Published online Jun 14, 2015. doi: 10.3748/wjg.v21.i22.6872
Peer-review started: December 21, 2014
First decision: January 8, 2015
Revised: January 28, 2015
Accepted: February 12, 2015
Article in press: February 13, 2015
Published online: June 14, 2015
Processing time: 181 Days and 3.6 Hours
AIM: To investigate the mechanism of endoplasmic reticulum (ER) stress induction by an occult infection related hepatitis B virus S surface antigen (HBsAg) variant.
METHODS: We used an HBsAg variant with lower secretion capacity, which was a KD variant from a Korean subject who was occultly infected with the genotype C. We compared the expression profiles of ER stress-related proteins between HuH-7 cells transfected with HBsAg plasmids of a wild-type and a KD variant using Western blot.
RESULTS: Confocal microscopy indicated that the KD variant had higher levels of co-localization with ER than the wild-type HBsAg. The KD variant up-regulated ER stress-related proteins and induced reactive oxygen species (ROS) compared to the wild-type via an increase in calcium. The KD variant also down-regulated anti-oxidant proteins (HO-1, catalase and SOD) compared to the wild-type, which indicates positive amplification loops of the ER-ROS axis. The KD variant also induced apoptotic cell death via the up-regulation of caspase proteins (caspase 6, 9 and 12). Furthermore, the KD variant induced a higher level of nitric oxide than wild-type HBsAg via the up-regulation of the iNOS protein.
CONCLUSION: Our data indicate that occult infection related HBsAg variants can lead to ER-derived oxidative stress and liver cell death in HuH-7 cells.
Core tip: The molecular mechanisms underlying the relationships between occult hepatitis B virus infection and liver disease progression remain a mystery. The present study demonstrated that the HBsAg variant KD, which exhibits a secretion defective phenotype, universally induced endoplasmic reticulum (ER) stress pathways in hepatocytes. This induction of ER stress may evoke ER stress-mediated biological actions that induce liver damaging processes, including ROS production, nitric oxide production, and apoptosis induction. In conclusion, occult infection related to hepatitis B virus S surface antigen variants may play a very pivotal role in the progression of liver diseases primarily via ER-derived oxidative stress and apoptosis in hepatocytes.
- Citation: Lee IK, Lee SA, Kim H, Won YS, Kim BJ. Induction of endoplasmic reticulum-derived oxidative stress by an occult infection related S surface antigen variant. World J Gastroenterol 2015; 21(22): 6872-6883
- URL: https://www.wjgnet.com/1007-9327/full/v21/i22/6872.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i22.6872
Hepatitis B virus (HBV) infection is a global health problem, and more than 350 million people are chronic carriers of the virus[1]. South Korea is an endemic area of HBV infection, and the Korean National Health and Nutrition Survey of 2007 listed the prevalence of hepatitis B virus S surface antigen (HBsAg) as 4.2% in men and 3.1% in women[2]. Moreover, an extraordinary prevalence of genotype C2 was reported in this area[3]. This genotype is more virulent than genotype B[4], and it may contribute to the distribution of characteristic HBV mutation patterns that are related to the progression of liver diseases[5-15].
HBV surface open reading frames (ORFs) encode 3 types of proteins that share C terminals, large (L), middle (M), and small (S) surface antigen. The HBsAg is expressed at high levels, and it can be secreted independently of L and M envelope proteins. The L and S envelope proteins are needed for virion secretion, but the M protein is dispensable[16]. Overexpression of the L protein blocks HBsAg secretion[17]. Proper stoichiometry between L and S envelope proteins is important for the secretion of HBsAg and virions.
Occult HBV infection is defined as an infection state that is negative for HBsAg serology, but the presence of HBsAg DNA is demonstrated using polymerase chain reaction (PCR). Generally, HBV infection is diagnosed using serological detection of the circulating HBsAg[18,19]. Occult HBV infection is highly prevalent, particularly in HBV endemic areas, and it is significantly related to severe forms of liver diseases, such as cirrhosis or hepatocellular carcinoma (HCC)[20]. However, the exact molecular mechanisms underlying the relationship between occult infection and severe liver diseases are not known.
The endoplasmic reticulum (ER) performs multiple important functions that are essential to cell survival and normal cellular function, including Ca2+ storage, post-translational modification, and the folding and assembly of newly synthesized secretory proteins. Various disturbances can cause an accumulation of unfolded/misfolded proteins in the ER, which triggers an evolutionarily conserved response termed the unfolded/misfolded protein response (UPR)[21,22]. Viral infection may also trigger the UPR because of an overloading of the ER, and viral infection is one of the ancient evolutionary pressures that links ER stress to cell suicide to avoid viral replication and spreading[23,24]. Several mutations in S ORFs lead to ER stress in hepatocytes through the accumulation of HBV virions because of failure of the appropriate production of 3 proteins in the S ORF, which may contribute to hepatocarcinogenesis and liver damage. However, most studies of HBV-induced ER stress focused on a specific deletion type of large surface proteins (LHBs)[25,26]. HBsAg has the most potent secretory capacity of the HBV encoding proteins. Therefore, it is reasonable that unfolded proteins as a result of an LHB mutation may increase the ER stress response. Nevertheless, to the best of our knowledge, ER stress mechanisms that focus on HBsAg variants of occult infection (occult HBsAgs) are rarely introduced[27].
Recently, we reported that a variety of novel HBsAg variants that are absent or rarely encountered in other areas were observed in Korean subjects with occult genotype C infections[13]. Notably, we found that some occult HBsAgs exhibited deficiencies in HBsAg secretion[15]. We hypothesized that these HBsAgs could induce strong ER stress in hepatocytes via UPR activation. Therefore, the current study elucidated the molecular mechanisms to provide a positive link between occult infection and liver disease progression. The current study focused on ER stress-linked pathways that are induced by occult HBsAg. The present study used a HBsAg KD variant from Korean subjects with occult infections of HBV genotype C, which was previously reported to exhibit very low levels of HBsAg secretion but a higher level of accumulated intracellular HBsAg compared to the wild-type HBsAg[15].
The serum for the HBsAg variant (KD) related to occult infection and the control HBsAg (wild-type or NOR) were acquired from a 54-year-old Korean occult subject showing HBsAg seronegativity and a Korean chronic patient, respectively[15]. The institutional review board of the Seoul National University Hospital approved this study (1202-051-398). Salubrinal (Santa cruz Biotech, CA, United States), thapsigargin (Sigma-Aldrich, MO, United States), and N-acetyl-cysteine (NAC; Sigma-Aldrich, MO, United States) were used as an ER stress inhibitor, an ER stress inducer, and an inhibitor of ROS, respectively.
Previously reported[15] plasmids encoding two types of HBsAg (NOR and KD variant) were used in this study. Briefly, the nested PCR method was used for HBsAg DNA amplification. First-round PCR was performed using the PreS2-Del-F2 and HB2R primers (PreS2-Del-F2: 5’-GGG TCA CCA TAT TCT TGG G-3’; HB2R: 5’-CAT ACT TTC CAA TCA ATA GG-3’), which target the large surface region of HBsAg. Second-round amplification was performed using the Cystein-S-F1 and Cystein-S-R1 primers (Cystein-S-F1: 5’-ATG GAG ARC ACM ACA TCA GGA TTC C-3’; Cystein-S-R1: 5’-TYA AAT GTA TAC CCA AAG ACA MAA G-3’), which amplify the small surface region. The amplified 681-bp products of the NOR and KD variant were cloned into the Topo TA cloning vector (Invitrogen, Massachusetts, United States) according to the manufacturer’s protocol. The inserted target region in the TA vector was digested using EcoRI (Takara, Shiga, Japan), and the target regions were finally re-cloned into the pIRES2-EGFP vector (Clontech, CA, United States).
The secretion capacity of the occult infection-related KD variant and NOR were compared using ELISA for HBsAg in the supernatant and lysed pellet using the commercial Bioelisa HBsAg Colour ELISA Kit (BIOKIT, Barcelona, Spain) according to the manufacturer’s protocol. Additionally, the pCMV-β-gal vector containing β-galactosidase was co-transfected and analyzed according to the recommendation of the β-Galactosidase Enzyme Assay System kit (Promega, WI, United States) to normalize the HBsAg ELISA in cloned HBsAg.
The human hepatoma cell line HuH-7 was used for in vitro cell culture studies. Cells were maintained at 37 °C in an atmosphere of 5% CO2 in RPMI-1640 (GibcoBRL, NY, United States) supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, and 100 U/mL penicillin/streptomycin (Gibco BRL). Cells were plated at 4 × 105 cells in six-well plates one day before transfection. Lipofectamine 2000 (Invitrogen, CA, United States) was used to transfect the three plasmid DNAs [void pIRES2 vector (MOCK), NOR, and KD] using opti-MEM (Gibco BRL) for starvation and incubation.
Proteins were quantified using a Qubit fluorometer (Invitrogen), and Western blotting was performed. Isolated proteins (50 μg) were loaded and separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Lysate proteins were transferred to nitrocellulose membranes. Each membrane was incubated for 1 h at room temperature in phosphate-buffered saline (PBS) containing 5% skim milk powder (blocking solution) prior to incubation with a primary antibody (1:1000 dilution, 1 h) and a secondary antibody (1:2000 dilution, 30 min). A commercial kit was used to obtain the nuclear fraction (Thermo, IL, United States). Protein analyses involved the following antibodies: activating transcription factor 6 (ATF6), phosphorylated pancreatic ER eIF2α kinase (pPERK), phosphorylated insulin-response element 1, X-box binding protein 1 (XBP1), phosphorylated eukaryotic initiation factor-α, heme oxygenase 1 (HO-1), CHOP, glucose-regulated protein, 78-kDa (GRP78), JNK, phosphorylated JNK, Bcl-2, Bax, caspase 12, caspase 9, caspase 6 (all from Cell Signaling, MA, United States), inducible nitric oxide synthase (iNOS; BD, NJ, United States), catalase (RD Systems, MN, United States), Cu/ZnSOD (BioDesign, NY, United States), and manganese superoxide dismutase (MnSOD) (Stressgen, Victoria, BC, Canada). Immunoreactive signals were detected using a WEST-oneTM Western Blot Detection System (iNtRON, Kyungkido, Republic of Korea) and LAS-3000 (Fujifilm, Tokyo, Japan).
Rhod-2 AM (Invitrogen, Carlsbad, United States) was used as an indicator of intracellular calcium concentration. Cells (5 × 104) were seeded in six-well plates, incubated overnight, and transiently transfected. Cells were incubated for 24 h, and Rhod-2 AM was applied at a final concentration of 4 μmol/L in PBS containing 0.1 g/L CaCl2 for 30 min. Intracellular calcium levels were analyzed using fluorescence-activated cell sorting (FACS) in a FACSCan II apparatus (BD, NJ, United States).
Dichlorodihydrofluoresein diacetate (DCF-DA; Molecular Probes, OR, United States) and dihydrogenrhodamine123 (DHR123; Calbiochem, CA, United States) were used to detect intracellular ROS levels. Cells (4 × 105) were seeded in six-well plates, and incubated for 24 h. Cells were transiently transfected with occult HBsAg DNA using Lipofectamine 2000 (Invitrogen, Carlsbad, United States) and incubated at 37 °C in 5% CO2 overnight. The day after transfection, cells were treated with ROS lysis buffer. Supernatants (100 μL) were transferred to 96-well plates and treated with DCF-DA or DHR123 at a final concentration of 10 μmol/L for 30 min. Supernatants were analyzed using an LS 55 luminescence spectrometer (Perkin-Elmer, MA, United States) and a FACSCalibur apparatus (BD, NJ, United States).
Samples were prepared after transient transfection and analyzed in triplicate to determine nitric oxide (NO) levels (Assay Designs, MI, United States). The quantitative determination of NO production was determined using the Griess reaction. NO levels were assessed spectrophotometrically at 540 nm.
Cells (5 × 104) were plated in 96-well plates for 24 h. Cells were transfected with plasmid DNA (MOCK, NOR, or KD) using opti-MEM medium for 24 h. Supernatants were recovered, and DNA fragmentation was quantified using the Cell Death Detection ELISAPLUS kit (Roche, Mannheim, Germany). For each assay, 20 μL of supernatant was added to each well containing 80 μL of immunoreagent. Mixtures were gently shaken for 2 h, and each well was rinsed three times with incubation buffer. The buffer after the final rinse was carefully removed, and the substrate solution was pipetted into each well. Each plate was incubated until the color development was sufficient for analysis. Color development was terminated by the addition of a stop solution to each well, and the absorbance was measured at an optical density of 405 nm.
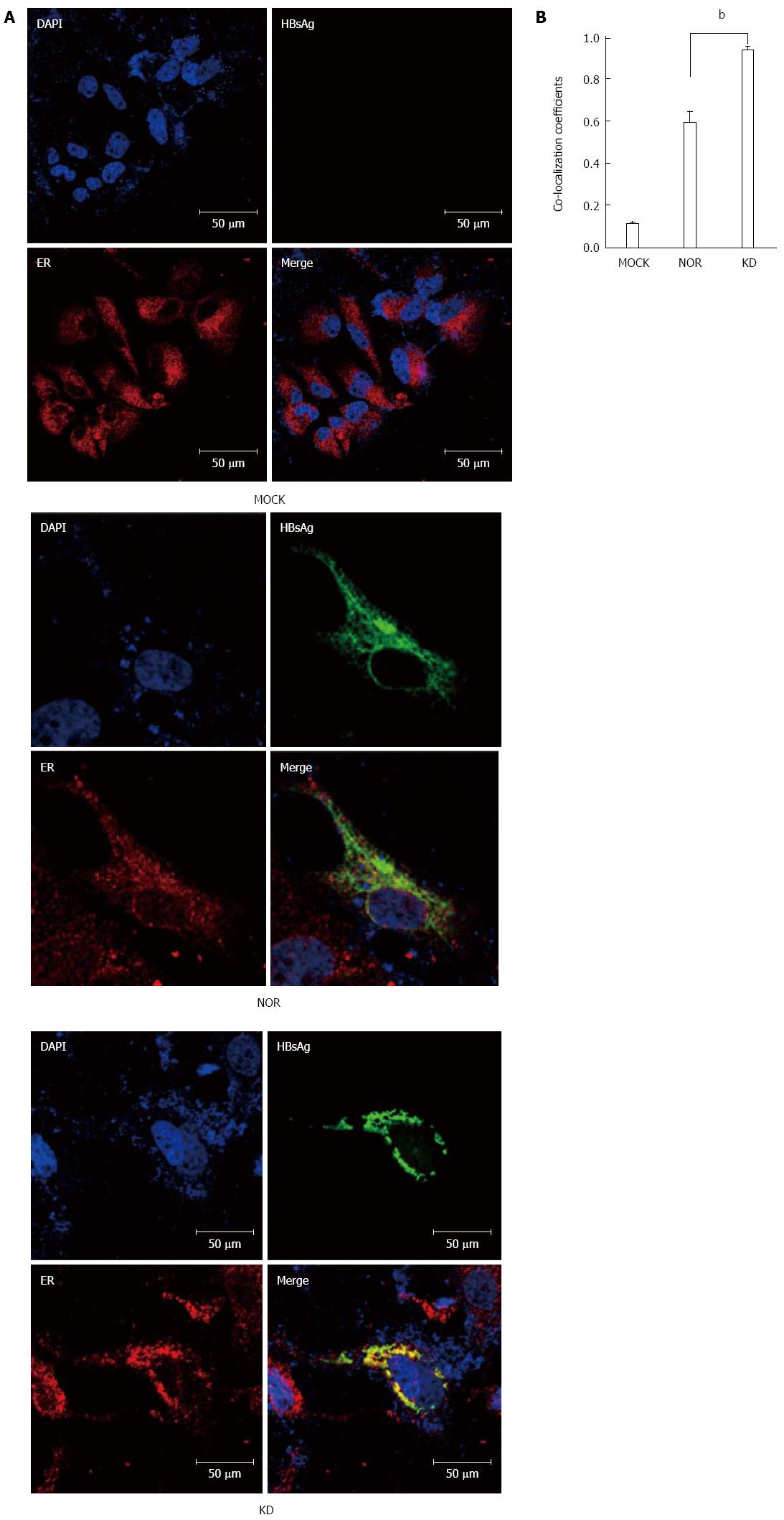
Immunofluorescence double-staining assays were performed using confocal microscopy to co-localize HBsAg in ER in KD variants and NOR in the HuH-7 cell line. HuH-7 cell lines were transiently transfected with one empty vector (pIRES2-EGFP-MOCK) or two different small surface protein expression vectors (pIRES2-EGFP-NOR and KD). Cells were harvested 2 d post-transfection, fixed with 4% paraformaldehyde, permeabilized using 10% fetal bovine serum-0.1% Triton X-100 in PBS for 1 h at room temperature, and stained for HBsAg (green) and the ER marker calnexin (red). DAPI stained the nucleus a blue color. Cells were incubated with a polyclonal Ab against HBsAg (Abcam, Cambridge, MA, United States) and a monoclonal Ab against the ER marker calnexin (Santa Cruz, CA, United States) to analyze the colocalization of HBsAg and the ER. Goat anti-rabbit-Alexa Fluor 488 (Invitrogen, Carlsbad, United States) and goat anti-mouse-Alexa Fluor 594 (Invitrogen, Carlsbad, United States) were used as secondary antibodies for the experiments. Cell nuclei were counterstained with 10 μg/mL of DAPI (Sigma-Aldrich, MO, United States). The results were visualized under a Confocal A1 (Nikon, Japan) confocal microscope. Comparisons of colocalization between HBsAg and ER in the cytoplasm were estimated using colocalization coefficients according to the lasso ROI selection. Statistical comparisons were performed using one-way ANOVA, and the average coefficients of ten images examined in a double-blind manner are shown.
This retrospective study was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB Grant No. C-0803-013-237), and the patients’ medical records were anonymized and de-identified prior to analyses.
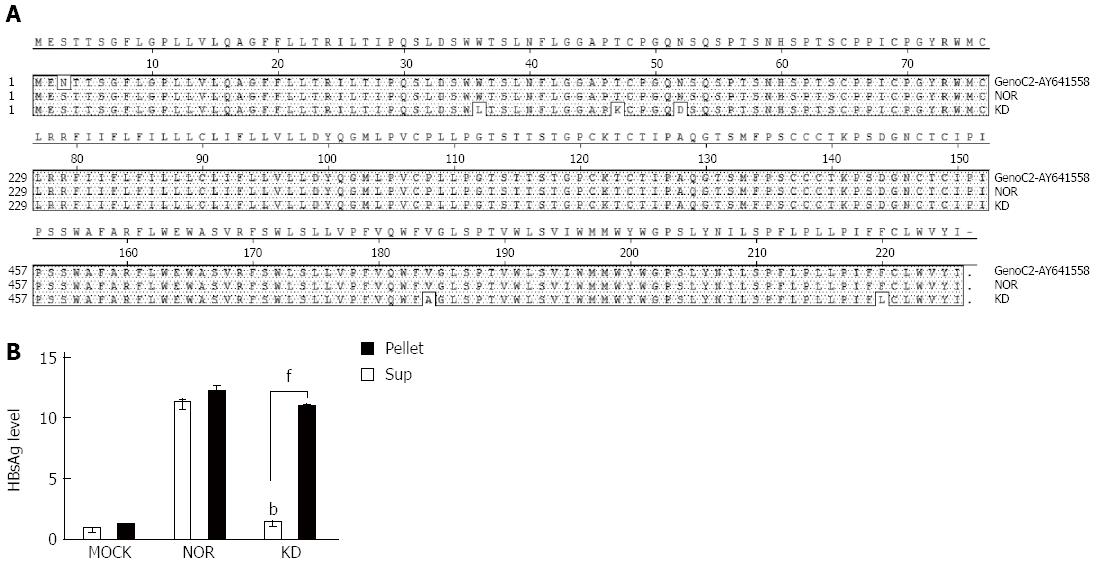
Sequencing analyses of cloned plasmids revealed no mutations in wild-type HBsAg from a chronic carrier (NOR). However, a total of five mutations (W36L, T47K, N52D, V184A, and F220L) were mainly concentrated in the N-terminal region in the KD variant, but no mutations were found in the major hydrophilic region (MHR), as previously reported[15] (Figure 1A). Comparisons of the 2 cloned HBsAg plasmids (wild-type (NOR) and KD variant) in the pellets and supernatants of HuH-7 cells using ELISA revealed that the KD variant had deficiencies in secretion capacity similar to the negative control compared to the wild-type (Figure 1B). These results suggest a higher level of ER accumulation of the KD variant compared to the wild-type. Colocalization coefficients of the ER and HBsAg between wild-type and KD variant were compared 2 d after transfection of HBsAg plasmids into HuH-7 cells to address this issue. HBsAg of the KD variant showed significantly higher colocalization coefficients with the ER marker calnexin compared to the wild-type (Figure 2A and B).
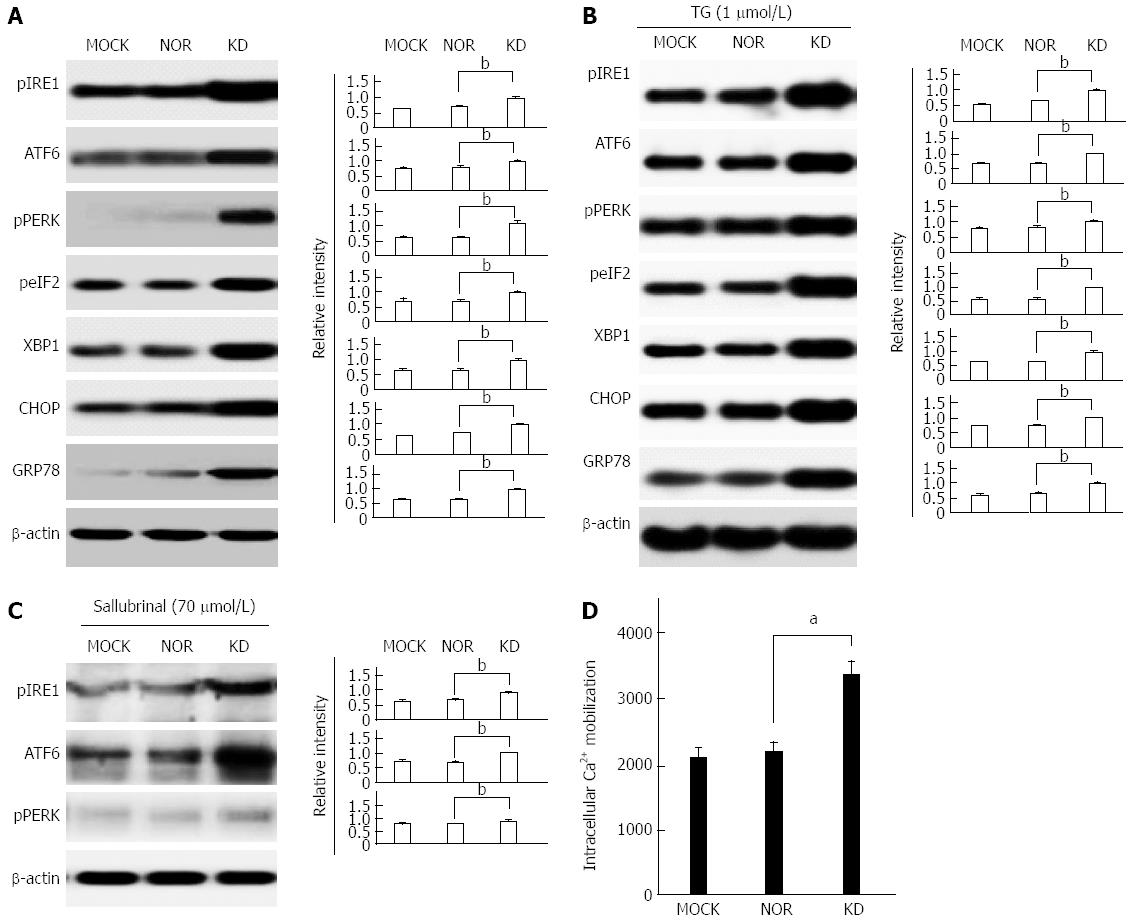
We compared the expression of ER stress-related proteins between the KD variant and wild-type to address issue of whether the compromising of normal HBsAg secretion function in the KD variant leads to ER stress response. The KD variant up-regulated the expression of the seven main ER stress-related proteins, namely, IRE1, ATF6, PERK, elF2, XBP1, CHOP, and GRP 78, in the absence (Figure 3A) or presence (Figure 3B) of the ER stress inducer TG compared to MOCK and NOR. These results indicate that the reduced secretion of HBsAg spontaneously induced ER stress in the absence of ER stress inducers. The ER stress inhibitor salubrinal was used after the transient transfection of HuH-7 cell lines to further investigate the effects of the KD variant on ER stress. The data show that ER stress transducers, which induce effects of IRE1, ATF6, and PERK, were greatly reduced in salubrinal-treated cells compared to cells without salubrinal treatment (Figure 3C). The up-regulation of ER stress pathways induces the release of Ca2+ from the ER lumen to the cytoplasm[26]. We monitored Ca2+ mobilization using the fluorescent membrane permeable fluorochrome Rhod2-AM to investigate this issue. The FACS data show that the KD variant induced an increase in intracellular Ca2+ concentrations, which contrasts MOCK and NOR cells (Figure 3D).
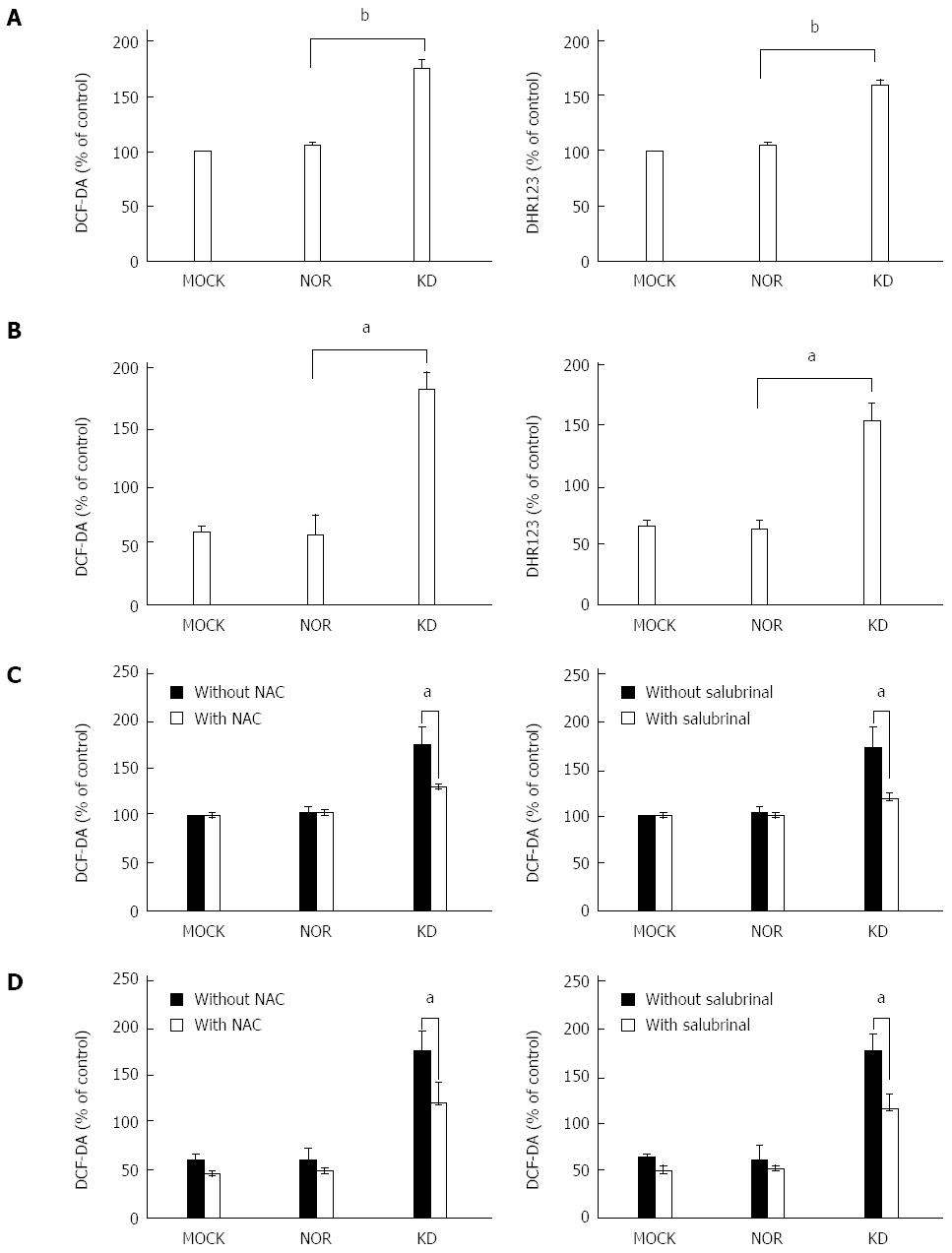
Released Ca2+ may induces ROS generation via mitochondrial membrane perturbation[27]. Therefore, an ROS assay was performed in transiently transfected HuH-7 cells with or without salubrinal treatment to investigate whether the KD variant induces ROS generation through ER stress pathways. Cells were loaded with DHR123 and DCF-DA to assess the levels of mitochondrial and intracellular ROS, respectively. Two different methods for ROS measurement, ELISA (Figure 4A) and FACS analysis (Figure 4B), were used in this study. The data show that the KD variant induced a higher level of ROS generation in mitochondria and cytoplasm compared to MOCK and NOR cells (Figure 4A and B). Salubrinal treatment reduced these inducing effects (Figure 4C and D), which suggests that the KD variant induces ROS generation in an ER stress-dependent manner[28]. The HBsAg variant may also play a key role in increased ROS generation within hepatocytes. Figure 4C and D shows that the ROS inhibitor N-acetyl-cysteine (NAC) reduced ROS induction by the KD variant to a level similar to MOCK and NOR, which verifies the validity of the above experiment.
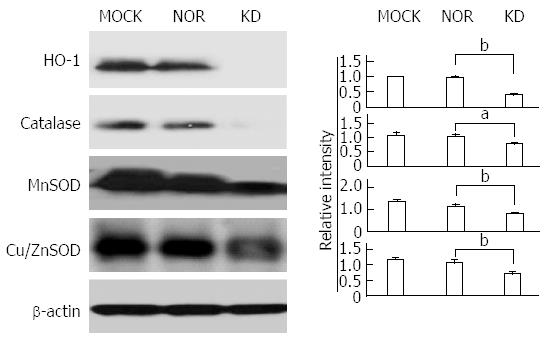
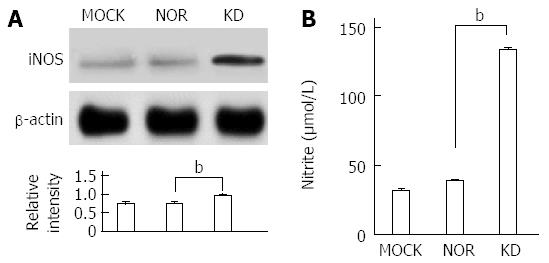
The balance between oxidants and anti-oxidants is a major concern in hepatocyte damage[29]. The expression of antioxidant-related enzymes after plasmid transfection was measured to analyze the effect of the KD variant on the expression of antioxidant proteins (Figure 5). Generally, the expression levels of anti-oxidant ROS scavenger proteins, such as SOD (MnSOD and CuZn SOD), HO-1, and catalase, were significantly down-regulated by the KD variant, which leads to a positive amplification loop of the ER stress-ROS axis. Experiments using iNOS protein expression and a commercially available nitrite assay were performed to investigate the effects of the KD variant on NO levels. The KD variant significantly up-regulated iNOS (Figure 6A) and increased nitrite production compared to MOCK and NOR (Figure 6B); these findings suggest that the KD variant may play a pivotal role in NO production in hepatocytes via up-regulation of the iNOS protein.
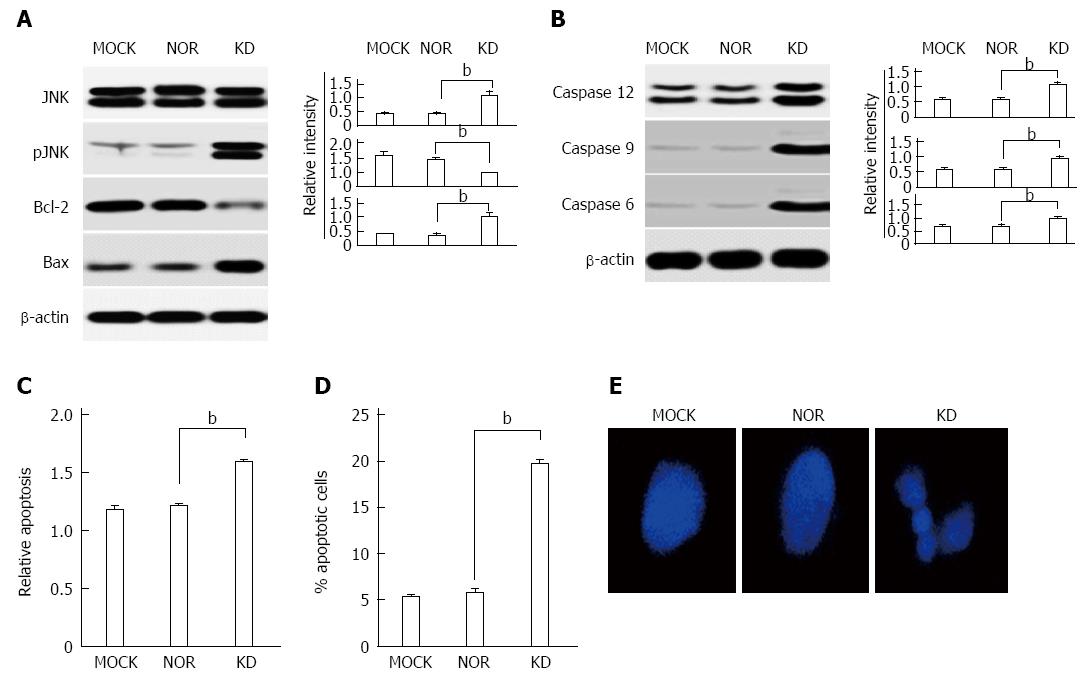
Apoptosis induction in hepatocytes by stress signals, such as ER stress, is closely related to the progression of liver diseases[30]. We investigated the effects of the KD variant on the expression of apoptosis-related genes to examine whether the KD variant induces apoptosis in hepatocytes. First, the data showed that phosphorylation of the JNK protein, which plays a key role in apoptosis induction because of ER stress, was up-regulated in the KD variant, but not MOCK or NOR. The up-regulation of the pro-apoptotic protein, Bax, and the down-regulation of the anti-apoptotic protein, Bcl-2, which followed JNK activation, were also observed in the KD variant (Figure 7A). Caspases 3, 9, and 12, which directly mediate apoptotic cell death, were also up-regulated in the KD variant but not MOCK or NOR (Figure 7B). Additionally, we conducted cell death ELISAs to investigate the effect of the KD variant on apoptosis by in vitro determination of cytoplasmic histone-associated DNA fragments. Highly elevated apoptosis levels were found in the KD variant compared to MOCK and NOR (Figure 7C). Additionally, PI staining using FACS was performed for apoptosis detection, and increased levels were observed in the KD variant (Figure 7D). Finally, DNA fragmentation associated with apoptosis was assessed for morphological identification. We found enhanced DNA fragmentation as a hallmark of apoptosis in the KD variant (Figure 7E). These results collectively indicate that occult infection related HBsAg variant (KD) induces apoptosis in hepatocytes via the ER stress-JNK activation axis.
HBsAg is the strongest secretory HBV protein. Therefore, it is reasonably expected that HBsAg variants with deficient secretory capacity could induce ER stress in liver cells via the UPR, which could lead to liver cell damage[22,31]. This process provides a plausible link between occult infection and the progression of liver diseases. Therefore, the present study investigated the above hypothesis by examining whether our KD variant showing a secretion defect could induce ER stress pathways and evoke ER stress-mediated biological actions that are associated with the progression of liver cell diseases. Our data indicated that the KD variant activated ER-related genes and induced ER stress, which mediated the induction of ROS, NO, and apoptosis in similar manners. These results suggest that the KD variant may affect an upstream signal of the ER stress pathway.
This study investigated possible links between a KD variant and the progression of liver disease and focused on three biological actions that could lead to liver damage, including ER stress and ROS production, NO production, and apoptosis induction. First, our data showed that the KD variant induced ER stress and ROS production via intracellular calcium increases in hepatocytes (Figures 3 and 4). Generally, a relatively mild level of ROS induction is advantageous in cell signaling, and it induced anti-oxidant genes that enabled elevated ROS levels to remain at normal levels in this case[32]. Increased ROS levels in hepatocytes due to the hepatitis C virus (HCV) core proteins induce the expression of anti-oxidant genes, which may maintain ROS homeostasis. This process may otherwise induce hepatocyte apoptosis or HCC[33]. In contrast to the HCV core protein, our KD variant down-regulated the anti-oxidant proteins HO-1, MnSOD, Cu/Zn SOD, and catalase (Figure 5), which suggests a positive amplification of the ER stress-ROS production axis. The differences between these two cases may be due to differences in the intensity of inducing ER stress or ROS production. Perhaps the excessive ER stress and ROS level induced by the KD variant were beyond the threshold necessary to maintain normal cell function, which facilitated cell death by increasing cytosolic ROS levels via the abrogation of anti-oxidant gene function. The excessive ER stress and ROS level also provide possible links between occult HBsAg variants and apoptosis.
Second, the KD variant increased NO production in hepatocytes in an iNOS expression-dependent manner. Recently, the role of NO-mediated oxidative stress was well established in chronic viral hepatitis induced by HBV and HCV[34,35]. Therefore, the iNOS-mediated NO synthesis that was observed by the KD variant in the present study (Figure 6) was likely induced by ER stress-ROS mediated inflammation. The elevated NO production by the HBsAg variant may be responsible for the generation of chronic hepatitis and the progression of liver diseases via peroxynitrite, which is a potential oxidant that is produced by the reduction of superoxide anion, a species of ROS, with NO[36].
Third, apoptosis plays a central role in liver diseases[37]. The apoptosis of hepatocytes, which are the major component of liver cells, is directly related to the failure of liver functions. Additionally, the regeneration of liver cells after chronic apoptosis in combination with the accumulation of mutated DNA because of ER stress-ROS production may induce hepatocarcinogenesis. Figure 7 shows that the KD variant induced apoptosis in hepatocytes via at least three potential pathways, which are more inter-connected than distinct. First, apoptosis may be mediated by CHOP activation via the major ER stress transducer PERK-ElF2α axis (Figure 3A). Second, apoptosis may be mediated by intracellular calcium increases via ER stress (Figure 3D). Finally, apoptosis may also be mediated by ER stress-mediated JNK activation, which induces anti-apoptotic BCL-2 down-regulation and pro-apoptotic Bax-2 activation (Figure 7A). The synergistic combination of these three pathways may lead to apoptosis in hepatocytes in a caspase-dependent manner (Figure 7B).
In conclusion, the KD variant that is related to occult infections may play a pivotal role in the progression of liver diseases, such as chronic hepatitis, liver fibrosis, cirrhosis, and HCC, during the natural course of HBV infections primarily via ER stress and ROS production. The possible down-regulation of HBV replication because of ROS and NO production by HBsAg variants may also provide a likely explanation for the relationship between occult HBV infection and ER stress-mediated liver diseases. The present study provides two primary observations. First, the results of this study emphasized the role of HBsAg among other HBV products in the pathogenesis of liver diseases. Second, the data obtained in this study strongly support a hypothesis that one resource of occult HBV infections, at least in the Korean population, may be chronic patients with advanced liver diseases. Therefore, horizontal transfer of occult HBV variants may occur between occult HBV patients, chronic HBV patients, and otherwise healthy people.
Occult hepatitis B virus (HBV) infection is highly prevalent, particularly in HBV endemic areas, and it is significantly related to severe forms of liver diseases, such as cirrhosis and hepatocellular carcinoma. However, the exact molecular mechanisms underlying occult infections that are related to severe liver diseases are not known.
The data indicated that an occult infection related to the S surface antigen (HBsAg) variant KD with lower secretion capacity led to endoplasmic reticulum (ER)-derived oxidative stress and liver cell death in HuH-7 cells. These results provide novel insight into relationships between occult infection and liver disease progression.
This report is the first study to demonstrate that an occult infection related to an HBsAg variant may have a pivotal function in liver disease progression via ER stress-related pathways.
An occult infection related to the HBsAg variant KD, which was introduced in this study, could be effectively used for further investigation of ER stress pathways.
Occult HBV infection is defined as an infection state that is negative for HBsAg serology, but viral genome persistence is observed in infected individuals. The ER has multiple important functions that are essential to cell survival and normal cellular functions, including Ca2+ storage, post-translational modification, and the folding and assembly of newly synthesized secretory proteins.
The authors examined the underlying mechanisms of the relationship between occult HBV infection and liver disease progression and focused on the induction of ER stress because of occult infection related to an HBsAg variant. The results revealed that an occult infection related to the HBsAg variant KD with lower secretion capacity enhanced ER-derived oxidative stress and liver cell death in HuH-7 cells compared to wild-type HBsAg. These results provide novel insight into the relationships between occult infection and liver disease progression.
P- Reviewer: Bock CT, Caboclo JLF S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | KCDC Trend in Seroprevalence of Hepatitis B surface Antigen (HBsAg) in Korea Report of National Health and Nutrition Survey. 2013. Available from: http://www.cdc.go.kr. |
| 3. | Kim H, Jee YM, Song BC, Shin JW, Yang SH, Mun HS, Kim HJ, Oh EJ, Yoon JH, Kim YJ. Molecular epidemiology of hepatitis B virus (HBV) genotypes and serotypes in patients with chronic HBV infection in Korea. Intervirology. 2007;50:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Orito E, Mizokami M, Sakugawa H, Michitaka K, Ishikawa K, Ichida T, Okanoue T, Yotsuyanagi H, Iino S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Japan HBV Genotype Research Group. Hepatology. 2001;33:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 315] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 5. | Song BC, Kim SH, Kim H, Ying YH, Kim HJ, Kim YJ, Yoon JH, Lee HS, Cha CY, Kook YH. Prevalence of naturally occurring surface antigen variants of hepatitis B virus in Korean patients infected chronically. J Med Virol. 2005;76:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Kim HJ, Park JH, Jee Y, Lee SA, Kim H, Song BC, Yang S, Lee M, Yoon JH, Kim YJ. Hepatitis B virus X mutations occurring naturally associated with clinical severity of liver disease among Korean patients with chronic genotype C infection. J Med Virol. 2008;80:1337-1343. [RCA] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Mun HS, Lee SA, Jee Y, Kim H, Park JH, Song BC, Yoon JH, Kim YJ, Lee HS, Hyun JW. The prevalence of hepatitis B virus preS deletions occurring naturally in Korean patients infected chronically with genotype C. J Med Virol. 2008;80:1189-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Mun HS, Lee SA, Kim H, Hwang ES, Kook YH, Kim BJ. Novel F141L pre-S2 mutation in hepatitis B virus increases the risk of hepatocellular carcinoma in patients with chronic genotype C infections. J Virol. 2011;85:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Lee SA, Mun HS, Kim H, Lee HK, Kim BJ, Hwang ES, Kook YH, Kim BJ. Naturally occurring hepatitis B virus X deletions and insertions among Korean chronic patients. J Med Virol. 2011;83:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Lee SA, Cho YK, Lee KH, Hwang ES, Kook YH, Kim BJ. Gender disparity in distribution of the major hydrophilic region variants of hepatitis B virus genotype C according to hepatitis B e antigen serostatus. J Med Virol. 2011;83:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Lee SA, Kim K, Kim H, Kim BJ. Nucleotide change of codon 182 in the surface gene of hepatitis B virus genotype C leading to truncated surface protein is associated with progression of liver diseases. J Hepatol. 2012;56:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Kim DW, Lee SA, Hwang ES, Kook YH, Kim BJ. Naturally occurring precore/core region mutations of hepatitis B virus genotype C related to hepatocellular carcinoma. PLoS One. 2012;7:e47372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Kim H, Lee SA, Kim DW, Lee SH, Kim BJ. Naturally occurring mutations in large surface genes related to occult infection of hepatitis B virus genotype C. PLoS One. 2013;8:e54486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Lee SA, Kim KJ, Kim DW, Kim BJ. Male-specific W4P/R mutation in the pre-S1 region of hepatitis B virus, increasing the risk of progression of liver diseases in chronic patients. J Clin Microbiol. 2013;51:3928-3936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Kim H, Lee SA, Won YS, Le HJ, Kim BJ. Occult infection related hepatitis B surface antigen variants showing lowered secretion capacity. World J Gastroenterol. 2014;21:1794-1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Bruss V, Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci USA. 1991;88:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 310] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Molnar-Kimber KL, Jarocki-Witek V, Dheer SK, Vernon SK, Conley AJ, Davis AR, Hung PP. Distinctive properties of the hepatitis B virus envelope proteins. J Virol. 1988;62:407-416. [PubMed] |
| 18. | Liang TJ, Baruch Y, Ben-Porath E, Enat R, Bassan L, Brown NV, Rimon N, Blum HE, Wands JR. Hepatitis B virus infection in patients with idiopathic liver disease. Hepatology. 1991;13:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Koike K, Kobayashi M, Gondo M, Hayashi I, Osuga T, Takada S. Hepatitis B virus DNA is frequently found in liver biopsy samples from hepatitis C virus-infected chronic hepatitis patients. J Med Virol. 1998;54:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Bánhegyi G, Baumeister P, Benedetti A, Dong D, Fu Y, Lee AS, Li J, Mao C, Margittai E, Ni M. Endoplasmic reticulum stress. Ann N Y Acad Sci. 2007;1113:58-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1342] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 22. | Ji C, Kaplowitz N. ER stress: can the liver cope? J Hepatol. 2006;45:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 638] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 24. | Wang HC, Huang W, Lai MD, Su IJ. Hepatitis B virus pre-S mutants, endoplasmic reticulum stress and hepatocarcinogenesis. Cancer Sci. 2006;97:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 26. | Chua PK, Wang RYL, Lin MH, Masuda T, Suk FM, Shih C. Reduced secretion of virions and hepatitis B virus (HBV) surface antigen of a naturally occurring HBV variant correlates with the accumulation of the small S envelope protein in the endoplasmic reticulum and Golgi apparatus. J Virol. 2005;79:13483-13496. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 511] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 28. | Yang H, Westland C, Xiong S, Delaney WE. In vitro antiviral susceptibility of full-length clinical hepatitis B virus isolates cloned with a novel expression vector. Antiviral Res. 2004;61:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Alisi A, Piemonte F, Pastore A, Panera N, Passarelli C, Tozzi G, Petrini S, Pietrobattista A, Bottazzo GF, Nobili V. Glutathionylation of p65NF-kappaB correlates with proliferating/apoptotic hepatoma cells exposed to pro- and anti-oxidants. Int J Mol Med. 2009;24:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Hara Y, Hino K, Okuda M, Furutani T, Hidaka I, Yamaguchi Y, Korenaga M, Weinman SA, Lemon SM, Okita K. Hepatitis C virus core protein inhibits deoxycholic acid-mediated apoptosis despite generating mitochondrial reactive oxygen species. J Gastroenterol. 2006;41:257-268. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Xu Z, Jensen G, Yen TS. Activation of hepatitis B virus S promoter by the viral large surface protein via induction of stress in the endoplasmic reticulum. J Virol. 1997;71:7387-7392. [PubMed] |
| 32. | Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3513] [Cited by in RCA: 4192] [Article Influence: 262.0] [Reference Citation Analysis (0)] |
| 33. | Mahmood S, Kawanaka M, Kamei A, Izumi A, Nakata K, Niiyama G, Ikeda H, Hanano S, Suehiro M, Togawa K. Immunohistochemical evaluation of oxidative stress markers in chronic hepatitis C. Antioxid Redox Signal. 2004;6:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | García-Monzón C, Majano PL, Zubia I, Sanz P, Apolinario A, Moreno-Otero R. Intrahepatic accumulation of nitrotyrosine in chronic viral hepatitis is associated with histological severity of liver disease. J Hepatol. 2000;32:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Kandemir O, Polat A, Kaya A. Inducible nitric oxide synthase expression in chronic viral hepatitis and its relation with histological severity of disease. J Viral Hepat. 2002;9:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Oishi P, Grobe A, Benavidez E, Ovadia B, Harmon C, Ross GA, Hendricks-Munoz K, Xu J, Black SM, Fineman JR. Inhaled nitric oxide induced NOS inhibition and rebound pulmonary hypertension: a role for superoxide and peroxynitrite in the intact lamb. Am J Physiol Lung Cell Mol Physiol. 2006;290:L359-L366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Fabregat I, Roncero C, Fernández M. Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int. 2007;27:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |