the included studies, and a reviewer (Lu HW) reviewed and confirmed the accuracy of the extracted data. Data from the included studies were recorded as follows: (1) authors, country, year; (2) publication; (3) number of participants in the SimR and SeqR groups; and (4) outcomes after liver transplantation.
The methodological quality of each randomized controlled trials (RCTs) was independently assessed using the Jadad score. This five-point quality scale includes points for randomization (0-2 points), double blinding (0-2 points), and withdrawals and dropouts (0-1 points). Total scores of 0-2 were considered indicative of low quality, whereas studies with total scores ≥ 3 were defined as high quality. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of each retrospective study. The NOS contains 8 items, which are divided into three categories: selection (4 items), comparability (1 item), and exposure for case-control studies (3 items). A star system was used to allow for a semi-quantitative assessment of study quality. A study can be awarded a maximum of one star for each item within the selection and exposure categories. A maximum of two stars can be given for comparability. Studies were defined as high quality if they had more than seven stars; as medium quality if they had between four and six stars; and as poor quality if they had fewer than four stars.
The statistical methods of this study were reviewed by Deyu Meng from the Faculty of Mathematics and Statistics, Xi’an Jiaotong University. Data were analyzed by the statistical software Review Manager 5.2 (The Cochrane Collaboration) using a fixed effects model. The results of continuous and discontinuous data were reported as MD with 95%CI and as OR with 95%CI, respectively. P < 0.05 was considered to indicate a statistically significant difference. The heterogeneity of the included studies was evaluated by the χ2 and I2 tests (Review Manager 5.2), and P < 0.10 or I2 > 25% was defined as indicating heterogeneity. A funnel plot (Review Manager 5.2) and Egger’s test (Stata 10.0) were used to assess the risk of publication bias, and P < 0.10 indicated statistical publication bias. The risk of bias in the studies was assessed by two independent reviewers (Wang JZ, Li YM) using a domain-based assessment tool recommended by the Cochrane collaboration. Two methods of sensitivity analysis were conducted by comparing the effect size between a fixed effects model and a random effects model; the fixed effect model was applied after excluding the greatest weight study.
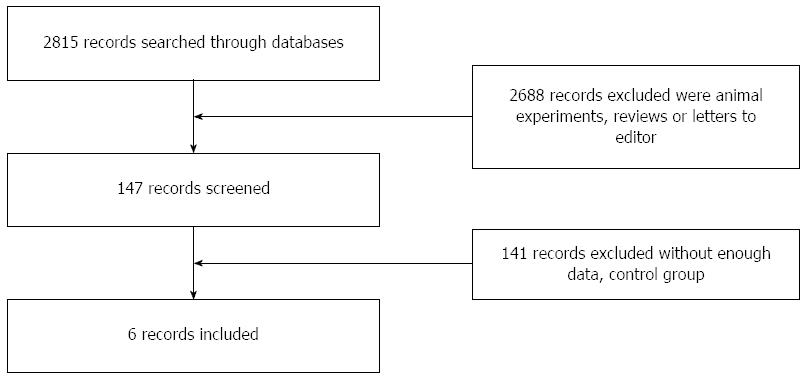
We screened the title and abstract of 2815 studies after performing the database search. Then, 2688 studies were excluded because they were animal experiments, reviews, or letters to the editor, and 141 clinical studies did not meet our criteria due to their lack of a control group or insufficient data on outcomes after liver transplantation. A total of six studies were included[12-17], comprising a total of 467 patients (Figure 1).
Six studies with a total of 467 patients were included. Two studies were RCTs[16,17], with a total of 120 patients; the other studies were retrospective, with a total of 347 patients[13-17]. The included studies were conducted in five countries: two in Italy, one in China, one in the United States, one in Brazil, and one in the Netherlands. None of the six studies was considered low quality (Table 1).
| Study | Year | Country | No. of SeqR/ SimR | Type of study | Jadad score | NOS |
| Sankary et al[12] | 1995 | United States | 83/45 | Retrospective study | - | 6 |
| Massarollo et al[13] | 1998 | Brazil | 26/50 | Retrospective study | - | 7 |
| Polak et al[14] | 2005 | the Netherlands | 71/31 | Retrospective study | - | 7 |
| Wu et al[15] | 2006 | China | 21/20 | Retrospective study | - | 6 |
| Adani et al[16] | 2011 | Italy | 19/21 | RCT | 3 | - |
| Baccarani et al[17] | 2012 | Italy | 38/42 | RCT | 3 | - |
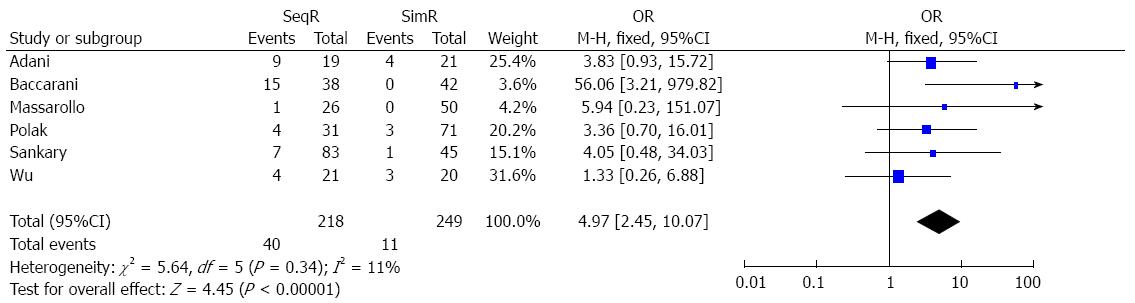
Simultaneous revascularization reduced the incidence of ITBLs: The incidence of ITBLs was significantly reduced in the SimR group compared to the SeqR group according to this meta-analysis (OR = 4.97, 95%CI: 2.45-10.07; P < 0.00001). Furthermore, the results of the χ2 and I2 tests did not indicate heterogeneity (P = 0.34, I2 = 11%) (Figure 2).
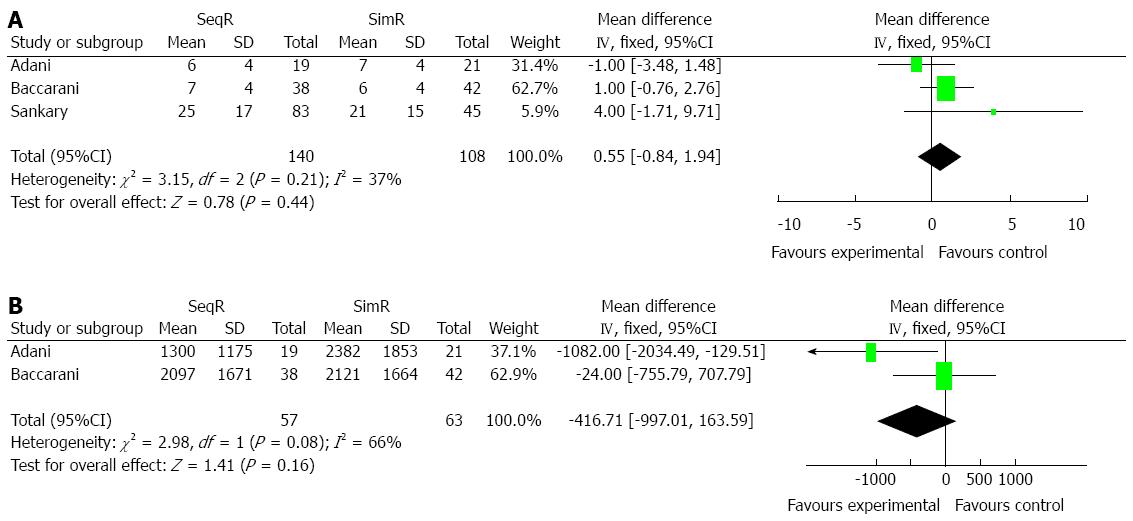
Simultaneous revascularization did not increase blood transfusions: Two and three studies, respectively, were conducted to compare units of blood cell and plasma transfusions between SeqR and SimR. There were no significant differences in units of blood cell transfusions (MD = 0.55, 95%CI: -0.84-1.94; P = 0.44) and plasma transfusions (MD = -416.71, 95%CI: -997.01-163.59; P = 0.16). The results of the χ2 and I2 tests were P = 0.21 (I2 = 37%) and P = 0.08 (I2 = 66%), respectively (Figure 3).
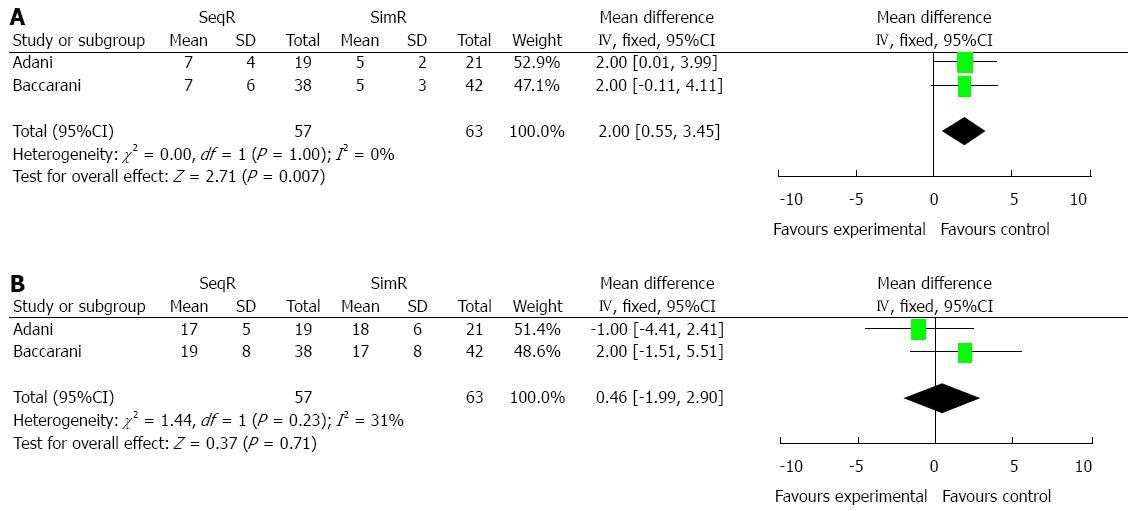
Simultaneous revascularization decreased ICU days: Two studies were conducted to compare ICU and total hospital days. SimR significantly decreased ICU days (MD = 2.00, 95%CI: 0.55-3.45; P = 0.007), while no significant difference was shown in total hospital days (MD = 0.46, 95%CI: -1.99-2.90; P = 0.71). The results of the χ2 and I2 tests did not indicate heterogeneity (P = 1.00, I2 = 0% and P = 0.23, I2 = 31%), respectively (Figure 4).
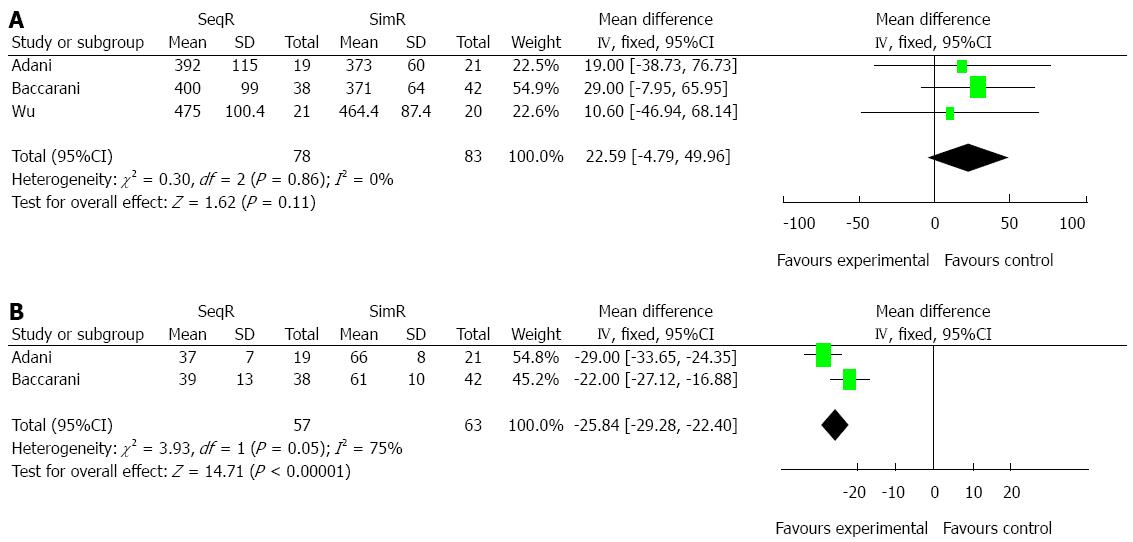
Simultaneous revascularization increased WIT: Although there was no significant difference in total operation time (MD = 22.59, 95%CI: -4.79-49.96; P = 0.11), SimR significantly prolonged WIT (MD = -25.84, 95%CI: -29.28-22.40; P < 0.00001). However, the results of χ2 and I2 tests in WIT showed heterogeneity (P = 0.05, I2 = 75%), which was not indicated in total operation time (P = 0.86, I2 = 0%) (Figure 5).
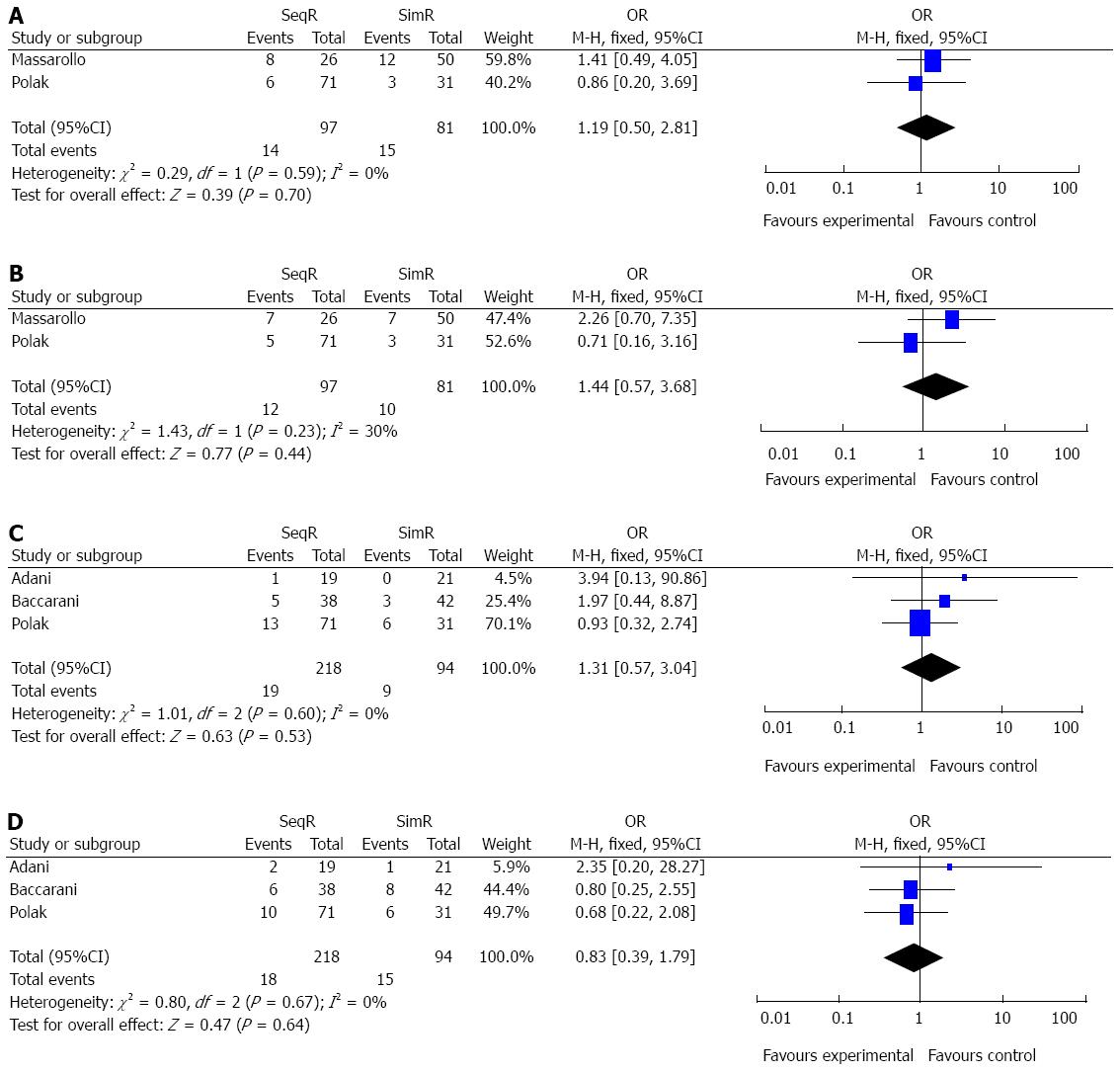
Simultaneous revascularization did not increase patient mortality and graft failure in one month and one year: There were two and three studies comparing graft failure and mortality in one month and one year between SeqR and SimR, respectively. There were no significant differences in patient graft failure and mortality in one month (OR = 1.19, 95%CI: 0.50-2.81; P = 0.70; OR = 1.44, 95%CI: 0.57-3.68, P = 0.44), or in one year (OR = 1.31, 95%CI: 0.57-3.04, P = 0.53; OR = 0.83, 95%CI: 0.39-1.78, P = 0.64). All results of χ2 and I2 tests did not indicate heterogeneity (P = 0.59, I2 = 0%; P = 0.23, I2 = 30%; P = 0.60, I2 = 0%; P = 0.67, I2 = 0%) (Figure 6).
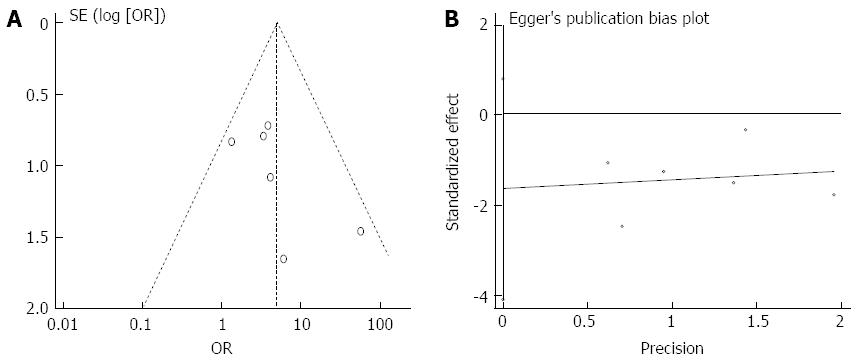
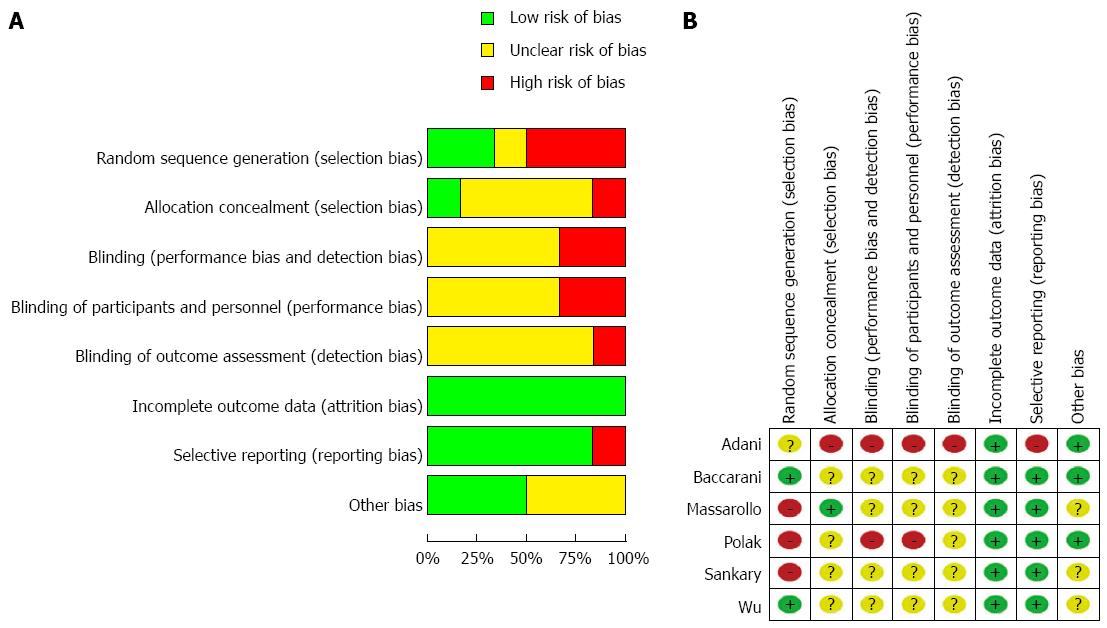
Although the funnel plot was not strictly symmetrical, Egger’s test did not show publication bias (P = 0.136) (Figure 7). The analyses indicated that random sequence generation and blinding, which were not properly described in these studies, might have been a source of bias (Figure 8). The sensitivity analysis showed the same effect sizes among a fixed effect model (P < 0.00001), a random effect model (P = 0.002) and a fixed effect model after excluding the largest weight study (P < 0.00001) (Figure 9).
The current methods of revascularization in liver transplantation can be divided into two main groups according to whether the liver graft is reperfused sequentially or simultaneously. In SeqR cases, the first reconstructed vessel of the liver graft is one of the portal vein, the hepatic artery, or the inferior vena cava, followed by subsequent revascularization of the remaining vessels. By contrast, in SimR cases, the graft is simultaneously reperfused via the portal vein and the hepatic artery[3]. Although the best method of revascularization in liver transplantation remains debatable[18], the most commonly used procedure for the revascularization of liver grafts is SeqR[19]. Theoretically, there are some potential advantages to SeqR: (1) the portal vein contributes approximately 3/4 of the blood supply and 1/2 of the oxygen supply to the liver; (2) the portal vein is easily anastomized to shorten the WIT and anhepatic phase during the operation; and (3) the arterial anastomosis is performed under better technical conditions, without retrograde bleeding from the graft hepatic artery and in a surgical field free of hemorrhages[20]. However, SeqR increases warm ischemic injury to the bile ducts, which depend on the hepatic artery. Because ischemic injuries to the bile ducts account for a majority of cases of morbidity, mortality, and graft failure in patients after liver transplantation[21-25], some researchers have advocated the use of SimR. Although some meta-analyses have demonstrated that SeqR increases total biliary complications[1,2], including anastomotic biliary complications and nonanastomotic biliary complications (i.e., ITBLs)[26,27], little is known about the relationship between revascularization and ITBLs.
We comprehensively reviewed the literature on the relationship between revascularization and ITBLs. The results of this meta-analysis on ITBLs imply that SimR reduces the incidence of ITBLs after liver transplantation, which is in accordance with previous clinical studies[12,13]. In addition, the heterogeneity tests did not show heterogeneity, and the sensitivity analysis showed the same effect sizes, which strengthened the confidence that can be placed in these results.
Although the specific mechanisms of SimR that reduce the incidence of ITBLs remain unclear, reduced arterial ischemic injury to the bile ducts may be the main reason. The peribiliary vascular plexus is composed of branches arising directly from the right and left hepatic arteries (and accessory hepatic arteries when present) and from their segmental branches as well as branches arising indirectly from the gastroduodenal artery via the arteries supplying the common bile duct[28]. In SeqR cases, the graft is exclusively perfused through the portal vein for at least 10 min until the completion of hepatic arterial anastomosis[29,30]. The delay of rearterialization in SeqR is associated with more pronounced microvascular disturbances and subsequent graft dysfunction, including ischemic injury to the bile ducts. In a rat model of liver transplantation, SimR resulted in the best microcirculatory perfusion of the graft. In addition, the authors found less leukocyte accumulation in the sinusoids and more abundant bile flow after simultaneous reperfusion compared to SeqR[31]. Moreover, SimR also elicits a remarkable improvement in oxygen tension and the maintenance of tissue ATP compared to SeqR, which may be helpful for reducing the incidence of ITBLs[32]. Although there are some potential benefits of SimR, the main disadvantages of SimR are the prolonged WIT and anhepatic phase, which can be detrimental to postoperative graft function, survival, and morbidity[3]. However, Adani et al[16] reported that delayed graft function was diagnosed in 10% vs 9% in SeqR and SimR, while Polak et al[14] reported that primary nonfunction was diagnosed in 3% (SeqR) vs 1% (SimR) and the rate of retransplantation was 9% in SeqR and 7% in SimR, respectively. Vascular complications were absent except for one case of hepatic artery thrombosis (HAT) leading to retransplantation in SeqR[16]. Liver ischemia-reperfusion injury, resulting in ITBLs during liver transplantation, triggers a cascade of events leading to biliary apoptosis, necrosis, and cholangitis which may even lead to graft failure. The main cause of prolonged WIT in SimR may be the simultaneous reconstruction of the portal vein and hepatic artery, which is inevitable due to this particular surgical technique. However, interestingly, SimR did not significantly prolong the total operation time or increase the incidence of blood transfusions, graft failure or mortality (one month and one year) in this study. Therefore, this is a burning issue to find the answer in the worlds of liver transplantation.
Besides different kinds of revascularization, some donor factors affect ITBLs, such as ABO incompatibility, gender, cytomegalovirus (CMV) infection and metalloproteinase-2 (MMP-2) polymorphism. The incidence of ITBLs after ABO-incompatible liver transplantation in adults is much higher than in ABO-compatible liver transplantation[33]. MMP-2 genotype in both donor and recipient is strongly and independently related to the development of ITBLs within 4 years after liver transplantation[34].
Although this systematic review and meta-analysis imply that SimR reduces the incidence of ITBLs after liver transplantation, there are undoubtedly some limitations: (1) the number of patients in the included studies ranged from 19 to 83; (2) although we do believe that our search strategy was sufficient, only six studies were included in this meta-analysis of ITBLs, which may lead to inaccurate conclusions[35,36]; and (3) assessment of risk of bias showed that the methods of random sequence generation and blinding, which were not properly described in these studies, might have been a source of bias, which may have led to an overstatement of the treatment effects of SimR[37].
In conclusion, the present meta-analysis included all current relevant clinical studies from various countries through July 2014, and the findings indicate that SimR reduces the incidence of ITBLs and decreases ICU days. Additional randomized and blinded clinical trials with a sufficient number of participants are needed to adequately compare SimR and SeqR in liver transplantation.
Sequential portal and arterial revascularization (SeqR) and simultaneous portal and arterial revascularization (SimR) have been advocated to improve outcomes after liver transplantation. However, little is known about the relationship between revascularization and ischemic-type biliary lesions (ITBLs). The authors undertook this meta-analysis to investigate the relationship between revascularization and outcomes after liver transplantation.
There are two main methods for revascularization of the liver graft: SimR and SeqR. The sequence of graft reperfusion may be relevant for the development of ITBLs. In some retrospective studies, the incidence of ITBLs in patients who underwent SimR of the graft was lower compared to patients who had SeqR. Some clinical studies are conducted to validate whether SimR is better than SeqR.
Although some meta-analyses have been conducted to compare the incidence of total biliary complications between SimR and SeqR in liver transplantation, the method that results in a greater reduction in the incidence of ITBLs and other outcomes remains unclear. The current study demonstrated that ITBLs and ICU days were significantly reduced in the SimR group compared with the SeqR group.
The present meta-analysis indicates that SimR reduces the incidence of ITBLs and may be more suitable to protect the integrity of the intrahepatic biliary tree.
In SimR, the liver graft is simultaneously reperfused by the portal vein and the hepatic artery. Some scientists have recommended the use of SimR for its reduction of the risk of arterial ischemic damage to biliary epithelial cells, which are more susceptible to ischemia-reperfusion injury than hepatocytes.
This is a well written paper analyzing a hot topic in liver transplantation technique and its outcomes. This work is a meta-analysis concerning the aspect of sequential vs simultaneous portal and arterial reperfusion in liver transplantation. The authors performed a structured literature review with a final analysis of six studies including 467 patients overall. As expected, in patients with simultaneous reperfusion a significantly longer warm ischemic time was found. In contrast, ischemic-type biliary lesions were significant reduced in the group of patients with simultaneous reperfusion. Graft failure and mortality were not different between both groups at one month and one year after liver transplantation.
P- Reviewer: Darius T, Frankova S, Herden U S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Liu XM
| 1. | Gurusamy KS, Naik P, Abu-Amara M, Fuller B, Davidson BR. Techniques of flushing and reperfusion for liver transplantation. Cochrane Database Syst Rev. 2012;3:CD007512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Manzini G, Kremer M, Houben P, Gondan M, Bechstein WO, Becker T, Berlakovich GA, Friess H, Guba M, Hohenberger W. Reperfusion of liver graft during transplantation: techniques used in transplant centres within Eurotransplant and meta-analysis of the literature. Transpl Int. 2013;26:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Polak WG, Porte RJ. The sequence of revascularization in liver transplantation: it does make a difference. Liver Transpl. 2006;12:1566-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sanchez-Perez B, Aranda-Narvaez JM, Suarez-Munoz MA, Fernandez-Aguilar JL, Perez-Daga JA, Gonzalez-Sanchez AJ, Montiel-Casado C, Becerra-Ortiz RM, Pulido-Roa I, Santoyo J. Simultaneous (Arterial/Portal) or Sequential (Portal) Revascularization of the Liver Graft: Does It Make Any Difference? Transpl Int. 2011;17:S236-S236. |
| 5. | Brockmann JG, August C, Wolters HH, Hömme R, Palmes D, Baba H, Spiegel HU, Dietl KH. Sequence of reperfusion influences ischemia/reperfusion injury and primary graft function following porcine liver transplantation. Liver Transpl. 2005;11:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Puhl G, Schaser KD, Pust D, Köhler K, Vollmar B, Menger MD, Neuhaus P, Settmacher U. The delay of rearterialization after initial portal reperfusion in living donor liver transplantation significantly determines the development of microvascular graft dysfunction. J Hepatol. 2004;41:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Moreno C, Sabaté A, Figueras J, Camprubí I, Dalmau A, Fabregat J, Koo M, Ramos E, Lladó L, Rafecas A. Hemodynamic profile and tissular oxygenation in orthotopic liver transplantation: Influence of hepatic artery or portal vein revascularization of the graft. Liver Transpl. 2006;12:1607-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Cursio R, Gugenheim J. Ischemia-Reperfusion Injury and Ischemic-Type Biliary Lesions following Liver Transplantation. J Transplant. 2012;2012:164329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Ayanoglu HO, Ulukaya S, Tokat Y. Causes of postreperfusion syndrome in living or cadaveric donor liver transplantations. Transplant Proc. 2003;35:1442-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8044] [Article Influence: 536.3] [Reference Citation Analysis (2)] |
| 12. | Sankary HN, McChesney L, Frye E, Cohn S, Foster P, Williams J. A simple modification in operative technique can reduce the incidence of nonanastomotic biliary strictures after orthotopic liver transplantation. Hepatology. 1995;21:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Massarollo PC, Mies S, Raia S. Simultaneous arterial and portal revascularization in liver transplantation. Transplant Proc. 1998;30:2883-2884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Polak WG, Miyamoto S, Nemes BA, Peeters PM, de Jong KP, Porte RJ, Slooff MJ. Sequential and simultaneous revascularization in adult orthotopic piggyback liver transplantation. Liver Transpl. 2005;11:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Wu D, Zheng S, Dong J, Wang S, Bie P, Yang Z. Comparative study of two intraoperative reperfusion modes affecting liver function at the early stage after orthotopic liver transplantation. J Digest Surg. 2006;3:175-178. |
| 16. | Adani GL, Rossetto A, Bresadola V, Lorenzin D, Baccarani U, De Anna D. Contemporaneous Portal-Arterial Reperfusion during Liver Transplantation: Preliminary Results. J Transplant. 2011;2011:251656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Baccarani U, Rossetto A, Lorenzin D, Bidinost S, Lugano M, Bresadola V, De Anna D, Della Rocca G, Adani GL. Protection of the Intrahepatic Biliary Tree by Contemporaneous Portal and Arterial Reperfusion during Liver Transplantation: Results of a Prospective Randomized Trial. Liver Transpl. 2012;18:S83-S83. |
| 18. | Walsh TS, Garden OJ, Lee A. Metabolic, cardiovascular, and acid-base status after hepatic artery or portal vein reperfusion during orthotopic liver transplantation. Liver Transpl. 2002;8:537-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Sadler KM, Walsh TS, Garden OJ, Lee A. Comparison of hepatic artery and portal vein reperfusion during orthotopic liver transplantation. Transplantation. 2001;72:1680-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Adani GL, Rossetto A, Lorenzin D, Lugano M, De Anna D, Della Rocca G, Donini A, Bresadola V, Risaliti A, Baccarani U. Sequential versus contemporaneous portal and arterial reperfusion during liver transplantation. Transplant Proc. 2011;43:1107-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Stahl JE, Kreke JE, Malek FA, Schaefer AJ, Vacanti J. Consequences of cold-ischemia time on primary nonfunction and patient and graft survival in liver transplantation: a meta-analysis. PLoS One. 2008;3:e2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Verdonk RC, Buis CI, van der Jagt EJ, Gouw AS, Limburg AJ, Slooff MJ, Kleibeuker JH, Porte RJ, Haagsma EB. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl. 2007;13:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Heidenhain C, Pratschke J, Puhl G, Neumann U, Pascher A, Veltzke-Schlieker W, Neuhaus P. Incidence of and risk factors for ischemic-type biliary lesions following orthotopic liver transplantation. Transpl Int. 2010;23:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Wang B, Zhang Q, Zhu B, Cui Z, Zhou J. Protective effect of gadolinium chloride on early warm ischemia/reperfusion injury in rat bile duct during liver transplantation. PLoS One. 2013;8:e52743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Esmat MEE, Buckels JA, Mirza DF, Mayer DA, Gunson BG, Yen CY, Hegab BH, Bramhall SR. Biliary complications after orthotopic liver transplantation; Ten years experience. Transpl Int. 2007;20:258-259. |
| 27. | Gastaca M, Matarranz A, Muñoz F, Valdivieso A, Aguinaga A, Testillano M, Bustamante J, Terreros I, Suarez MJ, Montejo M. Biliary complications in orthotopic liver transplantation using choledochocholedochostomy with a T-tube. Transplant Proc. 2012;44:1554-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Vellar ID. Preliminary study of the anatomy of the venous drainage of the intrahepatic and extrahepatic bile ducts and its relevance to the practice of hepatobiliary surgery. ANZ J Surg. 2001;71:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Baccarani U, Isola M, Adani GL, Avellini C, Lorenzin D, Rossetto A, Currò G, Comuzzi C, Toniutto P, Risaliti A. Steatosis of the hepatic graft as a risk factor for post-transplant biliary complications. Clin Transplant. 2010;24:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Adani GL, Lorenzin D, Avellini C, Isola M, Rossetto A, Toniutto P, Bresadola V, Risaliti A, Baccarani U. Steatosis of the Hepatic Graft as a Risk Factor for Post-Transplant Biliary Complications. Liver Transpl. 2009;15:S156-S156. |
| 31. | Post S, Palma P, Gonzalez AP, Rentsch M, Menger MD. Timing of arterialization in liver transplantation. Ann Surg. 1994;220:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Reck T, Steinbauer F, Steinbauer M, Schwille PO, Wittekind C, Hohenberger W, Köckerling F. Impact of arterialization on hepatic oxygen supply, tissue energy phosphates, and outcome after liver transplantation in the rat. Transplantation. 1996;62:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Wu J, Ye S, Xu X, Xie H, Zhou L, Zheng S. Recipient outcomes after ABO-incompatible liver transplantation: a systematic review and meta-analysis. PLoS One. 2011;6:e16521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Ten Hove WR, Korkmaz KS, op den Dries S, de Rooij BJ, van Hoek B, Porte RJ, van der Reijden JJ, Coenraad MJ, Dubbeld J, Hommes DW. Matrix metalloproteinase 2 genotype is associated with nonanastomotic biliary strictures after orthotopic liver transplantation. Liver Int. 2011;31:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Tseng TY, Dahm P, Poolman RW, Preminger GM, Canales BJ, Montori VM. How to use a systematic literature review and meta-analysis. J Urol. 2008;180:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Bhandari M, Devereaux PJ, Montori V, Cinà C, Tandan V, Guyatt GH. Users’ guide to the surgical literature: how to use a systematic literature review and meta-analysis. Can J Surg. 2004;47:60-67. [PubMed] |
| 37. | Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. QUOROM Group. Br J Surg. 2000;87:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |