Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6990
Peer-review started: December 7, 2014
First decision: January 22, 2015
Revised: January 27, 2015
Accepted: April 9, 2015
Article in press: April 9, 2015
Published online: June 14, 2015
Processing time: 193 Days and 12.2 Hours
AIM: To investigate the effects of bariatric surgery on metabolic parameters, incretin hormone secretion, and duodenal and ileal mucosal gene expression.
METHODS: Nine patients with type 2 diabetes mellitus (T2DM), chronic serum hyperglycemia for more than 2 years, and a body mass index (BMI) of 30-35 kg/m2 underwent metabolic surgery sleeve gastrectomy with transit bipartition between May 2011 and December 2011. Blood samples were collected pre and 3, 6 and 12 mo postsurgery. Duodenal and ileal mucosa samples were collected pre- and 3 mo postsurgery. Pre- and postoperative blood samples were collected in the fasting state before ingestion of a standard meal (520 kcal) and again 30, 60, 90, and 120 min after the meal to determine hemoglobin A1c (HbA1c) levels and the lipid profile, which consisted of triglyceride and total cholesterol levels. Intestinal gene expression of p53 and transforming growth factor (TGF)-β was analyzed using quantitative reverse-transcription PCR. Gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) were quantified using the enzyme-linked immunoassay method and analyzed pre- and postoperatively. Student’s t test or repeated measurements analysis of variance with Bonferroni corrections were performed as appropriate.
RESULTS: BMI values decreased by 15.7% within the initial 3 mo after surgery (31.29 ± 0.73 vs 26.398 ± 0.68, P < 0.05) and then stabilized at 22% at 6 mo postoperative, resulting in similar values 12 mo postoperatively (20-25 kg/m2). All of the patients experienced improved T2DM, with 7 patients (78%) achieving complete remission (HbA1c < 6.5%), and 2 patients (22%) achieving improved diabetes (HbA1c < 7.0% with or without the use of oral hypoglycemic agents). At 3 mo postoperatively, fasting plasma glucose had also decreased (59%) (269.55 ± 18.24 mg/dL vs 100.77 ± 3.13 mg/dL, P < 0.05) with no further significant changes at 6 or 12 mo postoperatively. In the first month postoperatively, there was a complete withdrawal of hypoglycemic medications in all patients, who were taking at least 2 hypoglycemic drugs preoperatively. GLP-1 levels significantly increased after surgery (149.96 ± 31.25 vs 220.23 ± 27.55) (P < 0.05), while GIP levels decreased but not significantly. p53 gene expression significantly increased in the duodenal mucosa (P < 0.05, 2.06 fold) whereas the tumor growth factor-β gene expression significantly increased (P < 0.05, 2.52 fold) in the ileal mucosa after surgery.
CONCLUSION: Metabolic surgery ameliorated diabetes in all of the patients, accompanied by increased anti-proliferative intestinal gene expression in non-excluded segments of the intestine.
Core tip: This study shows an improvement in expression of antiproliferative genes of intestinal mucosa after type 2 diabetes mellitus amelioration promoted by metabolic surgery procedure. We make a link between this outcome and morphological changes in intestinal mucosa on diabetes, that occurs mainly by insufficient negative control of mucosal growth in hyperglycemia. Metabolic and bariatric surgery promotes dramatic amelioration of glucose metabolism in diabetic patients by not completely understood means. This paper highlights for the first time, the intestinal absorptive capacity as the main focus for research of diabetes amelioration after metabolic surgery procedures.
- Citation: Rodrigues MRDS, Santo MA, Favero GM, Vieira EC, Artoni RF, Nogaroto V, Moura EG, Lisboa P, Milleo FQ. Metabolic surgery and intestinal gene expression: Digestive tract and diabetes evolution considerations. World J Gastroenterol 2015; 21(22): 6990-6998
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6990.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6990
It has been recently suggested that diabetes alters the gene expression of various DNA repair genes, including p53 and transforming growth factor (TGF)-β[1]. Both of these genes are considered to be cell division down-regulators[2], including for normal intestinal epithelium growth[3-5], and their expression might be altered in patients predisposed or affected by metabolic disease. It was established over 40 years ago that diabetes influences, or can be influenced by, morphological changes in the intestinal mucosa[6]. In experimental models of type 2 diabetes mellitus (T2DM), hyperplasia of the intestinal mucosa was observed in the early phases of the metabolic changes[7]. This phenomenon may be related to a transient interruption of normal cellular apoptosis[8], indicating altered intestinal regulatory gene functions for disease development.
Rat studies have demonstrated that hypertrophic changes occur primarily in the proximal gastrointestinal (GI) tract and enhance the carbohydrate absorptive capacity of the intestinal mucosa[9]. Furthermore, in experimental models of T2DM, postprandial hyperglycemia has been observed before the development of the metabolic disease, concomitantly with the morphofunctional changes in the intestinal epithelium[7]. In humans, postprandial hyperglycemia strongly predicts the development of T2DM[10]. Interestingly, with the resolution of diabetes, the intestinal epithelium also regains normal function and trophy, although the mechanisms underlying these changes are not understood[11].
Recently, Verdam et al[12] suggested that obese patients with T2DM have significantly more intestinal mass than their non-T2DM counterparts, suggesting that the hypertrophic changes previously observed in experimental models might be a cornerstone feature for T2DM development in humans as well.
Santoro et al[13] recently published the results of sleeve gastrectomy with transit bipartition (SGTB) or a partial duodenal switch, which is a metabolic surgery procedure. This innovative procedure brings the distal ileum in contact with undigested food through a gastro-ileal anastomosis, without excluding the duodenum from the passage of food; it has shown potential in resolving T2DM.
The present study aimed to investigate intestinal mucosal gene expression [p53 and tumor growth factor-β (TGF-β)] before and after SGTB in class I obese [body mass index (BMI) 30-35 kg/m2] patients with T2DM and resolution of hyperglycemia after surgery. Considerations about the role of the GI tract in T2DM are also highlighted.
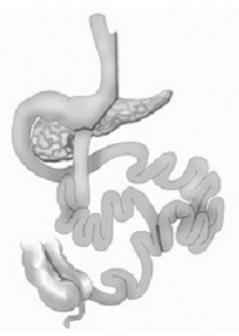
This observational study was conducted from May to December 2011. Nine class I obese patients with T2DM who had presented with chronic serum hyperglycemia for > 2 years were enrolled and underwent SGTB. This open access procedure involved a typical sleeve gastrectomy. After the SG, the ileocecal transition is located. A single stitch is used to mark the point at the ileum located 80 cm from the ileocecal valve. The point 260 cm proximal to the ileocecal valve is then located. At this point, the intestinal segment is sectioned. The distal end is brought to the gastric antrum, and a wide laterolateral gastroileal anastomosis is created. In the following sequence, the small bowel cranial to the gastroileal anastomosis is laterally and widely anastomosed to the ileum at an 80 cm distance from the ileocecal valve (previously marked) in a lateral-lateral mode (Figure 1).
All patients provided written informed consent before undergoing the surgery. The study was approved by the Institutional Review Board at the State University of Ponta Grossa, Paraná, Brazil.
Diabetes diagnoses were based on the following American Diabetes Association[14] criteria: a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L), symptoms of diabetes plus a casual plasma glucose level ≥ 200 mg/dL (11.1 mmol/L), or a 2-h postload glucose level ≥ 200 mg/dL during a 75-goral glucose tolerance test. The exclusion criteria included peptide C levels < 0.9 ng/dL, pregnancy, inflammatory bowel disease, drug or alcohol addiction, and psychiatric disturbances that precluded complete understanding of the surgical procedure.
Diabetes remission was defined as a hemoglobin A1c (HbA1c) level < 6.5% without the use of diabetes medications. Diabetes was considered to be improved if the patients had an HbA1c level < 7.0% with or without the use of oral hypoglycemic agents. The lipid profile consisted of fasting and postprandial triglyceride and total cholesterol levels. Clinical evolution was analyzed at 3, 6 and 12 postoperative months.
Pre- and postoperative blood samples were collected during the fasting state (8 h fasting) before the patients ingested a standard meal (520 kcal) and again 30, 60, 90, and 120 min after the meal. Blood was collected in tubes with the anticoagulant ethylenediaminetetraacetic acid (1 g/L). Plasma was separated in aliquots of 1 mL per vial and frozen at -80 °C. During the preoperative period (under deep sedation with 50 mg of propofol plus 50 μg of fentanil), biopsy specimens of the duodenal mucosa (4 specimens, approximately 2 mm3 each) were obtained using upper digestive endoscopy. Samples of the ileal mucosa (260 cm proximal to the ileocecal valve) were collected prior to the gastro-ileal anastomosis joining at the anastomosis site. After SGTB, access to the ileum and duodenum is possible during an upper endoscopy (Figure 1).Three months after surgery, mucosal samples were collected using endoscopic biopsy forceps with the patients under deep sedation with 5 mL of propofol and 1 mL of fentanil. The trans- and post-operative tissues were promptly placed in RNAlater solution (Qiagen), with subsequent freezing at -80 °C for storage until qRT-PCR analysis.
Gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are the two primary incretin hormones secreted from the intestine after ingesting glucose or nutrients that stimulate insulin secretion by pancreatic β cells. Quantification of plasma GLP-1 and GIP was performed using the enzyme-linked immunoassay method in the range of 450 nm (Biotek EL800, Winooski, VT, United States). The peptides were quantified using commercial kits from Phoenix Pharmaceuticals, Inc. (Belmont, CA, United States), according to the manufacturer’s instructions.
Total RNA from the duodenal and ileal samples was isolated using the IllustraRNAspin Mini RNA Isolation Kit (GE Healthcare, Buckinghamshire, United Kingdom), according to the manufacturer’s instructions. Reverse transcription of 1 μg of total RNA was performed using the First-Strand cDNA Synthesis Kit (GE Healthcare, Buckinghamshire, United Kingdom), according to the manufacturer’s protocol. Gene expression of the target genes was quantified with qRT-PCR in a StratageneMxPro3005P thermocycler (Agilent Technologies, Santa Clara, United States). cDNA was amplified in duplicate PCR reactions using 1× of the SYBR® Green Master Mix (Stratagen, La Jolla, CA, United States). A negative control was also included for each gene amplification assay. The PCR cycling conditions were as follows: 5 min at 94 °C; 40 cycles of 15 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C, with a dissociation curve. The 18S was used as an internal control. The values are expressed as the relative expression in terms of the control levels (2-ΔΔCt).
Data are shown as the mean ± SE. Student’s t test or repeated measurements ANOVA with Bonferroni corrections were performed as appropriate, with a level of significance set at P < 0.05.
The patients (5 women, 4 men) were similar in age (Table 1). The mean BMI was 31.18 ± 1.17 kg/m2. All patients had satisfactory postoperative evaluations, with a normal diet, normal bowel movements, and no hospital readmissions.
| mean ± SD | Range | |
| Age (yr) | 47.11 ± 7.84 | 30.00–53.00 |
| Gender (men), n (%) | 4 (44.4) | |
| Gender (women), n (%) | 5.01 (55.6) | |
| T2DM duration (yr) | 5.6 | 3.00–11.00 |
| Preoperative BMI (kg/m2) | 31.17 ± 2.18 | 26.47–33.39 |
Complete T2DM remission was observed in 7 (78%) patients, and improved diabetes was observed in 2 (22%) patients. Before surgery, all patients were taking at least 2 hypoglycemic drugs, and 6 (66.7%) were insulin dependent. In the first postoperative month, there was a complete withdrawal of hypoglycemic medications in all patients.
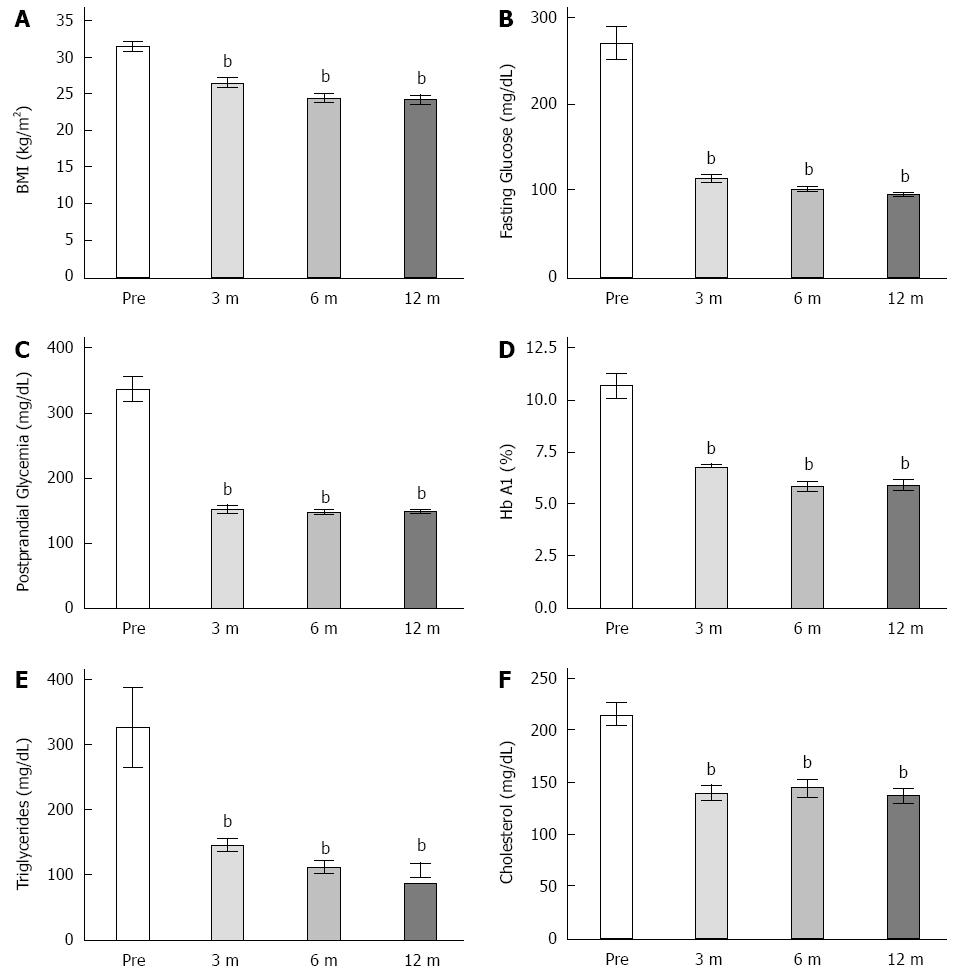
There was a statistically significant decrease in BMI at 3 mo (by 15.7%) (31.29 ± 0.73 vs 26.39 ± 0.683, P < 0.05), a further decrease at 6 postoperative months (22.2%), and a decrease of 22.8% at 12 postoperative months compared with thepre-operative values (Figure 2A). At 3 postoperative months, the fasting plasma glucose had also decreased (59%) (269.55 ± 18.24 mg/dL vs 100.77 ± 3.13 mg/dL, P < 0,05); there were no further changes in the fasting glucose levels at 6 or 12 postoperative months surgery (Figure 2B). Three months postoperatively, postprandial glycaemia had decreased by 55% (334.88 ± 19.24 mg/dL vs 150.75 ± 6.84 mg/dL, P < 0,05), and this level was maintained at 6 and 12 postoperative months (Figure 2C). The HbA1c levels before surgery were 10.7%, and they decreased to 6.8% at 3 postoperative months (10.66% ± 0.59% vs 6.78% ± 0.10%, P < 0.05) (Figure 2D). Serum triglyceride and cholesterol levels also decreased 3 mo after surgery (325.11 ± 60.29 mg/dL vs 144.25 ± 10.11 mg/dL and 214.33 ± 10.26 mg/dL vs 139.12 ± 7.63 mg/dL (55% and 27%, respectively) and remained at this level at 6 and 12 postoperative months (Figure 2E and F).
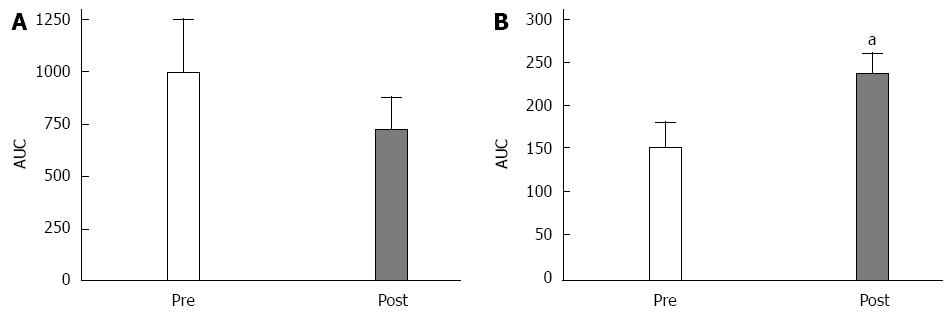
Eight patients completed the fasting and postprandial blood sample collection protocol. There was a significant increase in the postoperative GLP-1 levels of each patient [compared with the preoperative levels (P < 0.05; Figure 3A)] when analyzed with the area under the curve (AUC)analysis. There was a slight decrease in the GIP concentrations, but this decrease was not significant (Figure 3B).
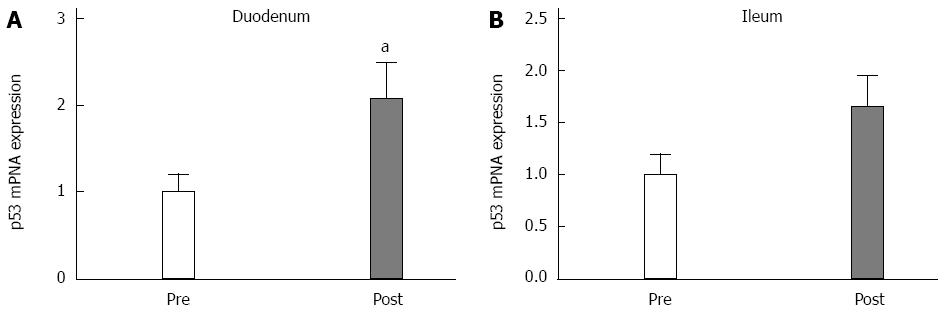
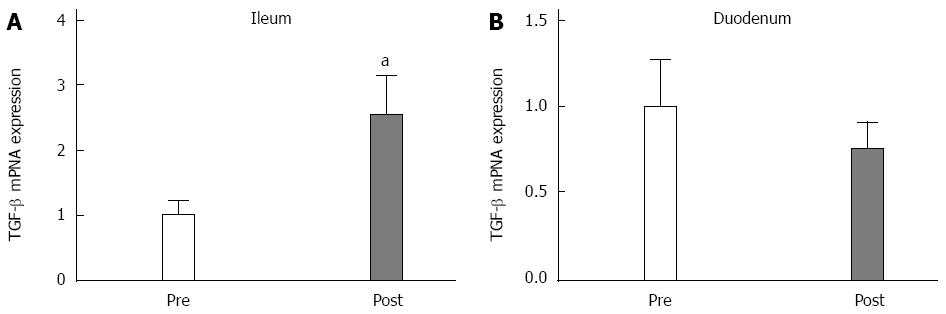
Three months after surgery, the p53 expression significantly increased in the duodenum (P < 0.05; Figure 4A), and there was a trend of increased expression in the ileal mucosa (P = 0.1; Figure 4B). The TGF-β expression levels did not change in the duodenum (Figure 5A). However, TGF-β expression significantly increased in the ileum (Figure 5B) at 3 postoperative months.
The present study demonstrated that SGTB improves glucose and lipid metabolism in patients with T2DM and leads to complete T2DM remission in the majority of patients. In addition, there was increased expression of the anti-proliferative intestinal genes after metabolic improvement, suggesting that these genes play a possible role in the intestinal mucosa with T2DM progression.
Surgeries that are performed in the digestive tract, particularly bariatric or metabolic surgical procedures, are reportedly effective not only in treating obesity[15] but also for T2DM[16-18], although the specific mechanisms underlying the latter finding are not completely understood. Two different theories have been posited, namely the foregut and hindgut hypothesis[19]. Research has also been conducted to understand how improvements in T2DM occur even before weight loss following these surgeries[20]. The effects of GI surgery on metabolic processes reinforce the importance of the GI tract in T2DM development.
In morbidly obese patients, bariatric surgery results in up to a 70% loss of excess weight[21]. In the present study, the mean postoperative BMI was < 25 kg/m2, a result that we considered to be attributed to a 100% loss of excess weight. In addition, we observed significant improvements in other metabolic parameters, such as triglyceride and total cholesterol levels. Different bariatric surgery procedures promote amelioration of dyslipidemia, potentially because of the ingestion of less food and the improved insulin resistance[22].
There were significant improvements in fasting and postprandial glycemia after surgery. Pancreatic beta cell behavior depends on serum glucose levels, and phasic or chronic hyperglycemia may lead to desensitization, exhaustion, and apoptosis of the beta cells (glucotoxicity)[23,24]. Loss of beta cell mass is a cornerstone feature for the development of chronic hyperglycemia[25]. Hyperabsorption in the epithelium in diabetes patients promotes postprandial hyperglycemia, an event that is potentially toxic to beta cell mass. Studies in different experimental models have demonstrated improvements in beta cell function after metabolic surgery[26], and this effect might be a result not only from improved incretin hormone function but also from less glucotoxicity in the beta cells, which are frequently exposed to postprandial hyperglycemia caused by the consumption of high glycemic foods. In the present group of T2DM patients, lower levels of postprandial glycemia might have been promoted by partial or complete deviation of hyperplastic and hyper absorption in the proximal segments of the intestine; however, further research is required to clarify this hypothesis.
GIP and GLP-1 are the two primary incretin hormones secreted from the intestine, and T2DM patients generally have lower levels of GLP-1[27]. The postoperative improvement in T2DM in the present study, at least partially, might be a result of enhanced GLP-1. Schirra et al[28] have demonstrated that the load of ingested carbohydrates must overcome the absorptive capacity of the proximal intestine to enhance the GLP-1 secretion by mucosal L cells, which are primarily located in the distal small intestine. The intestinal hyperabsorption in T2DM patients might prevent food from reaching these distal segments of the intestine. When direct contact of the duodenal and proximal jejunal epithelium with food is avoided through an endoluminal plastic prostheses (EndoBarrier® GI Dynamics), improvements are observed in insulin resistance, fasting glycemic levels, and postprandial glycemic levels[29,30]; these outcomes are similar to those obtained from bariatric surgery. These results, in addition to increased GLP-1 secretion, are also observed in glucose intolerant patients through the inhibition of disaccharidase alpha by orally administered acarbose, which partially prevents the absorption of carbohydrates in the proximal intestine[31]. Similarly, studies have shown that metabolic surgery improves GLP-1 secretion, mainly by stimulating the distal intestine with undigested food[32,33], and SGTB promotes stimulation of the ileum by ingested food through a gastroileal anastomosis. Isolated sleeve gastrectomy without transit bipartition also elevates postprandial GLP-1 levels and improves glucose metabolism, although it does not alter the intestinal flow. After sleeve gastrectomy, gastric emptying is accelerated[34], and this acceleration might prevent complete absorption of food in the proximal intestine. The proximal intestine absorption capacity and flow intensity (amount of substrate/time) are important tasks for distal intestine stimulations and GLP-1 secretion[28].
In contrast, the GIP levels tended to decrease in the postoperative period in the present study. In T2DM patients, there is a resistance to GIP action[35], and surgery might influence its function. Højberg et al[36], while studying rats, demonstrated that resolution of hyperglycemia restores GIP function by stimulating the expression of GIP receptors in pancreatic tissue. However, there is considerable variation in the reported results regarding GIP secretion after bariatric and metabolic surgery; thus, the relationship remains unclear[37].
Comparing two groups of patients with the same weight loss (9.5 kg) achieved through bariatric surgery and a hypocaloric diet, Laferrère[38] shows better metabolic results (incretin secretion, postprandial glucose levels) in operated patients instead of having the same amount of fat tissue loss. This result reinforces the importance of metabolic surgery or the rearrangement of the gastrointestinal tract on metabolic profile amelioration.
According to Osborne et al[11], diabetes promotes morphological changes in the intestinal mucosa, and hyperglycemia correction reverses the hyperplasia. The mechanism underlying hyperplasia in T2DM appears to differ from that of other models of intestinal hyperplasia. In rats subjected to Roux-en-Y gastric bypass, the hyperplasia was only observed on the roux limb and not on the biliopancreatic limb, suggesting that overstimulation of the distal segments of the bowel by undigested food, previously poorly stimulated, might be the primary mechanism for the hyperplasia[39]. In T2DM models, the hyperplasia occurs as an early step in the evolution of diabetes in non-surgically modified intestines, suggesting different mechanisms of hyperplasia induction[7]. Furthermore, Noda et al[8] predicted that overfeeding is a necessary condition to intestinal hypertrophy, a serious concern in modern society[40], particularly with the increased consumption of high glycemic index foods, which parallels the increase in T2DM incidence[41]. Food rich in carbohydrates is the primary stimulant for the growth of intestinal cells[42]. The inability of the anti-proliferative genes in the intestinal mucosa to counteract the stimulation for growth promoted by food ingestion could be one factor in the pathophysiology of intestinal hypertrophy in diabetes. p53 and TGF-β are important down-regulators of epithelial growth[3-5] and are influenced by diabetes[10]. Alvarado-Vásquez et al[43] have reported lower levels of p53 in the endothelial cells of the umbilical cords of mothers with a strong family predisposition for T2DM. Interestingly, a cohort study of 55000 subjects demonstrated an association between the development of hyperglycemia and p53 polymorphisms, leading us to suggest a possible link between the expression levels of this gene and the progression of T2DM[44].
Few studies have analyzed the gene expression profile of intestinal epithelial cells in the presence of metabolic disease. In the present study, we analyzed the mucosal gene expression of p53 and TGF-β in non-excluded segments of the intestine and at the same intestinal site, pre- and postoperatively. Both genes were down-regulated before surgery (before improvements in T2DM). Interestingly, p53 expression was enhanced postoperatively, and this enhancement was more pronounced in the duodenum than in the ileum. At the same time, the expression of TGF-β was not altered in the duodenum but was significantly enhanced in the ileum. The small sample size may have resulted in these expression differences based on the intestinal site. However, lower levels of the anti-proliferative genes in T2DM (as in the preoperative state) could be explained by the direct effect of diabetes on p53 and TGF-β[1]. In normal and tumor cells, p53 regulates the energy source, and it favors phosphorylative oxidation instead of glycolytic pathways. One method by which p53 favors phosphorylative oxidation is the down-regulation of the expression of glucose transporter (GLUT)-1 receptors[45], which are responsible for basolateral glucose transport. p53 also influences the expression of GLUT 3 and GLUT 4 receptors[46]. A recent report has indicated that bariatric surgery significantly enhances mucosal expression of GLUT-1 receptors[47], thereby enhancing glucose consumption by enterocytes and acting as a primary factor for glucose homeostasis after surgery.
This study has certain limitations. One important limitation is the small sample size, as well as the lack of morphological experiments of the epithelium before and after surgery. Despite these limitations, we believe that our results will encourage future research regarding the relationship between the digestive tract and T2DM, particularly relating to higher proximal intestine absorptive capacity.
In summary, our data demonstrated that SGTB improves T2DM in a group of class I obese patients, and the metabolic improvements were accompanied by increased expression of the anti-proliferative intestinal genes. We propose a new theory regarding the resolution of T2DM after metabolic surgery, focusing on the hyper absorption capacity of the proximal intestine.
We thank Natalia Lima, PhD, and Paulo Svidnick, MSc, for important contributions developing the laboratory experiments.
Bariatric surgery, by not completely understood means, promotes type 2 diabetes mellitus (T2DM) resolution. Hyperplasia of intestinal epithelium in diabetic subjects can enhance the absorptive capacity and might be a cornerstone in the evolution of diabetes. The authors highlight the differential expression of antiproliferative genes of intestinal mucosa in T2DM patients as a marker for metabolic disease development.
Gene expression is influenced by hyperglycemia. In patients predisposed to developing T2DM, underexpression of antiproliferative genes on intestinal mucosa might promote morphofunctional intestinal alterations that influence disease development. The correlation between enhanced absorptive capacity of proximal intestine and bariatric surgery capacity in promoting T2DM resolution should be an important research task.
In this study, the authors analysis expression of antiproliferative genes on intestinal mucosa, particularly p53 and TGF-β, on the hyperglycemic state and after resolving metabolic disease with metabolic surgery. The authors highlight and correlate gene expression with intestinal morphofunctional behavior as a main pathophysiology target in T2DM research.
The study suggests that altered gene expression on intestinal mucosa might be a predictor of T2DM development. The authors discuss the role of the absorptive capacity of the proximal intestine in T2DM as a target for disease treatment, and they also propose an alternative explanation for the ability of bariatric surgery to resolve T2DM.
Sleeve gastrectomy with transit bipartition, a metabolic surgery procedure that involves a gastroileal anastomosis in the antrum after sleeve gastrectomy; nutrient transit is maintained in the duodenum, thereby avoiding blind loops and minimizing malabsorption. The stomach retains 2 outflow pathways. A lateral enteroanastomosis connects both segments at 80 cm proximal to the cecum.
This study examines the metabolic markers before and after bariatric surgery in T2DM patients and also focuses on differential intestinal mucosal gene expression as a predictor of metabolic improvement. This study is useful, although the cohort size is too small to draw definitive conclusions.
P- Reviewer: Vossenkamper A, Zhang DF S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Ye C, Li X, Wang Y, Zhang Y, Cai M, Zhu B, Mu P, Xia X, Zhao Y, Weng J. Diabetes causes multiple genetic alterations and downregulates expression of DNA repair genes in the prostate. Lab Invest. 2011;91:1363-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Hong S, Lee HJ, Kim SJ, Hahm KB. Connection between inflammation and carcinogenesis in gastrointestinal tract: focus on TGF-beta signaling. World J Gastroenterol. 2010;16:2080-2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Li L, Li J, Rao JN, Li M, Bass BL, Wang JY. Inhibition of polyamine synthesis induces p53 gene expression but not apoptosis. Am J Physiol. 1999;276:C946-C954. [PubMed] |
| 4. | Li L, Rao JN, Guo X, Liu L, Santora R, Bass BL, Wang JY. Polyamine depletion stabilizes p53 resulting in inhibition of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol. 2001;281:C941-C953. [PubMed] |
| 5. | Liu L, Santora R, Rao JN, Guo X, Zou T, Zhang HM, Turner DJ, Wang JY. Activation of TGF-beta-Smad signaling pathway following polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1056-G1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Schedl HP, Wilson HD. Effects of diabetes on intestinal growth in the rat. J Exp Zool. 1971;176:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Fujita Y, Kojima H, Hidaka H, Fujimiya M, Kashiwagi A, Kikkawa R. Increased intestinal glucose absorption and postprandial hyperglycaemia at the early step of glucose intolerance in Otsuka Long-Evans Tokushima Fatty rats. Diabetologia. 1998;41:1459-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Noda T, Iwakiri R, Fujimoto K, Yoshida T, Utsumi H, Sakata H, Hisatomi A, Aw TY. Suppression of apoptosis is responsible for increased thickness of intestinal mucosa in streptozotocin-induced diabetic rats. Metabolism. 2001;50:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Adachi T, Mori C, Sakurai K, Shihara N, Tsuda K, Yasuda K. Morphological changes and increased sucrase and isomaltase activity in small intestines of insulin-deficient and type 2 diabetic rats. Endocr J. 2003;50:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988;319:1500-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 289] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Osborne DL, Payne SC, Russ RD, Tobin B. Comparison of therapeutic regimens in the amelioration of alterations in rat gastrointestinal mucosal DNA, RNA and protein induced by streptozotocin diabetes mellitus. Life Sci. 2000;66:2405-2417. [PubMed] |
| 12. | Verdam FJ, Greve JW, Roosta S, van Eijk H, Bouvy N, Buurman WA, Rensen SS. Small intestinal alterations in severely obese hyperglycemic subjects. J Clin Endocrinol Metab. 2011;96:E379-E383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Santoro S, Castro LC, Velhote MC, Malzoni CE, Klajner S, Castro LP, Lacombe A, Santo MA. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg. 2012;256:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35 Suppl 1:S64-S71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1005] [Cited by in RCA: 1114] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 15. | Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416-423; discussion 423-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 859] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 16. | Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 1590] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 17. | Sasaki A, Wakabayashi G, Yonei Y. Current status of bariatric surgery in Japan and effectiveness in obesity and diabetes. J Gastroenterol. 2014;49:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Pok EH, Lee WJ. Gastrointestinal metabolic surgery for the treatment of type 2 diabetes mellitus. World J Gastroenterol. 2014;20:14315-14328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Liu S, Zhang G, Wang L, Sun D, Chen W, Yan Z, Sun Y, Hu S. The entire small intestine mediates the changes in glucose homeostasis after intestinal surgery in Goto-Kakizaki rats. Ann Surg. 2012;256:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1816] [Cited by in RCA: 1733] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 22. | Kaul A, Sharma J. Impact of bariatric surgery on comorbidities. Surg Clin North Am. 2011;91:1295-312, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 284] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 447] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 25. | Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 626] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 26. | Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143:897-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 626] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 28. | Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, Göke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 292] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | de Moura EG, Orso IR, Martins BC, Lopes GS, de Oliveira SL, Galvão-Neto Mdos P, Mancini MC, Santo MA, Sakai P, Ramos AC. Improvement of insulin resistance and reduction of cardiovascular risk among obese patients with type 2 diabetes with the duodenojejunal bypass liner. Obes Surg. 2011;21:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Escalona A, Pimentel F, Sharp A, Becerra P, Slako M, Turiel D, Muñoz R, Bambs C, Guzmán S, Ibáñez L. Weight loss and metabolic improvement in morbidly obese subjects implanted for 1 year with an endoscopic duodenal-jejunal bypass liner. Ann Surg. 2012;255:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 31. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072-2077. [PubMed] |
| 32. | Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond). 2009;33 Suppl 1:S33-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | DePaula AL, Macedo AL, Schraibman V, Mota BR, Vencio S. Hormonal evaluation following laparoscopic treatment of type 2 diabetes mellitus patients with BMI 20-34. Surg Endosc. 2009;23:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Braghetto I, Davanzo C, Korn O, Csendes A, Valladares H, Herrera E, Gonzalez P, Papapietro K. Scintigraphic evaluation of gastric emptying in obese patients submitted to sleeve gastrectomy compared to normal subjects. Obes Surg. 2009;11:1515-1521. [RCA] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Theodorakis MJ, Carlson O, Muller DC, Egan JM. Elevated plasma glucose-dependent insulinotropic polypeptide associates with hyperinsulinemia in impaired glucose tolerance. Diabetes Care. 2004;27:1692-1698. [PubMed] |
| 36. | Højberg PV, Vilsbøll T, Rabøl R, Knop FK, Bache M, Krarup T, Holst JJ, Madsbad S. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 328] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 37. | Rao RS, Kini S. GIP and bariatric surgery. Obes Surg. 2011;21:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479-2485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 501] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 39. | Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25:e70-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 40. | Gupta D, Krueger CB, Lastra G. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr Diabetes Rev. 2012;8:76-83. [PubMed] |
| 41. | Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774-779. [PubMed] |
| 42. | Weser E, Babbitt J, Vandeventer A. Relationship between enteral glucose load and adaptive mucosal growth in the small bowel. Dig Dis Sci. 1985;30:675-681. [PubMed] |
| 43. | Alvarado-Vásquez N, Zapata E, Alcázar-Leyva S, Massó F, Montaño LF. Reduced NO synthesis and eNOS mRNA expression in endothelial cells from newborns with a strong family history of type 2 diabetes. Diabetes Metab Res Rev. 2007;23:559-566. [PubMed] |
| 44. | Burgdorf KS, Grarup N, Justesen JM, Harder MN, Witte DR, Jørgensen T, Sandbæk A, Lauritzen T, Madsbad S, Hansen T. Studies of the association of Arg72Pro of tumor suppressor protein p53 with type 2 diabetes in a combined analysis of 55,521 Europeans. PLoS One. 2011;6:e15813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627-2633. [PubMed] |
| 46. | Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 759] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 47. | Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |