Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6965
Peer-review started: October 28, 2014
First decision: December 26, 2014
Revised: January 19, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: June 14, 2015
Processing time: 233 Days and 13.7 Hours
AIM: To compare symptom control with esomeprazole regimens for non-erosive reflux disease and chronic gastritis in patients with a negative endoscopy.
METHODS: This randomized, open-label study was designed in line with clinical practice in China. Patients with typical reflux symptoms for ≥ 3 mo and a negative endoscopy who had a Gastroesophageal Reflux Disease Questionnaire score ≥ 8 were randomized to initial treatment with esomeprazole 20 mg once daily either for 8 wk or for 2 wk. Patients with symptom relief could enter another 24 wk of maintenance/on-demand treatment, where further courses of esomeprazole 20 mg once daily were given if symptoms recurred. The primary endpoint was the symptom control rate at week 24 of the maintenance/on-demand treatment period. Secondary endpoints were symptom relief rate, success rate (defined as patients who had symptom relief after initial treatment and after 24 wk of maintenance treatment), time-to-first-relapse and satisfaction rate.
RESULTS: Based on the data collected in the modified intention-to-treat population (MITT; patients in the ITT population with symptom relief after initial esomeprazole treatment, n = 262), the symptom control rate showed a small but statistically significant difference in favor of the 8-wk regimen (94.9% vs 87.3%, P = 0.0473). Among the secondary endpoints, based on the data collected in the ITT population (n = 305), the 8-wk group presented marginally better results in symptom relief after initial esomeprazole treatment (88.3% vs 83.4%, P = 0.2513) and success rate over the whole study (83.8% vs 72.8%, P = 0.0258). The 8-wk regimen was found to provide a 46% reduction in risk of relapse vs the 2-wk regimen (HR = 0.543; 95%CI: 0.388-0.761). In addition, fewer unscheduled visits and higher patient satisfaction supported the therapeutic benefits of the 8-wk regimen over the 2-wk regimen. Safety was comparable between the two groups, with both regimens being well tolerated.
CONCLUSION: Chinese patients diagnosed with chronic gastritis achieved marginally better control of reflux symptoms with an 8-wk vs a 2-wk esomeprazole regimen, with a similar safety profile.
Core tip: In China, physicians tend to perform endoscopies on patients presenting with typical symptoms of gastro-esophageal reflux disease, such as reflux or heartburn. If endoscopy findings are negative, patients are usually diagnosed with chronic gastritis rather than non-erosive reflux disease (NERD) and treated with a 2-wk course of proton pump inhibitors (PPIs). We compared symptom control rates when patients were treated with a 2-wk course of PPIs vs an 8-wk course, as recommended for patients with NERD. In this multicenter, randomized, open-label study, the 8-wk PPI regimen provided marginally better symptom control and relief rates than the 2-wk regimen, with a similar safety profile.
- Citation: Sun J, Yuan YZ, Hou XH, Zou DW, Lu B, Chen MH, Liu F, Wu KC, Zou XP, Li YQ, Zhou LY. Esomeprazole regimens for reflux symptoms in Chinese patients with chronic gastritis. World J Gastroenterol 2015; 21(22): 6965-6973
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6965.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6965
Gastro-esophageal reflux disease (GERD) can be routinely diagnosed on the basis of typical reflux symptoms[1]. Approximately 60% of patients with GERD have non-erosive reflux disease (NERD)[2,3], which is characterized by typical reflux symptoms and normal esophageal findings.
Recent studies have shown that the prevalence of GERD in Asia is increasing[4,5]. In China, the majority of patients with reflux symptoms are investigated by endoscopy and, if no clear endoscopic findings are identified, they are often diagnosed as having chronic gastritis (CG) rather than NERD because this diagnosis is thought to be more acceptable to the patient. Patients are then treated according to the recommended treatment algorithm for CG in China, which involves a 2-wk course of proton pump inhibitors (PPIs), rather than the treatment algorithm for NERD, which involves a 8-wk course, as proposed by the 2007 Chinese GERD consensus statement[6].
The aim of this study was to compare symptom control rates between the NERD regimen (8 wk of PPI treatment) and the CG regimen (2 wk) in patients who have typical reflux symptoms and a negative endoscopy.
This multicenter, randomized, open-label phase IV study (clinicaltrials.gov study code: D9612L00127) was conducted in 10 centers in China. The study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with International Conference on Harmonization/Good Clinical Practice guidelines. All participants gave written informed consent before any study procedures were performed.
Adult patients (aged 18-75 years) with a Gastroesophageal Reflux Disease Questionnaire (GerdQ) score ≥ 8 were enrolled if they had typical reflux symptoms (i.e., heartburn, regurgitation or both) as their main gastrointestinal symptoms for ≥ 3 mo. Patients were required to have undergone endoscopy in the 2 wk before randomization, without any endoscopic findings. The main exclusion criteria were as follows: involvement in the planning and/or conduct of the study; previous enrollment or randomization in the present study; participation in another clinical study with an investigational product during the last 3 mo; and endoscopically visible reflux esophagitis, esophageal varices, Barrett’s esophagus, malignancy or peptic ulcer. Patients were ineligible if they had: unintentional weight loss > 3 kg in the previous 3 mo; hematemesis, melena or per-rectum blood loss in the previous year; progressive dysphagia, anemia or any other symptom suggestive of malignancy; PPI or histamine-2 receptor antagonist (H2RA) therapy in the 2 wk before enrollment; known intolerance/allergy to PPIs; or history of esophageal, gastric or duodenal surgery.
Treatment of Helicobacter pylori (H. pylori) infection was not allowed during the study. If H. pylori infection was detected, treatment could start after the final visit, as assessed by the investigator.
Patients who met the inclusion criteria were randomized into either the 8-wk treatment group or the 2-wk treatment group. Treatment of the 8-wk group was divided into two phases on the basis of the 2007 Chinese consensus on GERD[6]: initial treatment phase and maintenance/on-demand phase. Treatment of the 2-wk group was designed to reflect treatment practice in China.
For the initial phase, patients were randomized to treatment with esomeprazole 20 mg once daily for 8 wk or 2 wk. Patients with symptom relief (defined as no more than 1 day with mild symptoms of GERD during the past 7 d) at the end of the initial 2- or 8-wk treatment phase entered a 24-wk maintenance/on-demand phase. The total study period was therefore 26 wk for the CG regimen and 32 wk for the NERD regimen.
During the maintenance/on-demand phase, patients were given esomeprazole if their symptoms relapsed (i.e., they had a recurrence of their symptoms that was sufficient to require treatment in the opinion of the investigator). In the event of symptom relapse in the 8-wk group, patients were instructed to take esomeprazole 20 mg once daily for at least 3 d consecutively until symptom control was reached. If relapse occurred before week 8 of the on-demand/maintenance period, patients received 14 tablets of esomeprazole at each of the scheduled week 8 and week 16 visits. If this was not sufficient, patients were instructed to visit the study center to receive extra tablets. Such visits were not considered unscheduled if their purpose was limited to obtaining on-demand drugs. If the first relapse occurred after week 8, the patient was withdrawn from the study and treated according to standard clinical practice. In the 2-wk group, patients were treated with another 2-wk course of esomeprazole 20 mg once daily if their symptoms recurred. In this group, there was no limitation on the number of repeated treatments that could be given during the 24-wk maintenance period. As with the 8-wk group, hospital visits were not considered unscheduled if their purpose was to obtain on-demand drugs.
In the 2-wk and 8-wk treatment periods, assessments occurred at screening (0 wk), and then at 2 and 8 wk, respectively. There were three scheduled visits (at 8, 16 and 24 wk) during the 24-wk maintenance/on-demand period. GerdQ[7] was administered when the patients entered the study and at the week-8, week-16 and week-24 visits to assess symptom control status in the 7 d before each visit.
The primary endpoint was defined as the symptom control rate at week 24 of the maintenance/on-demand period. Symptom control was defined as a score ≤ 1 for GerdQ items relating to frequency of heartburn, regurgitation, nocturnal reflux and rescue medication use.
Secondary endpoints included symptom relief rate, success rate, time-to-first-relapse and satisfaction. Symptom relief was defined as no more than 1 d with mild symptoms of GERD during the past 7 d, which was reported retrospectively by the patient. Success was defined as symptom relief after initial treatment and symptom control at 24 wk of maintenance treatment. Symptom relief rate and success rate were used as efficacy variables for initial and overall treatment, respectively. Time-to-first-relapse was defined as the period from the date of the last dose of initial treatment to the date when for the first time the patient visited the investigator owing to symptom relapse. Unscheduled visits were indicated by the recurrence of a patient’s symptoms, need for extra treatment or medical consultation. Satisfaction rate was self-reported using a 7-point scale, which showed the patients’ subjective attitudes towards both regimens.
Safety assessments included monitoring of serious adverse events (AEs) and discontinuation due to AEs of any severity.
The required sample size was calculated based on clinical experience, as there were insufficient data available from past studies for the symptom control rates in both treatment regimens to be estimated. With a total of 170 evaluable patients, 85 in the 8-wk group and 85 in the 2-wk group, the power would be greater than 80% to detect a difference of 20% in symptom control rates between two treatment groups at a 2-sided significance level of 0.05 using Fisher’s exact test, assuming that symptom control rates were about 66% in the 2-wk group and 86% in the 8-wk group (in reference to data from the as-yet unpublished BU-NEG-0005 study, which used the same definition of symptom control as the present study). Considering PPI response rates of around 70% and drop-out rates of around 20%, approximately 300 patients needed to be randomized.
Analysis on efficacy endpoints was performed for the intention-to-treat (ITT) population (all randomized patients who received at least one dose of study medication), the modified intention-to-treat (MITT) population (patients in the ITT population with symptom relief after initial esomeprazole treatment) and the per-protocol population (patients in the ITT population without significant protocol deviations/violations), with the MITT as the primary analysis population. The safety population was defined as all patients who took at least one dose of trial medication and for whom post-dose data were collected.
All treatment comparisons were two-sided and the nominal level of significance was 5%. The Fisher’s exact test was used to analyze the primary efficacy endpoint of symptom control rate at 24 wk in the MITT population and the secondary efficacy endpoints of symptom relief rate at initial treatment and success rate in the whole regimen, both of which were analyzed using the ITT population. The Kaplan-Meier survival analysis method[8] and the log-rank test were used to assess time-to-first-relapse. A weighted least-squares regression analysis was also performed to compare mean number of unscheduled visits between the two regimens. Comparison of patients who were satisfied (scores of 1-4) or very satisfied (scores of 1-2) in the groups at 24 wk was performed using Fisher’s exact test.
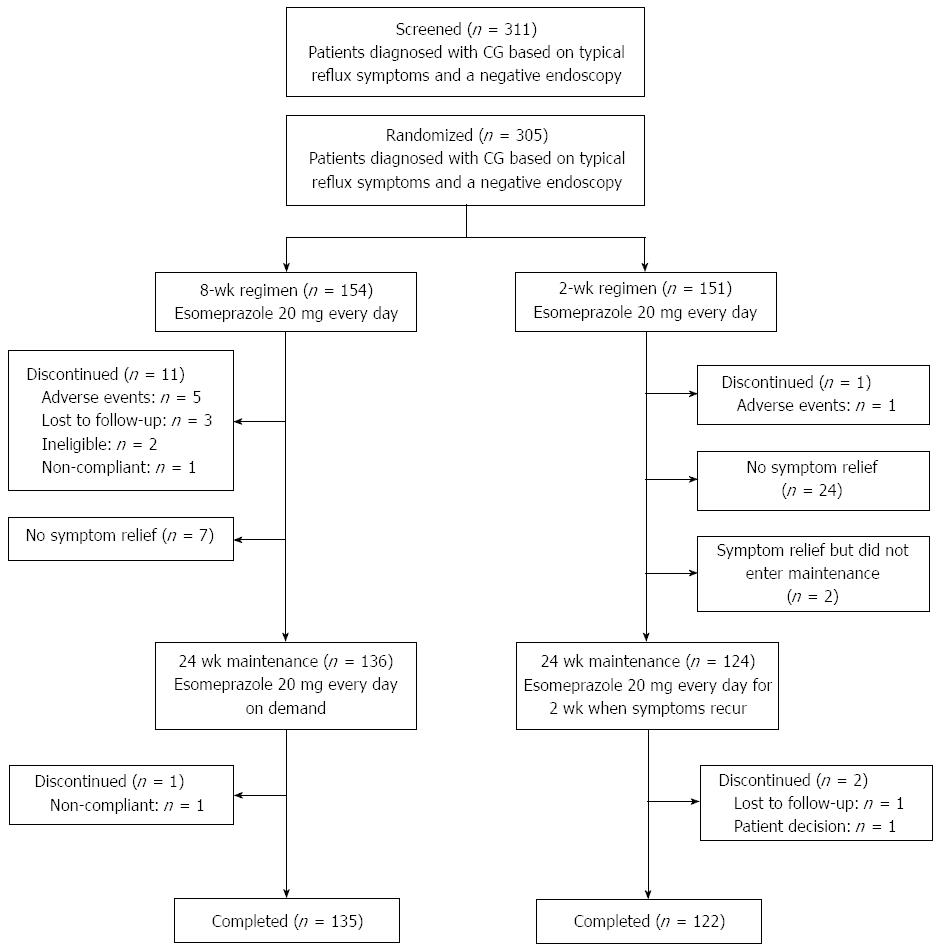
In total, 154 patients were randomized into the 8-wk group and 151 into the 2-wk group (Figure 1). The patients were enrolled from April 2010 to June 2011. Six patients discontinued treatment owing to AEs (five in the 8-wk group and one in the 2-wk group; Figure 1). The completion rates for the initial 8- and 2-wk treatment phases were 92.9% (143/154) and 99.3% (150/151), and the completion rates for the whole study were 87.7% (135/154) and 80.8% (122/151), respectively.
Overall, both groups were generally comparable between ITT and MITT populations with respect to baseline GERD characteristics. Patient demographics were well balanced between both regimens except for sex (Table 1).
| Parameter | ITT (n = 305) | MITT (n = 262) | ||||
| 8-wk group | 2-wk group | Total | 8-wk group | 2-wk group | Total | |
| Age, yr, mean ± SD | 45.9 ± 12.72 | 44.9 ± 13.12 | 45.4 ± 12.91 | 45.3 ± 12.88 | 45.1 ± 13.36 | 45.2 ± 13.09 |
| Sex | ||||||
| Men | 80 (51.9) | 54 (35.8) | 134 (43.9) | 74 (54.4) | 46 (36.5) | 120 (45.8) |
| Women | 74 (48.1) | 97 (64.2) | 171 (56.1) | 62 (45.6) | 80 (63.5) | 142 (54.2) |
| Symptom | ||||||
| Heartburn | 142 (92.2) | 136 (90.1) | 278 (91.1) | 126 (92.6) | 113 (89.7) | 239 (91.2) |
| Regurgitation | 138 (89.6) | 135 (89.4) | 273 (89.5) | 124 (91.2) | 113 (89.7) | 237 (90.5) |
| Duration, mo, mean ± SD | ||||||
| Heartburn | 36.3 ± 56.47 | 35.8 ± 49.05 | 36.1 ± 52.87 | 39.0 ± 59.20 | 39.1 ± 52.55 | 39.0 ± 56.04 |
| Regurgitation | 36.4 ± 56.72 | 34.9 ± 47.41 | 35.6 ± 52.24 | 38.4 ± 59.28 | 37.7 ± 50.71 | 38.1 ± 55.24 |
| HP test positive | 48 (31.2) | 44 (29.1) | 92 (30.2) | 39 (28.7) | 35 (27.8) | 74 (28.2) |
| GerdQ score, mean ± SD | 10.7 ± 1.78 | 10.4 ± 1.89 | 10.5 ± 1.84 | 10.7 ± 1.76 | 10.4 ± 1.94 | 10.6 ± 1.85 |
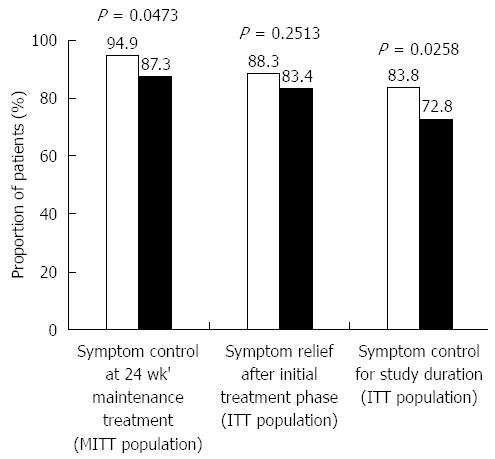
The proportions of patients with symptom control after 24 wk of maintenance/on-demand treatment (MITT population) in the 8-wk and 2-wk groups were 94.9% (129/136) and 87.3% (110/126), respectively, with a significant difference favoring the 8-wk regimen (P = 0.0473; Figure 2). The proportions of patients with symptom relief after the initial phase of treatment (ITT population) in the 8-wk and 2-wk groups were 88.3% (136/154) and 83.4% (126/151), respectively; this result numerically favored the 8-wk regimen with no significant difference (P = 0.2513; Figure 2). With regard to success rates in the whole study (ITT population), 83.8% (129/154) and 72.8% (110/151) of patients in the 8-wk and 2-wk groups, respectively, had successful symptom control, with a significant difference favoring the 8-wk regimen (P = 0.0258; Figure 2).
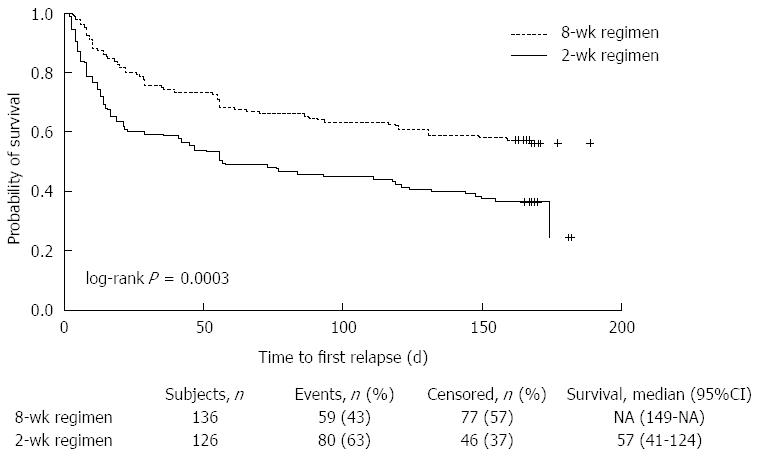
A total of 136 patients in the 8-wk group and 126 patients in the 2-wk group (MITT population) were included in the survival analysis. The median time-to-first-relapse for patients in the 2-wk group was 57 d, and the median for patients in the 8-wk group had not been reached at the end of the study. There were 59 patients with relapse in the 8-wk group (43.4%) and 80 (63.5%) in the 2-wk group. Significantly more patients in the 8-wk regimen stayed relapse-free in the maintenance/on-demand phase than in the 2-wk regimen (68.4% vs 50.6%, P = 0.003 at week 8; 63.2% vs 44.1%, P = 0.0016 at week 16; 56.3% vs 36.6%, P = 0.0012 at week 24). A log-rank test showed that symptom relapse occurred significantly later in the 8-wk group than in the 2-wk group (P = 0.0003; Figure 3). To quantify the reduction in risk of relapse with the 8-wk regimen, a post hoc Cox regression analysis with the treatment as the only covariate was performed and showed a 46% reduction (HR = 0.543; 95%CI: 0.388-0.761).
The proportions of patients who were satisfied or very satisfied with their symptom control after 24 wk of maintenance were significantly higher in the 8-wk group than in the 2-wk group (100% vs 96%, P = 0.0247; 48.5% vs 24.6%, P < 0.0001, respectively).
Both regimens were well tolerated. No serious AEs or deaths were reported in this study (Table 2). A total of five patients from the 8-wk group discontinued owing to AEs that were of mild or moderate intensity, while one patient in the 2-wk group discontinued owing to two AEs of moderate intensity.
| 2-wk group (n = 151) | 8 wk group (n = 154) | Total (n = 305) | ||||
| Mild | Moderate | Mild | Moderate | Mild | Moderate | |
| Patients with at least one AE | 0 | 1 (0.7) | 3 (1.9) | 2 (1.3) | 3 (1.0) | 3 (1.0) |
| Gastrointestinal disorders | 0 | 0 | 3 (1.9) | 2 (1.3) | 3 (1.0) | 2 (0.7) |
| Nausea | 0 | 0 | 1 (0.6)1 | 2 (1.3)2 | 1 (0.3) | 2 (0.7) |
| Abdominal discomfort | 0 | 0 | 1 (0.6)1 | 1 (0.6)1 | 1 (0.3) | 1 (0.3) |
| Constipation | 0 | 0 | 0 | 1 (0.6)1 | 0 | 1 (0.3) |
| Frequent bowel movements | 0 | 0 | 1 (0.6)1 | 0 | 1 (0.3) | 0 |
| Abdominal pain, upper | 0 | 0 | 0 | 1 (0.6)1 | 0 | 1 (0.3) |
| Eye disorders | 0 | 0 | 1 (0.6) | 0 | 1 (0.3) | 0 |
| Vision blurred | 0 | 0 | 1 (0.6) | 0 | 1 (0.3) | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 1 (0.7) | 0 | 0 | 0 | 1 (0.3) |
| Back pain | 0 | 1 (0.7) | 0 | 0 | 0 | 1 (0.3) |
| Renal and urinary disorders | 0 | 1 (0.7) | 0 | 0 | 0 | 1 (0.3) |
| Dysuria | 0 | 1 (0.7) | 0 | 0 | 0 | 1 (0.3) |
| Respiratory, thoracic and mediastinal disorders | 0 | 0 | 0 | 1 (0.6) | 0 | 1 (0.3) |
| Cough | 0 | 0 | 0 | 1 (0.6) | 0 | 1 (0.3) |
This study compared the efficacy of an 8-wk regimen of esomeprazole 20 mg once daily vs a 2-wk regimen in patients with typical reflux symptoms and a negative endoscopy. Our results showed that high rates of symptom control were seen in both groups, but that the 8-wk regimen was significantly superior to the 2-wk regimen. Several previous studies have suggested that patients with NERD are less responsive to PPIs than patients with reflux esophagitis[9-11]; however, the high rate of symptom control found in both arms of our study suggests that patients with NERD can be responsive to highly effective PPIs such as esomeprazole.
The study design for the 8-wk regimen in our study was similar to that in the esomeprazole group of the COMMAND study[12], which compared on-demand treatment with esomeprazole 20 mg with continuous therapy with lansoprazole 15 mg in patients with NERD in the United Kingdom. In the COMMAND study, after 4 wk of initial treatment and 6 mo of on-demand treatment, more than 85% of patients reported to have no or only mild symptoms in the previous 7 d, while the results from the 8-wk arm in our study indicated that 94.9% of patients achieved symptom control after 24 wk. This suggests the superiority of at least 8 wk of initial treatment with esomeprazole over either 2 or 4 wk of initial treatment.
With regard to symptom relief rate in the present study, a numerically higher symptom relief rate of 88.3% was observed for the 8-wk regimen vs 83.4% in the 2-wk regimen. This finding is in line with the hypothesis raised in a randomized, double-blind study of esomeprazole compared with rabeprazole, which found that by extending the duration of initial therapy beyond 4 wk more patients would have symptom relief[13]. In the COMMAND study[12], after the initial 2 wk of treatment, 47% of patients had complete resolution of GERD symptoms for 7 consecutive days. This increased to 77% of patients after treatment for 4 wk[12]. Symptom relief rate in our study was higher numerically; however, our definition of symptom relief did allow 1 d with mild symptoms of GERD during the past 7 d, so a higher rate should be expected. Success rate was also analyzed across the whole duration of our study with a statistically significant difference of 10.9% favoring the 8-wk regimen over the 2-wk regimen (P = 0.0258).
In clinical practice, therapeutic options are mainly driven by relapse frequency[14], and one possible limitation of on-demand therapy is that it allows symptoms to recur[15]. Therefore, relapse has become a crucial predictor of the efficacy of initial treatment for most patients with NERD. In our study, we observed relapse rates of 63.5% and 43.4% in the 2-wk and 8-wk groups, respectively, showing that fewer relapses occurred in the 8-wk group. Time-to-first-relapse was also measured as another quantitative endpoint to analyze the effectiveness of initial treatments. The longer the time-to-first-relapse, as demonstrated in the 8-wk group, the better the efficacy of the regimen was. Moreover, the median time-to-first-relapse for patients in the 8-wk group had not been reached at the time of analysis, supporting the superiority of that treatment regimen.
Patient satisfaction evaluation in NERD studies has been used as an important outcome measure[16]. Ideally, this should be evaluated using a validated questionnaire[17]. In our study, a questionnaire on patient satisfaction was measured on a 7-point scale, and a higher level of patient satisfaction was reported with the 8-wk regimen than the 2-wk. However, it should be noted that the satisfaction rates were very high in both regimens, with 100% in the 8-wk group and 96.0% in the 2-wk group, so this finding should not be over-interpreted. In the COMMAND study[12], a high satisfaction rate (93.2%) suggested that a transient recurrence of heartburn symptoms (predominantly mild in severity) was only a minor inconvenience to patients. It may also be attributed to the nature of on-demand treatment, which enabled patients to feel more in control of their treatment and tailor it to their own particular needs[12]. On-demand treatment may be more acceptable to patients than the requirement to take medication every day on a long-term basis[10].
In placebo-controlled trials, esomeprazole 20 mg on demand has been shown to be effective and well tolerated in the maintenance of symptom control in patients with NERD[18,19]. In the present study, more patients discontinued from the 8-wk than the 2-wk treatment arm, which may have been the result of the different drug exposures in the treatment period and the difference in assigning maintenance/on-demand treatment in both groups. However, it should be noted that few patients discontinued from either arm. Overall, no safety concerns were associated with the use of esomeprazole 20 mg once daily for up to 8 wk followed by a 24-wk esomeprazole 20 mg once daily maintenance/on-demand treatment.
A key strength of this study is that it is the first randomized, comparative, clinical trial of patients with NERD ever conducted in China, and that it was designed on the basis of the 2-phase treatment algorithm proposed by the 2007 Chinese consensus statement on GERD[6]. The main limitation of our study lies in the open-label design, which may lead to bias[20]. We tried to minimize the possibility of bias by rigorous quality-control management. In terms of the randomization, unlike the COMMAND study[12], where randomization was set after the initial phase, we performed the randomization before the initial phase. We believe the potential bias arising from this randomization is limited as the MITT patients showed a well-balanced demographic and clinical characteristic profile that was similar to that of ITT patients. However, the proportion of women was higher in the 2-wk group than in the 8-wk group. The implications of this are unclear. A further limitation is that satisfaction rates were measured with a patient-reported questionnaire that used a 1-wk recall period and are therefore subject to greater self-reporting bias than if a daily diary had been used[21].
In conclusion, in Chinese patients who have typical reflux symptoms and a negative endoscopy, 8 wk of treatment with esomeprazole provides marginally better symptom control and symptom relief rates than a 2-wk regimen, with a similar safety profile.
We thank Jiaxing Tigermed Data Co., who provided medical writing support, which was funded by AstraZeneca. Catherine Hill from PharmaGenesis™ London and Richard Claes from Oxford PharmaGenesis™ Ltd provided medical writing and editorial support, which was funded by AstraZeneca.
Recent studies have shown that the prevalence of gastro-esophageal reflux disease (GERD) in Asia is increasing. In China, physicians tend to perform endoscopies on patients presenting with typical symptoms of GERD, such as reflux or heartburn. If endoscopy findings are negative, patients are usually diagnosed with chronic gastritis rather than non-erosive reflux disease (NERD) because this diagnosis is thought to be more acceptable to both the doctor and the patient. These patients are then given the recommended treatment regimen for chronic gastritis [a 2-wk course of proton pump inhibitors (PPIs)] rather than the regimen for NERD (an 8-wk course of PPIs)
Selection of the most appropriate PPI treatment regimen for GERD symptoms is of importance to patients, physicians and payers. This was the first randomized, comparative, clinical trial of patients with NERD ever conducted in China. The authors compared symptom control when patients were treated with a 2-wk regimen of esomeprazole vs an 8-wk regimen.
High rates of symptom control were seen in both groups, but the 8-wk regimen of esomeprazole was significantly superior to the 2-wk regimen. The 8-wk regimen was also found to provide a reduction in the risk of relapse vs the 2-wk regimen. In addition, fewer unscheduled visits and higher levels of patient satisfaction supported the therapeutic benefits of the 8-wk regimen over the 2-wk regimen. Safety was similar between the two groups, with both regimens being well tolerated.
An initial 8-wk regimen of esomeprazole treatment was found to provide slightly superior control and relief of GERD symptoms compared with an initial 2-wk regimen. The findings of this study may help physicians to optimize future treatment of GERD.
GERD symptoms such as regurgitation and heartburn are caused by reflux of acid from the stomach into the esophagus. Proton pump inhibitors such as esomeprazole reduce the amount of acid produced in the stomach and are the mainstay of pharmacological treatment for GERD.
This very well designed and performed multicenter, randomized, open-label study considers the comparison of symptom control between the recommended PPI treatment regimens for non-erosive reflux disease (8 wk) and chronic gastritis (2 wks) in 305 Chinese patients who have typical reflux symptoms and negative endoscopy. This study confirms that the 8-wk PPI regimen provided marginally better symptom control and relief rates than the 2-wk regimen, with a similar safety profile. This study is making a great contribution to randomized clinical trial studies leading to optimization of GERD treatment.
P- Reviewer: Chmiela M, Vorobjova T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. [The Montreal definition and classification of gastroesophageal reflux disease: a global, evidence-based consensus paper]. Z Gastroenterol. 2007;45:1125-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2448] [Article Influence: 128.8] [Reference Citation Analysis (2)] |
| 2. | Hershcovici T, Fass R. Nonerosive Reflux Disease (NERD) - An Update. J Neurogastroenterol Motil. 2010;16:8-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Labenz J, Jaspersen D, Kulig M, Leodolter A, Lind T, Meyer-Sabellek W, Stolte M, Vieth M, Willich S, Malfertheiner P. Risk factors for erosive esophagitis: a multivariate analysis based on the ProGERD study initiative. Am J Gastroenterol. 2004;99:1652-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Goh KL. Changing epidemiology of gastroesophageal reflux disease in the Asian-Pacific region: an overview. J Gastroenterol Hepatol. 2004;19 Suppl 3:S22-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Wong BC, Kinoshita Y. Systematic review on epidemiology of gastroesophageal reflux disease in Asia. Clin Gastroenterol Hepatol. 2006;4:398-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Chinese Medical Association, GI Branch. Treatment consensus for gastroesophageal reflux disease. Zhonghua Xiaohua Zazhi. 2007;27:689-690. |
| 7. | Jones R, Junghard O, Dent J, Vakil N, Halling K, Wernersson B, Lind T. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 8. | Klein JP, Moesschberger ML. Survival analysis - Techniques for censored and truncated data. New York: Springer 2003; 234-238. [DOI] [Full Text] |
| 9. | Carlsson R, Dent J, Watts R, Riley S, Sheikh R, Hatlebakk J, Haug K, de Groot G, van Oudvorst A, Dalväg A. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. International GORD Study Group. Eur J Gastroenterol Hepatol. 1998;10:119-124. [PubMed] |
| 10. | Galmiche JP, Barthelemy P, Hamelin B. Treating the symptoms of gastro-oesophageal reflux disease: a double-blind comparison of omeprazole and cisapride. Aliment Pharmacol Ther. 1997;11:765-773. [PubMed] |
| 11. | Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro-oesophageal reflux disease in general practice. Scand J Gastroenterol. 1997;32:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 185] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Tsai HH, Chapman R, Shepherd A, McKeith D, Anderson M, Vearer D, Duggan S, Rosen JP. Esomeprazole 20 mg on-demand is more acceptable to patients than continuous lansoprazole 15 mg in the long-term maintenance of endoscopy-negative gastro-oesophageal reflux patients: the COMMAND Study. Aliment Pharmacol Ther. 2004;20:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Fock KM, Teo EK, Ang TL, Chua TS, Ng TM, Tan YL. Rabeprazole vs esomeprazole in non-erosive gastro-esophageal reflux disease: a randomized, double-blind study in urban Asia. World J Gastroenterol. 2005;11:3091-3098. [PubMed] |
| 14. | Tytgat GN. Review article: management of mild and severe gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;17 Suppl 2:52-56. [PubMed] |
| 15. | Talley NJ, Vakil N, Lauritsen K, van Zanten SV, Flook N, Bolling-Sternevald E, Persson T, Björck E, Lind T. Randomized-controlled trial of esomeprazole in functional dyspepsia patients with epigastric pain or burning: does a 1-week trial of acid suppression predict symptom response? Aliment Pharmacol Ther. 2007;26:673-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Modlin IM, Hunt RH, Malfertheiner P, Moayyedi P, Quigley EM, Tytgat GN, Tack J, Heading RC, Holtman G, Moss SF. Diagnosis and management of non-erosive reflux disease--the Vevey NERD Consensus Group. Digestion. 2009;80:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Coyne KS, Wiklund I, Schmier J, Halling K, Degl’ Innocenti A, Revicki D. Development and validation of a disease-specific treatment satisfaction questionnaire for gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;18:907-915. [PubMed] |
| 18. | Talley NJ, Lauritsen K, Tunturi-Hihnala H, Lind T, Moum B, Bang C, Schulz T, Omland TM, Delle M, Junghard O. Esomeprazole 20 mg maintains symptom control in endoscopy-negative gastro-oesophageal reflux disease: a controlled trial of ‘on-demand’ therapy for 6 months. Aliment Pharmacol Ther. 2001;15:347-354. [PubMed] |
| 19. | Talley NJ, Venables TL, Green JR, Armstrong D, O’Kane KP, Giaffer M, Bardhan KD, Carlsson RG, Chen S, Hasselgren GS. Esomeprazole 40 mg and 20 mg is efficacious in the long-term management of patients with endoscopy-negative gastro-oesophageal reflux disease: a placebo-controlled trial of on-demand therapy for 6 months. Eur J Gastroenterol Hepatol. 2002;14:857-863. [PubMed] |
| 20. | Megan B, Pickering RM, Weatherall M. Design, objectives, execution and reporting of published open-label extension studies. J Eval Clin Pract. 2012;18:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Massof RW. A general theoretical framework for interpreting patient-reported outcomes estimated from ordinally scaled item responses. Stat Methods Med Res. 2014;23:409-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |