Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5421
Peer-review started: October 30, 2014
First decision: November 14, 2014
Revised: December 20, 2014
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 7, 2015
Processing time: 195 Days and 10.6 Hours
Telaprevir and Boceprevir are the first direct acting antivirals approved for chronic hepatitis C in combination with peg-interferon alfa and ribavirin. Pancytopenia due to myelotoxicity caused by these drugs may occur, but severe hematological abnormalities or aplastic anemia (AA) have not been described. We collected all cases of severe pancytopenia observed during triple therapy with telaprevir in four Spanish centers since approval of the drug in 2011. Among 142 cirrhotic patients receiving treatment, 7 cases of severe pancytopenia (5%) were identified and three were consistent with the diagnosis of AA. Mean age was 59 years, five patients had compensated cirrhosis and two patients had severe hepatitis C recurrence after liver transplantation. Severe pancytopenia was diagnosed a median of 10 wk after the initiation of therapy. Three patients had pre-treatment hematological abnormalities related to splenomegaly. In six patients, antiviral treatment was interrupted at the onset of hematological abnormalities. Two patients died due to septic complications and one patient due to acute alveolar hemorrhage. The remaining patients recovered. Severe pancytopenia and especially AA, are not rare during triple therapy with telaprevir in patients with advanced liver disease. Close monitoring is imperative in this setting to promptly detect serious hematological disorders and to prevent further complications.
Core tip: Addition of the new directly acting antivirals, Telaprevir and Boceprevir, clearly improved sustained virological response rates in patients with chronic hepatitis C. However, these combinations have also increased the risk of serious adverse events, especially in patients with advanced liver fibrosis. We describe the development of severe pancytopenia and aplastic anemia during triple therapy with telaprevir in patients with advanced liver disease (before or after liver transplantation). Close monitoring is imperative in this setting to promptly detect serious hematological disorders and to prevent further complications.
- Citation: Lens S, Calleja JL, Campillo A, Carrión JA, Broquetas T, Perello C, de la Revilla J, Mariño Z, Londoño MC, Sánchez-Tapias JM, Urbano-Ispizua &, Forns X. Aplastic anemia and severe pancytopenia during treatment with peg-interferon, ribavirin and telaprevir for chronic hepatitis C. World J Gastroenterol 2015; 21(17): 5421-5426
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5421.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5421
Addition of the new directly acting antivirals (DAAs), Telaprevir and Boceprevir, clearly improved sustained virological response (SVR) rates in patients with chronic hepatitis C[1-5]. However, these combinations also increased the risk of serious adverse events (SAEs). One of the most common adverse events associated with triple therapy compared to standard peg-interferon and ribavirin (PR) regimens is anemia[6]. Interferon-related bone marrow suppression and ribavirin-related hemolytic anemia are common and may lead to dose-reduction, especially in patients with baseline cytopenia[7]. Nonetheless, very few cases of severe pancytopenia and only one case of aplastic anemia related to interferon therapy have been reported in patients with chronic hepatitis C virus (HCV)[8,9]. Thus, severe hematological disorders appear to be anecdotic as a great number of patients have been treated with these drugs during the last decades. As far as we know, cases of aplastic anemia related to telaprevir or boceprevir combination therapy have not been reported. Instead, a case of successful triple therapy in a patient with severe aplastic anemia has been recently reported[10].
We describe 7 cases of severe pancytopenia in patients receiving antiviral treatment with PR and telaprevir, three being consistent with the diagnosis of aplastic anemia (AA).
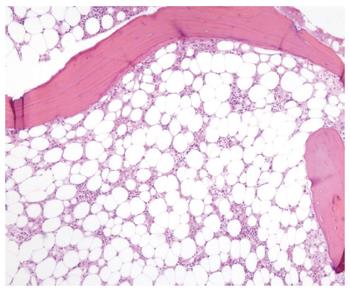
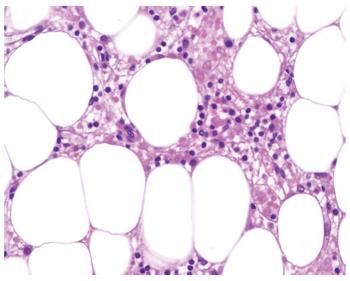
This patient was a 51-year-old woman with genotype 1b HCV-related cirrhosis. She had stable neutropenia (1500 cells/μL) and thrombocytopenia (74 x 109/L) that were likely related to portal hypertension (hepatic venous portal pressure gradient (HVPG) of 16 mmHg). Esophageal varices were not found on upper gastrointestinal endoscopy. She had no comorbidities and she did not take any medications. Viral load (VL) was undetectable at week 4 of triple therapy including telaprevir. At this time, blood analysis revealed worsening of neutropenia (1000 cells/μL), thrombocytopenia (42 x 109/L) and a decrease of 2.5 g/dL in hemoglobin (Hb) level, leading to interferon and ribavirin dose reduction. One week later, she complained of worsening of general condition, epistaxis, and mild rash. Blood tests revealed severe pancytopenia (Table 1). The patient was admitted to hospital; antiviral therapy was immediately interrupted and blood and platelet transfusions were administered. Despite supportive blood transfusion and granulocyte colony-stimulating factor (GCSF) the hematological abnormalities did not improve. A bone marrow aspirate showed a leucocyte population formed by lymphocytes (41%) and normal phenotypic plasma cells (55%) with the absence of neutrophils. Bone marrow biopsy was compatible with AA (Figures 1 and 2). Other acquired causes of bone marrow failure such as myelodysplastic syndromes, leukemia, megaloblastic anemia, paroxysmal nocturnal hemoglobinuria and viral hemophagocytic syndrome were excluded. Due to the lack of improvement of hematological parameters and the contraindication of bone marrow transplantation, cyclosporine was started with no improvement. The patient developed pulmonary aspergillosis and subsequently presented progressive liver and renal failure and died 50 d after admission.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 (LT) | Case 7 (LT) | |
| Features at baseline | |||||||
| Hemoglobin (g/dL) | 14.3 | 14.2 | 12.9 | 13.5 | 13.4 | 13.9 | 14 |
| Neutrophils (mm3) | 1500 | 1320 | 1900 | 900 | 3810 | 2880 | 1700 |
| Platelets (mm3) | 74000 | 96000 | 108000 | 101000 | 199000 | 102000 | 78000 |
| ALT (IU/L) | 59 | 77 | 46 | 96 | 77 | 120 | 536 |
| Bilirubin (mg/dL) | 1.3 | 0.93 | 0.6 | 1.1 | 0.5 | 1.4 | 1.2 |
| Albumin (g/dL) | 32 | 47 | 37 | 40 | 45 | 35 | 36 |
| INR | 1.1 | 1.1 | 1 | 1 | 1 | 1.1 | 1.28 |
| Liver stiffness (kPa) | 50 | 10.8 | 16 | 11 | 16.8 | 14.2 | 12 |
| HVPG (mmHg) | 16 | - | - | 8.5 | - | 8.5 | - |
| Child-Pugh score | 6 | 5 | 5 | 5 | 5 | 6 | 5 |
| Features at diagnosis of pancytopenia | |||||||
| Triple therapy (wk) | 10 | 4 | 7 | 5 | 16 | 16 | 8 |
| Hemoglobin (g/dL) | 7.5 | 7 | 7.8 | 7.6 | 7.2 | 7.9 | 8.5 |
| Neutrophils (cells/μL) | 400 | 0 | 250 | 200 | 400 | 400 | 300 |
| Platelets (109/L) | 5000 | 19000 | 2400 | 23000 | 12000 | 9000 | 30000 |
| Reticulocytes (109/L) | 0.2% | 0.1% | 0.6% | - | < 1% | < 1% | - |
| Bone Marrow Biopsy | Yes | Yes | Yes | - | - | - | - |
| Specific treatment | |||||||
| Treatment interruption | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Blood transfusion (units) | 14 | 7 | NA | 6 | 20 | 10 | 0 |
| EPO (days of therapy) | 30 | 60 | 60 | 6 | 240 | 90 | 30 |
| GCSF (days of therapy) | 30 | 60 | 30 | 6 | 90 | 90 | 45 |
| Outcome | Death | Recovered | Death | Death | Recovered | Recovered | Recovered |
This patient was a 56-year-old woman with HCV genotype 1a-related cirrhosis that was diagnosed 6 years earlier by liver biopsy and remained compensated. She was on oral therapy for diabetes mellitus. Two days after the initiation of triple therapy with telaprevir she developed a grade I micropapular rash which was successfully treated with topical corticoids and anti-histamines. At week 4 of therapy she complained of fever, odynophagia and cough. Physical examination showed an erythematous tonsillitis with candidiasis lesions. Blood tests showed a total leukocyte count of 570 cells/μL with 0% neutrophils, a platelet count of 19 x 109/L, Hb of 7 g/dL, and 0.1% reticulocytes (Table 1). The chest X-ray was normal. The patient was admitted to hospital and antiviral therapy was withdrawn. A bone marrow biopsy showed marked hypocellularity without morphologic abnormalities, consistent with AA. Supportive treatment with erythropoietin (EPO), GCSF and blood and platelet transfusion was initiated. Oral candidiasis due to Candida albicans was successfully eradicated. Two months later the patient’s hematological abnormalities had fully recovered.
This patient was a 67-year-old woman with HCV genotype 1a compensated cirrhosis, autoimmune hypothyroidism, arterial hypertension, and well controlled diabetes mellitus. Concomitant medications were metformin, levothyroxine, atenolol and eprosartan. Four weeks after initiation of antiviral therapy with PR and telaprevir, the VL was undetectable. There was an Hb concentration decrease of 5 g/dL from baseline and a blood transfusion was administered at this time. Ribavirin dose was adjusted and treatment with EPO was initiated. Three weeks later she was admitted to the Intensive Care Unit with the diagnosis of septic shock requiring vasoactive drug support and severe pancytopenia. Antiviral therapy was stopped at admission. Methicillin-sensitive Staphylococcus aureus was isolated in blood cultures within 48 h and specific antibiotic therapy was started (cloxacillin and piperacillin-tazobactam). Despite extensive investigations, the source of the infection was not identified. Pancytopenia progressively worsened (Table 1). The patient developed renal failure requiring hemodialysis as well as liver failure with bilirubin increasing up to 18 mg/dL and ascitic decompensation. The patient presented a multiorgan invasive zygomycosis and died one month after admission. Necropsy confirmed AA with severe hypocellularity in the bone marrow.
This patient was a 62-year-old woman with HCV genotype 1b-related cirrhosis. She had durable neutropenia (about 900 cells/μL) possibly related to hypersplenism due to portal hypertension (HVPG 8 mmHg). Upper gastrointestinal endoscopy detected a small esophageal varicose vein. She did not have comorbidities and did not take medications. VL was undetectable at week four of triple therapy with telaprevir. At this point, total neutrophil and platelet counts were 700 cells/μL and 47 x 109/L, respectively. One week later she was admitted to the hospital due to fever and chills lasting 48 h. The chest X-ray showed a right inferior lobe pneumonia and broad spectrum coverage antibiotic therapy was started. Blood tests disclosed severe pancytopenia (Table 1). Antiviral therapy was interrupted and supportive treatment with red blood cell and platelet transfusion was initiated. Seventy-two hours after admission, she suddenly presented a massive hemoptysis with alveolar hemorrhage. Despite orotracheal intubation and mechanical ventilation the patient suffered a cardiac arrest and did not recover. Necropsy was not performed.
This patient was a 68-year-old woman with HCV genotype 1b-related cirrhosis and cryoglobulinemia. She had no comorbidities and she did not take any medications. VL was undetectable at week 4 of triple therapy with telaprevir. The patient was hospitalized at week 8 due to acute pyelonephritis caused by Escherichia coli. The infection resolved after 2 wk of intravenous antibiotic therapy. Due to grade 3 anemia, the patient required a blood transfusion at week 12, and treatment with EPO and ribavirin dose reduction was required at this time. The patient completed 12 wk of telaprevir therapy and then continued on treatment with PR. During the following 4 wk, the patient developed severe pancytopenia (Table 1) despite supportive treatment with transfusions, EPO and GCSF administration. Reticulocyte count was < 1% x 109/L. Other causes of pancytopenia were ruled out. The patient continued on antiviral therapy until week 48, at reduced doses of PR. She achieved a sustained virological response (SVR) and is currently recovering from the hematological disorder.
This patient was a 51-year-old man who underwent orthotopic liver transplantation (LT) 24 mo before due to advanced HCV genotype 1b-related cirrhosis. Immunosuppressive therapy consisted of tacrolimus monotherapy with stable levels and no dose modifications during the previous months. He was also taking statins for hypercholesterolemia. No other comorbidities were present and the patient received no other medications. The patient developed HCV recurrence with severe liver injury at 21 mo after LT, as indicated by cholestasis, high VL (4 x 106 IU/mL) and increasing transient elastography values (14 kPa). The HVPG value was 8.5 mmHg, and a transjugular liver biopsy showed necroinflammatory activity and periportal fibrosis (F2) on the METAVIR scale, but no signs of rejection. At week 16 of triple therapy with telaprevir, 4 wk after telaprevir was stopped he presented with grade 3 anemia, neutropenia and severe thrombocytopenia. Antiviral therapy was withdrawn and blood and platelet transfusions were administered (Table 1). Supportive care with EPO and GCSF was maintained for a further 3 months. The patient achieved a SVR despite early discontinuation of antivirals, and he also recovered from the hematological abnormalities.
This patient was a 63-year-old woman whose liver histology showed advanced fibrosis (F3) 18 mo after LT. She had a genotype 1b infection, VL was 2 x 106 IU/L, and ALT and AST values were 235 and 536 IU/L, respectively. Immunosuppressive treatment consisted of tacrolimus and low-dose prednisone. She had developed diabetes mellitus after LT and was receiving insulin therapy. When triple therapy with PR and telaprevir was initiated, she presented a stable mild thrombocytopenia of 78 x 109/L. At week 4 of therapy, there was a marked decrease in neutrophils (500 cells/μL) along with anemia (Hb 10 g/L) and a further decrease in platelet count (50 x 109/L). The VL was undetectable. Doses of PR were adjusted and supplementary therapy with EPO and GCSF was given. Despite these measures, the neutrophil count continued to decrease and antiviral therapy was interrupted at week 6 (Table 1). One month later, the hematological abnormalities disappeared and this was followed by a SVR in spite of early discontinuation of therapy.
The approval of first generation protease inhibitors (PIs), boceprevir and telaprevir, has been a major step forward in the treatment of chronic hepatitis C[1,2,4,5]. Beyond the efficacy results, PIs-based regimens in patients with compensated cirrhosis may be associated with SAEs such as severe infections (4%-6%), clinical decompensation (3%-4%) and even death. These events were not reported in the registration trials, possibly because patients included in these studies were mainly very well compensated cirrhotics without significant portal hypertension. Indeed, a low platelet count was an exclusion criterion (< 90 × 109/L for telaprevir and < 100 × 109/L for boceprevir) in these trials. The results of triple therapy in cirrhotic patients demonstrated that a low platelet count (< 100 × 109/L) and low serum albumin (< 35 g/L) were strong predictors of severe complications. Importantly, the risk of severe complications was 44% in patients with both factors as compared to 3.4% in patients with normal platelet and albumin values[11].
The mechanism of hematological toxicity during triple therapy is still not completely understood, but is probably related to the concomitant administration of all three drugs. Interferon results in bone marrow suppression and ribavirin leads to hemolysis, while PIs may cause direct bone marrow toxicity (as suggested in a few reports in the setting of HIV infection[12,13]) in patients with portal hypertension or advanced cirrhosis. Finally, the risk of hematological abnormalities could also be influenced by genetic factors[14].
Severe AA is defined by a bone marrow cellularity < 25%, or 25%-50% with < 30% residual hematopoietic cells, and at least 2 of the following factors: neutrophils < 0.5 cells/μL, platelets < 20 × 109/L and low reticulocyte count. The overall incidence of this disease is low, as it is estimated to occur in only 2-4 million people per year[15]. Antibiotics, anti-inflammatory drugs and anticonvulsants are among the currently licensed drugs which have been associated with AA[16].
Severe bone marrow hypocellularity related to interferon administration has been reported not only in the setting of HCV infection[8,9]. However, taking into account the huge number of patients treated with this drug worldwide, the incidence of this adverse event appears to be extremely low. Indeed, in our center, of 1700 HCV patients treated with PR therapy in the last decades, there have been no cases of AA. The only reported case in the literature is a non-cirrhotic 46-year-old man who developed AA 4 mo after the initiation of PR therapy and had a fatal outcome despite bone marrow transplantation[8].
Among 142 cirrhotic patients receiving treatment in four Spanish centers since the approval of triple therapy in 2011, 7 cases of severe pancytopenia (5%) were identified and three had diagnostic features consistent with AA (2%). The mean age of these patients was was 59 years, 5 were Child A cirrhotics and two had severe HCV recurrence after LT. The mean liver stiffness value was 18 kPa, three patients had portal hypertension (range: 8.5-16 mmHg) and 3 patients had abnormal pre-treatment hematological values possibly related to splenomegaly (Table 1). All patients were closely monitored from the beginning of treatment (at least biweekly) and PR dose adjustments were made depending on hematological values. The diagnosis of severe pancytopenia was made a median of 10 wk after telaprevir initiation and antiviral therapy was interrupted at the time of this diagnosis in all but one patient (case 5). At diagnosis, the MeDRA classification of adverse events was > 3 in all cases (severe or medically significant, but not immediately life-threatening with hospitalization indicated).
Importantly, when these patients were offered triple therapy, data on predictive factors associated with severe complications were not yet available. However, when retrospectively analysing if these patients would have been candidates for antiviral treatment with telaprevir, only case 1 would have been excluded due to the presence of both thrombocytopenia and hypoalbuminemia (case 1).
Three patients had diagnostic features of AA (cases 1-3). In case 3, AA developed during septic shock and antibiotic treatment, thus, an influence on the development of bone marrow toxicity cannot be disregarded. In the remaining patients, other potential causes of AA were excluded by anamnesis and laboratory tests. Bone marrow biopsy histology revealed hypocellularity and excluded an infiltrative disease (Figures 1 and 2).
Despite all patients being diagnosed very early and receiving support in referral centers; two patients had septic complications and died due to multiorgan failure and one patient had a massive hemoptysis and did not recover despite vital support measures.
In conclusion, hematological adverse events are frequent during antiviral therapy with protease inhibitors in patients with chronic hepatitis C. A specialized center with expertise should be contacted as early as possible in order to initiate measures aimed at obtaining the best supportive care and exclude other possible causes of pancytopenia. Although AA is a rare condition, it has now been diagnosed during triple therapy with telaprevir in patients with advanced liver disease. Close monitoring of hematological tests is advised during treatment and therapy should be promptly interrupted in the case of a decrease in the three hematopoietic series to prevent the establishment of AA and its complications.
Seven cases of severe pancytopenia were identified and three had diagnostic features consistent with aplastic anemia (AA) during triple therapy with telaprevir. The mean age of these patients was 59 years, 5 were Child A cirrhotics and two had severe hepatitis C virus (HCV) recurrence after liver transplantation. The mean liver stiffness value was 18 kPa, and three patients had portal hypertension (range: 8.5-16 mmHg).
The diagnosis of severe pancytopenia was made a median of 10 wk after telaprevir initiation.
Other causes of hematological disorders were excluded (drug toxicity, vitamin deficiency, bone marrow infiltration, malignancy).
Severe AA was defined by a bone marrow cellularity < 25%, or 25%-50% with < 30% residual haematopoietic cells, and at least 2 of the following factors: neutrophils < 0.5 cells/μL, platelets < 20 × 109/L and low reticulocyte count. The remaining cases had severe pancytopenia (hemoglobin levels < 10 g/dL, neutrophils < 0.75 cells/μL and platelets < 50 × 109/L).
Bone marrow biopsy histology revealed hypocellularity and excluded an infiltrative disease.
Early discontinuation of antiviral therapy after AA diagnosis. Supportive care with G-CSF and blood and platelet transfusions was administered. One patient received cyclosporine.
Interferon-related bone marrow suppression and ribavirin-related hemolytic anemia are common and may lead to dose-reduction, especially in patients with baseline cytopenia. Nonetheless, very few cases of severe pancytopenia and only one case of AA related to interferon therapy have been reported in patients with chronic hepatitis C infection.
The addition of a protease inhibitor to antiviral treatment may induce bone-marrow toxicity, especially in patients with advanced liver disease.
In conclusion, hematological adverse events are frequent during antiviral therapy with protease inhibitors in chronic hepatitis C. A specialized center with expertise should be contacted as soon as possible in order to initiate measures aimed at obtaining the best supportive care and to exclude other possible causes of pancytopenia. Although AA is a rare condition, it has now been diagnosed during triple therapy with telaprevir in patients with advanced liver disease. Close monitoring of hematological tests is advised during treatment and therapy should be promptly interrupted in the case of a decrease in the three hematopoietic series to prevent the establishment of AA and its complications.
The authors presents seven cases of severe pancytopenia (three of them with AA) occurred in patients with HCV related cirrhosis during triple therapy (peginterferon + ribavirin + telaprevir). The adverse effects associated with triple therapy are common and well-known.
P- Reviewer: Abenavoli L, Stasi C, Trifan A S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Ma S
| 1. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 2. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1971] [Article Influence: 140.8] [Reference Citation Analysis (0)] |
| 3. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 4. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 5. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 6. | Zeuzem S, DeMasi R, Baldini A, Coate B, Luo D, Mrus J, Witek J. Risk factors predictive of anemia development during telaprevir plus peginterferon/ribavirin therapy in treatment-experienced patients. J Hepatol. 2014;60:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 8. | Ioannou S, Hatzis G, Vlahadami I, Voulgarelis M. Aplastic anemia associated with interferon alpha 2a in a patient with chronic hepatitis C virus infection: a case report. J Med Case Rep. 2010;4:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Hoffmann A, Kirn E, Krueger GR, Fischer R. Bone marrow hypoplasia and fibrosis following interferon treatment. In Vivo. 1994;8:605-612. [PubMed] |
| 10. | Balkan II, Bozcan S, Yemisen M, Kutlubay Z, Ozaras R. Chronic hepatitis C successfully treated with telaprevir, pegylated interferon and ribavirin in severe aplastic anemia. Ann Hepatol. 2014;13:843-844. [PubMed] |
| 11. | Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 12. | Fernández Ibieta M, Ramos Amador JT, González Tomé MI, Guillén Martín S, Bellón Cano JM, Navarro Gómez M, de José MI, Beceiro J, Iglesias E, Rubio B. [Anaemia and neutropenia in a cohort of non-infected children of HIV-positive mothers]. An Pediatr (Barc). 2008;69:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Cingolani A, Torti L, Pinnetti C, de Gaetano Donati K, Murri R, Tacconelli E, Larocca LM, Teofili L. Detrimental clinical interaction between ritonavir-boosted protease inhibitors and vinblastine in HIV-infected patients with Hodgkin’s lymphoma. AIDS. 2010;24:2408-2412. [PubMed] |
| 14. | Thompson AJ, Clark PJ, Singh A, Ge D, Fellay J, Zhu M, Zhu Q, Urban TJ, Patel K, Tillmann HL. Genome-wide association study of interferon-related cytopenia in chronic hepatitis C patients. J Hepatol. 2012;56:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136:534-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147:43-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |