Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5345
Peer-review started: October 29, 2014
First decision: November 14, 2014
Revised: December 6, 2014
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 7, 2015
Processing time: 198 Days and 18.9 Hours
AIM: To investigate the feasibility and clinical value of magnetic resonance imaging (MRI)-MRI image fusion in assessing the ablative margin (AM) for hepatocellular carcinoma (HCC).
METHODS: A newly developed ultrasound workstation for MRI-MRI image fusion was used to evaluate the AM of 62 tumors in 52 HCC patients after radiofrequency ablation (RFA). The lesions were divided into two groups: group A, in which the tumor was completely ablated and 5 mm AM was achieved (n = 32); and group B, in which the tumor was completely ablated but 5 mm AM was not achieved (n = 29). To detect local tumor progression (LTP), all patients were followed every two months by contrast-enhanced ultrasound, contrast-enhanced MRI or computed tomography (CT) in the first year after RFA. Then, the follow-up interval was prolonged to every three months after the first year.
RESULTS: Of the 62 tumors, MRI-MRI image fusion was successful in 61 (98.4%); the remaining case had significant deformation of the liver and massive ascites after RFA. The time required for creating image fusion and AM evaluation was 15.5 ± 5.5 min (range: 8-22 min) and 9.6 ± 3.2 min (range: 6-14 min), respectively. The follow-up period ranged from 1-23 mo (14.2 ± 5.4 mo). In group A, no LTP was detected in 32 lesions, whereas in group B, LTP was detected in 4 of 29 tumors, which occurred at 2, 7, 9, and 15 mo after RFA. The frequency of LTP in group B (13.8%; 4/29) was significantly higher than that in group A (0/32, P = 0.046). All of the LTPs occurred in the area in which the 5 mm AM was not achieved.
CONCLUSION: The MRI-MRI image fusion using an ultrasound workstation is feasible and useful for evaluating the AM after RFA for HCC.
Core tip: One of the major factors that impact the therapeutic effectiveness of radiofrequency ablation (RFA) for hepatocellular carcinoma (HCC) is local tumor progression (LTP), which is primarily attributed to insufficient ablative margins (AMs). Traditional image evaluation methods, including contrast-enhanced ultrasound and side-by-side comparison with computed tomography/magnetic resonance imaging (MRI) images, cannot quantitatively determine the AM in three dimensions. Our study was the first to report the feasibility of MRI-MRI image fusion using a commercial ultrasound workstation to assess the RFA AM for HCC. The results demonstrated that this method specifically describes the direction of the insufficient ablative zone and would be helpful for the early detection of LTP.
- Citation: Wang XL, Li K, Su ZZ, Huang ZP, Wang P, Zheng RQ. Assessment of radiofrequency ablation margin by MRI-MRI image fusion in hepatocellular carcinoma. World J Gastroenterol 2015; 21(17): 5345-5351
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5345
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common cause of cancer deaths in the world. Radiofrequency ablation (RFA) has been widely performed as a minimally invasive treatment modality for HCC with a high success rate of complete tumor ablation. However, recent studies reported high incidences (1.7%-41%) of local tumor progression (LTP) after RFA for HCC[1-10]. The main risk factors for LTP include large tumor size, insufficient ablative margins (AMs) and poor histological grade[11-17]. In previous studies, the AM was assessed by comparing the axial images of computed tomography (CT)-magnetic resonance imaging (MRI) or contrast-enhanced ultrasound (CEUS) obtained before and after RFA using a side-by-side approach[18-20]. These approaches are somewhat subjective and have limited ability to accurately assess the AM. Commonly, the index tumor and its surrounding hepatic parenchyma are mixed after ablation and appear on routine CT/MRI or CEUS, an area that lacks contrast enhancement. No conventional imaging methods can be used to differentiate the index tumor from the surrounding hepatic parenchyma in the ablative area. In addition, the original position of the index tumor in the ablative area cannot be clearly determined after RFA. Thus, accurately determining whether the ablative area overlaps with the entire AM range using routine imaging methods is difficult.
The recent development of new image fusion techniques has made it possible to accurately assess the AM and therapeutic responses to RFA for HCC[21,22]. Kim et al[21] quantitatively assessed the ablative area and margins using 3D CT image fusion before and after RFA for HCC. Their study demonstrated that the minimal AM should be ≥ 3 mm for preventing LTP. Fujioka et al[22] evaluated the therapeutic response to RFA for HCC using image registration of preoperative and postoperative CT. They found that this imaging technique can facilitate illustrating the relationship between tumor and ablation zone. More recently, Su et al[23] assessed the HCC AM after ablation using 3DCEUS-CT/MRI image fusion with encouraging results that also indicated the promising value of image fusion in determining the AM. Compared with CT and CEUS, MRI is more sensitive and frequently used in the evaluation of liver lesions and tumor treatment response. To the best of our knowledge, the use of MRI-MRI image fusion for evaluating the AM after RFA has not been reported.
This study aimed to explore the feasibility and clinical value of a newly developed MRI-MRI image fusion method in assessing AM after RFA for HCC.
The study was approved by the Institutional Review Board, and written informed consent was obtained from all patients. Between October 2009 and September 2011, 52 patients (46 men and 6 women, aged 50.2 ± 17.5 years, range: 27-74 years) with 62 HCC tumors who underwent RFA with confirmed complete ablation by MRI in the first month after RFA were enrolled into this study. All of the 62 liver lesions were pathologically or clinically diagnosed as HCC[24]. The baseline characteristics of the patients are shown in Table 1.
| Variable | Value |
| Hepatocellular carcinoma | n = 52 |
| Age (mean ± SD) | 50.2 ± 17.5 |
| Sex | |
| Male | 46 (88.5) |
| Female | 6 (11.5) |
| AFP | |
| ≥ 200 μg/L | 32 (61.5) |
| < 200 μg/L | 20 (38.5) |
| HbsAg | |
| Positive | 52 (100) |
| Negative | 0 (0) |
| Child-Pugh classification | |
| A | 35 (67.3) |
| B | 17 (32.7) |
| Tumor size cm (mean ± SD) | 2.0 ± 1.0 (1.0-3.1) |
| Multiple tumors | |
| Yes | 10 (19.2) |
| No | 42 (80.8) |
| Tumor location | |
| Liver segment I | 0 (0) |
| Liver segment II | 3 (4.8) |
| Liver segment III | 7 (11.3) |
| Liver segment IV | 7 (11.3) |
| Liver segment V | 9 (14.5) |
| Liver segment VI | 11 (17.7) |
| Liver segment VII | 12 (19.4) |
| Liver segment VIII | 13 (21.0) |
| Background liver cirrhosis | |
| Present | 46 (88.5) |
| Absent | 6 (11.5) |
All RFA procedures were performed by using a Cool-Tip radiofrequency system (Radionics Inc, Burlington, MA) under ultrasound guidance (My Lab Twice, Esoate, Genoa, Italy). A total of 59 lesions in 49 patients underwent percutaneous RFA, whereas 3 cases underwent laparotomy RFA. RFA combined with percutaneous ethanol injection treatment (PEIT) was performed in 3 cases, and transcatheter hepatic arterial chemoembolization (TACE) 3 wk before RFA was performed in 6 cases. All procedures were performed by 2 experienced ultrasound physicians who had at least 5 years of experience with the RFA procedure. For early evaluation of the possible complications, ultrasound examination was performed within 24 h after ablation.
Before and one month after RFA, contrast-enhanced MRI was performed for all the patients using a 1.5-T MR imaging unit (Gyroscan Intera; Philips Medical Systems, Best, The Netherlands). If MRI at one month after RFA showed that the lesion had been completely ablated, AM was further evaluated using MRI-MRI image fusion.
All MR imaging was performed by using a 1.5 T MR scanner (Signa Excite; GE Medical Systems, United States) with an 8-channel torso phased-array coil. Imaging included an axial T1-weighted fast field echo (FFE), axial T2-weighted single-shot turbo spin-echo (SSTSE), axial in-phase and out-phase chemical shift GRE T1-weighted images, and a gadolinium enhanced dynamic study. The parameters of FFE were as follow: repetition time/echo time, 129/238 and 476 ms; number of sections, 60; field of view, 380 mm; matrix, 158 × 256; flip angle, 70°; gap, 15%; section thickness, 3 mm; two signals acquired. The parameters of SSTSE were as follow: repetition time/echo time, 2100/84 ms; number of sections, 60; field of view, 350 mm; matrix, 207 × 384; flip angle, 150°; gap, 10%; section thickness, 3 mm; one signal acquired. For the gadolinium enhanced dynamic study, a multiphase dynamic study including arterial, portal, and delayed phases was performed before unenhanced MR imaging. The parameters of enhanced study were as follow: repetition time/echo time, 3.89/1.51 ms; number of sections, 52; field of view, 420 mm; matrix, 144 × 384; flip angle, 25°; gap, 20%; section thickness, 3 mm; one signal acquired. Gadolinium (Primovist; Bayer, Shanghai, China) at 8 mol/kg (0.016 mL/kg) was injected through the antecubital vein using a power injector, followed by a bolus administration of 20 mL saline at the same injection rate. An axial T2*-weighted image was performed by using the three-dimensional (3D) sensitivity-encoding water-excitation multishot echo-planar (SWEEP) sequence 10 mn later.
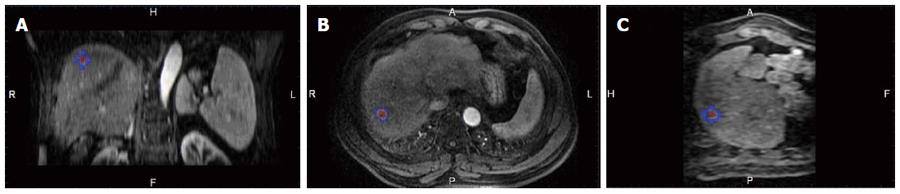
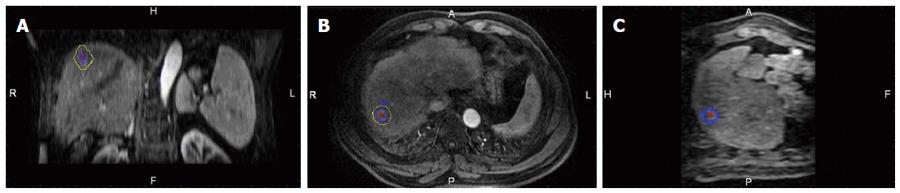
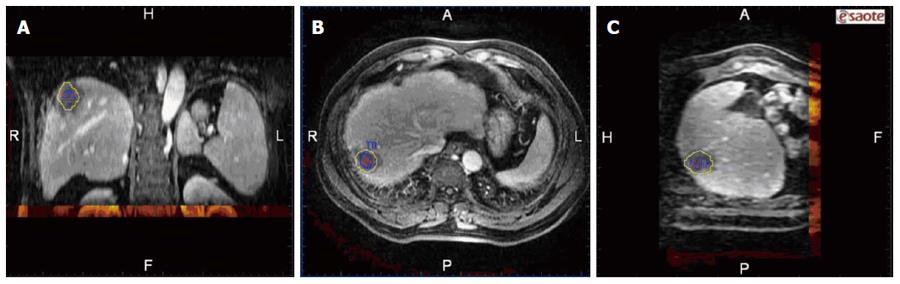
A commercially available image fusion system (My Lab Twice, Esoate, Genoa, Italy) was used to perform MRI-MRI image fusion. One series of MRI images before RFA with clearly demonstrated hepatic vessels, as well as HCC lesions in the portal vein phase or late phase, were selected, and the images in DICOM format were imported into the image fusion system. Another series of MRI images one month after RFA with clearly demonstrated hepatic vessel and ablative area in DICOM format were also imported into the image fusion system. Then, the system automatically displayed 6 pictures in 2 rows; the upper row included the transaction, coronal and vertical plane MRI images before RFA, and the lower row demonstrated the corresponding MRI images after RFA. The HCC lesion in the MRI before RFA was outlined manually, and then, a 5 mm AM was set automatically. The system marked the HCC lesion and AM using different colors (Figures 1 and 2).
Image registration was performed by aligning 2 overlaid MRI images with 6 parameters; translation and rotation were performed in 3 reformed planes to maximize image similarity around the HCC lesion and ablation area. The hepatic vein, hepatic artery-portal complex and hepatic contour near the lesion were used as landmarks for fine adjustments to obtain satisfactory registration. The pre- and post-RFA MRI images were then overlapped to assess whether the ablative area could cover the HCC lesion and 5 mm AM. If multiple tumors were present in the same patient, the above image processing of the registration was repeated for each tumor. The standards of complete registration include that three corresponding anatomic landmarks adjacent to the tumor were fully matched, and the offset was less than 5 mm in each plane. Failure of registration was assigned if the above standard was not met after three attempts. The time spent on registration for each lesion was recorded.
After image registration, post-RFA image was fused to the pre-RFA one in three different planes, then the relationship between the tumor/AM and ablation zone in three dimensions was clearly observed (Figure 3). Based on the results of the evaluation of the RFA procedure by image fusion, the lesions were divided into two groups: (1) the tumor and AM were completely covered by the ablation zone (group A); and (2) the tumor was completely covered by the ablation zone but an AM was not ensured (group B). To describe the position of the unachieved AM, the tumor was divided into 8 quadrants by three orthogonal planes (axial, coronal and vertical planes) crossing at the center of the tumor. The 4 cephaled quadrants of the 8 total quadrants were numbered 1-4, and the 4 caudal quadrants were numbered 5-8, both in a clockwise direction. The time spent on evaluating the AM for each lesion was recorded.
The patients were followed every 2 mo in the first year after RFA by CEUS, contrast-enhanced MRI or CT. Then, the follow-up interval was extended to every 3 mo after the first year. LTP was diagnosed when a follow-up MRI or CT revealed the development of the tumor adjacent to the ablative area with arterial hypervascularity and wash-out in the early or delayed venous phase. The LTP position was recorded according to the 1-8 quadrants mentioned above. The unachieved AM and LTP were considered consistent when they were observed in the same quadrant.
The patient data were exported into Microsoft Excel, where the mean ± SD was calculated. Statistical analyses were performed using the commercially available SPSS software package version 13.0 (SPSS, Inc, Chicago, IL). Categorical variables were compared using χ2 test. All P-values were two sided. The difference was considered significant at P < 0.05.
MRI-MRI image fusion was accurately achieved in 61 of the 62 tumors; the case without fusion was due to significant deformation of the liver and massive ascites after RFA. The success rate of MRI-MRI image fusion was 98.4% (61/62). Examples of the image registration and MRI-MRI image fusion before and after RFA are shown in Figures 1, 2 and 3. The total time required for the creation of image fusion was 15.5 ± 5.5 min (range: 8-22 min) in all cases.
At one month after RFA, MRI revealed that 61 of the 62 tumors had been completely ablated. Another session of RFA combined with TACE was performed in the case without complete ablation 5 wk after the first session of RFA. One month after the second RFA session, MRI revealed complete ablation; in this case, the second RFA instead of the first RFA session was enrolled.
Of the 61 lesions in 51 HCC patients, 32 tumors were completely ablated and 5 mm AM was achieved (group A), whereas 29 tumors were completely ablated but 5 mm AM was not achieved (group B). Of the 29 tumors in the group B, 23 cases were attributed to hepatic vessel-induced indentation of the ablation zone, and 6 cases were due to the insufficient ablation extent. The total time spent on AM evaluation was 9.6 ± 3.2 min (range: 6-14 min).
Of the 51 HCC patients, 4 were lost to follow-up because of an incorrect phone number or postal address or noncooperation; these patients included 2 in group A (at 1 mo and 7 mo after RFA, respectively) and 2 in group B (at 2 mo after RFA). The follow-up period ranged from 1 to 23 mo (14.2 ± 5.4 mo). In group A, no LTP was detected in 32 lesions, whereas in group B, LTP was detected in 4 of 29 tumors which occurred at 2, 7, 9, and 15 mo after RFA. The frequency of LTP in group B (13.8%; 4/29) was significantly higher than that in group A (0/32, P = 0.046). All of the LTPs occurred in the area in which the 5 mm AM was not achieved. Among the patients, distant intrahepatic metastatic lesions were detected in 17 cases, 10 in group A and 7 in group B.
Hepatectomy, liver transplantation and ablation were well accepted as potentially curative interventions for early stage HCC. However, only 5%-40% of HCC patients were eligible for hepatectomy due to late clinical presentation or serious impairment of liver function[24,25]. For liver transplantation, only a few patients have such opportunities due to the shortage of liver donators. Thus, ablation has become the first choice for patients with early stage HCC in whom the cirrhotic liver cannot tolerate hepatectomy. A randomized control trial has showed that RFA can achieve survival rates similar to those achieved by liver resection in patients with HCC ≤ 5 cm in diameter[26].
One of the major impacting factors on the therapeutic effectiveness of ablation for HCC is LTP, which is primarily ascribed to insufficient AM. Therefore, the assessment of AM plays an important role in RFA scheduling[16-18]. If tumors with insufficient AM are detected in a timely manner using precise image evaluation, an effective method could be implemented to improve the therapeutic effectiveness or determine whether closer follow-up is required. Traditional image evaluation methods, including CEUS and side-by-side comparison with CT/MRI images, cannot quantitatively determine the AM in three dimensions[19,20].
In previous studies, the results of CT image fusion with a CT workstation demonstrated the feasibility of the image fusion technique in the assessment of the RFA procedure for HCC[21,22]. In this study, we used a commercially available ultrasound workstation for the MRI-MRI image fusion before and after RFA. Of the 62 lesions in 52 HCC patients, the image fusion failed in only one case due to the significant deformation of the liver and massive ascites after RFA. The success rate of MRI-MRI image fusion was 98.4% (61/62). The total time required for the creation of fusion images was approximately 15 min in 40 all cases. The success rate of the MRI-MRI image fusion was similar to those based on other image fusion methods[21,22].
In the new image fusion system, an AM around the tumor and the performed image fusion after the image registration could be determined. The system automatically and simultaneously displays the ablative zone and AM in the same fusion image. Thus, whether the AM was achieved in all directions could be directly and easily assessed.
Similar to the other image fusion techniques, the accuracy of the MRI-MRI image fusion in this workstation was also dependent on the quality of the MRI volume data. To reduce the influence of liver deformation, the interval between RFA and subsequent collection of follow-up image data should be limited. To enhance the consistency of consecutive MRI localization, respiratory gating and image matching techniques can be adopted to reduce the influence of relative movement of the abdominal organs.
Our study demonstrated that the frequency of LTP in patients without a 5 mm AM was significantly higher than that in those with a 5 mm AM. All LTPs occurred in the section in which the AM was not confirmed. In one case, the fusion image showed that the tumor was not included within the ablation zone after the first RFA intervention, and this case exhibited incomplete ablation. A second RFA session combined with TACE was performed in this patients. In this second session, the tumor was completely ablated, however still, the AM could not be ensured at the 5th district. Therefore, we closely followed the patient and detected LTP in the unachieved AM area 4 mo later. Another session of RFA combined with TACE was performed on the patient. Our results demonstrated that the MRI-MRI image fusion technique could precisely describe the direction of the insufficient ablative zone, and would be helpful for the early detection of LTP.
However, this study had three disadvantages. First, a relatively small sample size and short-term follow-up in the present study may limit its external validity, which may require enrollment of more patients and follow-up extension. Second, the diagnosis of HCC in most cases was made clinically without pathological confirmation; thus, solid validation of relationship between AM and LTP remained inconclusive. Third, our research initially intended to evaluate the feasibility of MRI-MRI image fusion technique for determining AM through a pilot study. Future validation experiment using tissue mimicking phantoms or animals and including conventional side-by-side comparison methods and other image fusion methods are necessary to quantitatively determine AM and further confirm our results.
The present study showed that image fusion using pre- and post-RFA MRI images can evaluate the treatment outcome of RFA in HCC. We also demonstrated that this imaging technique can facilitate illustrating tumor and ablation zone simultaneously, and determining if a 5-mm AM was achieved.
In patients in whom the entire visible tumor was ablated and a 5-mm AM was not established, a closer follow-up schedule is recommended for the early detection of tumor recurrence. Thus, cases in which the optimal intervention window was missed could benefit from the possible salvage treatment.
One of the major impacting factors on the therapeutic effectiveness of ablation for hepatocellular carcinoma (HCC) is local tumor progression (LTP), which is primarily ascribed to insufficient ablative margins (AMs). Therefore, the assessment of AM plays an important role in the radiofrequency ablation (RFA) schedule. Traditional image evaluation methods, including contrast-enhanced ultrasound (CEUS) and side-by-side comparison with computed tomography (CT)/magnetic resonance imaging (MRI) images, cannot quantitatively determine the AM in three dimensions.
Image fusion is a postprocessing technique that is currently used to merge cross-sectional images from different modalities of the same subject location into a single composite image. The current important research aim is to determine how to use image fusion techniques to quantitatively evaluate the ablation margin after RFA of HCC in three dimensions.
Precious applications of 3D CT image fusion and 3DCEUS-CT/MRI image fusion have found that fuse images from CT and MR imaging provide clear view of the spatial relationship between a hepatic tumor and the ablation zone, and could be used to quantitatively determine AM after RFA of HCC in three dimensions. Compared with CT and CEUS, MRI is more sensitive and frequently used in the evaluation of liver lesions and tumor treatment response. This study is the first to report the feasibility of MRI-MRI image fusion using a commercial ultrasound workstation in the assessment of RFA in HCC. The results demonstrated that this image fusion technique could precisely describe the direction of the insufficient ablative zone and would be helpful in the early detection of LTP.
The study results suggest that MRI-MRI image fusion technique can facilitate illustrating tumor and ablation zone simultaneously and help determine whether a 5 mm AM was achieved. In cases in which the entire visible tumor was ablated but a 5 mm AM was not established, a closer follow-up schedule is recommended for the early detection of tumor recurrence. Thus, cases in which the optimal intervention window was missed could benefit from the possible salvage treatment.
Radiofrequency ablation: coagulation induction from all electromagnetic energy sources with frequencies less than 30 MHz. Complete ablation: the tumor was treated according to protocol and completely covered by the ablation zone. Ablative margin: in HCC, the ablation of the appropriate margins beyond the borders of tumor is necessary to achieve complete tumor destruction. The term ablative margin is proposed to describe the 0.5-1.0 cm-wide region that should ideally be ablated in these cases. Local tumor progression: the appearance over follow-up of foci of untreated disease in tumors previously considered to be completely ablated.
This is a good study in which the authors investigated the clinical usefulness of MRI-MRI image fusion method in evaluating the ablative margin in HCC lesions treated by RFA. Sixty-two lesions from 52 patients were investigated in this study. The results are interesting and suggest that this image fusion technique could precisely describe the direction of the insufficient ablative zone and would be helpful in the early detection of LTP.
P- Reviewer: Xia F, Yan LN S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Liu Z, Zhou Y, Zhang P, Qin H. Meta-analysis of the therapeutic effect of hepatectomy versus radiofrequency ablation for the treatment of hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2010;20:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759-768. [PubMed] |
| 4. | Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: long-term results. Eur Radiol. 2001;11:914-921. [PubMed] |
| 5. | Choi D, Lim HK, Kim MJ, Lee SH, Kim SH, Lee WJ, Lim JH, Joh JW, Kim YI. Recurrent hepatocellular carcinoma: percutaneous radiofrequency ablation after hepatectomy. Radiology. 2004;230:135-141. [PubMed] |
| 6. | Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130-1137. [PubMed] |
| 7. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [PubMed] |
| 8. | Solmi L, Nigro G, Roda E. Therapeutic effectiveness of echo-guided percutaneous radiofrequency ablation therapy with a LeVeen needle electrode in hepatocellular carcinoma. World J Gastroenterol. 2006;12:1098-1104. [PubMed] |
| 9. | Hänsler J, Frieser M, Tietz V, Uhlke D, Wissniowski T, Bernatik T, Hothorn T, Hahn EG, Strobel D. Percutaneous radiofrequency ablation of liver tumors using multiple saline-perfused electrodes. J Vasc Interv Radiol. 2007;18:405-410. [PubMed] |
| 10. | Waki K, Aikata H, Katamura Y, Kawaoka T, Takaki S, Hiramatsu A, Takahashi S, Toyota N, Ito K, Chayama K. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol. 2010;25:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [PubMed] |
| 12. | Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, Iwakiri H, Uto H, Kato J, Ido A. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977-981. [PubMed] |
| 13. | Kim SH, Lim HK, Choi D, Lee WJ, Kim SH, Kim MJ, Kim CK, Jeon YH, Lee JM, Rhim H. Percutaneous radiofrequency ablation of hepatocellular carcinoma: effect of histologic grade on therapeutic results. AJR Am J Roentgenol. 2006;186:S327-S333. [PubMed] |
| 14. | Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432-441. [PubMed] |
| 15. | Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253-1262. [PubMed] |
| 16. | Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, Tsuchihashi T, Saigenji K. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480-488. [PubMed] |
| 17. | Liu CH, Arellano RS, Uppot RN, Samir AE, Gervais DA, Mueller PR. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol. 2010;20:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT. Image-guided tumor ablation: standardization of terminology and reporting criteria; Society of Interventional Radiology Technology Assessment Committee; International Working Group on Image-Guided Tumor. Radiology. 2005;235:728-739. [PubMed] |
| 19. | Rhim H, Goldberg SN, Dodd GD, Solbiati L, Lim HK, Tonolini M, Cho OK. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics. 2001;21 Spec No:S17-35; discussion S36-39. [PubMed] |
| 20. | Curley SA. Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol. 2003;10:338-347. [PubMed] |
| 21. | Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (& gt; 2 and & lt; 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 22. | Fujioka C, Horiguchi J, Ishifuro M, Kakizawa H, Kiguchi M, Matsuura N, Hieda M, Tachikake T, Alam F, Furukawa T. A feasibility study: evaluation of radiofrequency ablation therapy to hepatocellular carcinoma using image registration of preoperative and postoperative CT. Acad Radiol. 2006;13:986-994. [PubMed] |
| 23. | Su ZZ, Li K, Zheng RQ, Xu EJ. Zhang T, Zhang AH, Yuan SF, He XQ. A feasibility study for determining ablative margin with 3D-CEUS-CT/MR image fusion after radiofrequency ablation of hepatocellular carcinoma. Ultraschall Med. 2012;33:E250-E255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [PubMed] |
| 25. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [PubMed] |