Published online May 7, 2015. doi: 10.3748/wjg.v21.i17.5242
Peer-review started: November 8, 2014
First decision: December 11, 2014
Revised: December 24, 2014
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: May 7, 2015
Processing time: 185 Days and 17.4 Hours
AIM: To create a new rat model for drug administration, cell transplantation, and endoscopic examination for the treatment of intestinal diseases.
METHODS: F344/NJc l-rnu/rnu rats (10-wk-old males, 350-400 g) were used in this study. The rats were anesthetized via 2% isoflurane inhalation. The rat’s cecum was isolated from the intestines, and a pouch was created. The remainder of the intestines was rejoined to create an anastomosis. The “side-to-side” anastomosis (SSA) technique initially involves the creation of a 2-cm longitudinal incision into each intestinal wall. To create an anastomosis along the ileal and colonic walls, both intestines were cut, and a continuous suture procedure was performed that included all layers of both intestines. The serous membrane was sutured along the edge and on the anterior wall of the anastomosis. The “end-to-end” anastomosis (EEA) technique was compared with the SSA technique. In the EEA technique, the frontal surfaces of both cut intestinal lumens were joined together by continuous sutures. Additional sutures were made at the serosa. After the anastomotic intestine was successfully constructed, the two intestinal lumens that were cut at the isolated cecum were managed. In addition, one luminal side of the pouch remained open to create an artificial anus on the dorsum as a passage for the residual substances in the pouch. Finally, small animal endoscopy was used to observe the inside of the pouch.
RESULTS: In this animal model, mucus and feces are excreted through the reconstructed passage. Accordingly, the cecal pouch mucosa was not obstructed or contaminated by feces, thus facilitating observations of the luminal surface of the intestine. The endoscopic observation of the cecal pouch provided clear visualization given the absence of feces. The membrane surface of the cecum was clearly observed. Two methods of creating an anastomotic intestine, the “SSA” and “EEA” techniques, were compared with regard to animal survival rate, complication rate, and operation time. The SSA technique resulted in a significantly increased survival rate and a lower incidence of complications in rat models compared with the EEA technique. The complications of stenosis and leakage resulted in death in the EEA technique. Thus, the EEA technique exhibited a lower survival rate compared with the SSA technique. However, the SSA technique required a significantly longer operation time compared with the EEA technique.
CONCLUSION: Our new rat model is potentially useful for the development of a novel treatment for intestinal diseases.
Core tip: The most innovative feature of this study involved the creation of a cecal pouch that was isolated from the intestines and rejoined to create an anastomosis and an artificial anus on the rat’s dorsum that consisted of one side of a cut that remained open on the lumen of the cecal pouch. Feces were excreted via the anastomosis. Thus, the pouch mucosa was not contaminated by feces, and the luminal surface remained clean. This feature enables endoscopic observation via the artificial anus. Furthermore, drug administration or cell transplantation into the pouch can be easily and repeatedly performed through the artificial anus, and temporal changes can be observed via endoscopy.
- Citation: Koshino K, Kanai N, Yamato M, Okano T, Yamamoto M. Novel isolated cecal pouch model for endoscopic observation in rats. World J Gastroenterol 2015; 21(17): 5242-5249
- URL: https://www.wjgnet.com/1007-9327/full/v21/i17/5242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i17.5242
Animal models allow researchers to better understand various processes related to human diseases, development, and physiology. In addition, these models provide valuable information leading to the development of human therapies. Therefore, well-developed animal models are crucial to ensure successful experiments[1-7]. In gastroenterological research, trinitrobenzenesulfonic acid (TNBS)-induced colitis is a conventional experimental colitis model that is widely used to understand the pathophysiology of inflammatory bowel disease (IBD)[8]. This animal model was also recently used in stem cell transplantation studies that involve the transplantation of in vitro cultured stem cells into an ulcerative lesion in animal intestines[9]. Typically, drugs and cells are delivered via enema in such studies. However, the use of this technique to deliver drugs and cells to target sites of the luminal surface is difficult and inefficient due to limited visualization and obstruction from the animals’ waste products. Even if drug and cell delivery is achieved, routine excretion is still unavoidable and often hampers drug absorption and cell engraftment. Therefore, it is necessary to develop a novel animal model that can facilitate efficient drug and cell administration in an experimental animal study.
Unlike human surgeries, operations in rat models are typically performed by a single operator under a microscope[10]. Therefore, a clear and stable field of operation, particularly while suturing intestinal tissues, is indispensable to the operator in terms of maintaining a meticulous surgical technique[11]. In the present, study we developed a new rat intestinal model wherein a cecal-isolated intestinal pouch was created to provide an experimental site for the gastrointestinal study. A separate excretory passage was simultaneously created using the rejoined intestine. The use of surgical procedures to rejoin the intestines were proposed and compared.
The animal protocol was designed to minimize pain or discomfort to the animals. The rats were housed and cared for in individual cages. The room was maintained at 22-24 °C and approximately 45% humidity on a 12 h light and dark cycle. Rats were provided with ad libitum access to food and water. The rats were anesthetized with 2% isoflurane inhalation before the procedures.
Animal care was performed according to the protocol approved by the Tokyo Women’s Medical University Animal Experimentation Committee (IACUC protocol number: 14-69). A total of 33 F344/NJc l-rnu/rnu rats (10-wk-old males, 350-400 g) purchased from Charles River Laboratories Japan (Tokyo, Japan) were used for this study. Prior to anesthesia, the animals were injected with a normal saline solution containing 10% penicillin/streptomycin (InvitrogenTM, Life Technologies, 1600 Faraday Avenue Carlsbad, CA 92008 United States) through the superficial dorsal vein of the penis to avoid infection. Subsequently, the rats were anesthetized via 2% isoflurane inhalation. The animals were restrained in the supine position on a micro warm plate (Kitazato Corporation, 81 Nakajima, Fuji, Shizuoka, 416-0907 Japan) set at 37 °C. Their dorsal hair was shaved, and the surgical area was sterilized with 70% ethanol.
A 2.5-cm incision was created on the right side of the rat dorsum. Due to the increased anatomical size of the rats (compared with humans), the cecum and small intestine were easily recognizable after passing through the retroperitoneal layer.
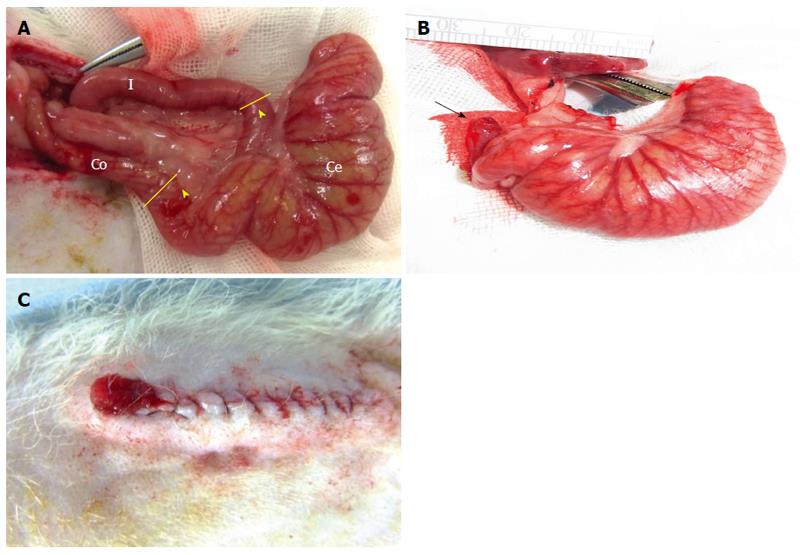
Two incision points were marked 2 cm away from the ileocecal and cecocolonic junctions (Figure 1A). To stabilize and secure an operative field, the posterior wall of the ileum and colon were placed in parallel. Under a surgical microscope, both intestinal walls were then fixed together with five interrupted 7-0 PDS*II (polydioxanone) sutures (Ethicon®, Johnson and Johnson, Medical PTY. Ltd., NSW, United States). The incisions (which were made as previously described) were located to isolate the cecum. Subsequently, the ileum and colon were rejoined to create an anastomotic intestine using each of the two newly proposed techniques, specifically, the “side-to-side” and “end-to-end” anastomosis techniques (see the “Results” section for the comparison of these two methods in detail). After the anastomotic intestine was successfully constructed, the 2 intestinal lumens that were cut at the isolated cecum were managed. The ileal lumen was closed with 7-0 PDS*II sutures, whereas the colonic lumen remained open to create an artificial anus as a passage for residual substances in the pouch. The cecal pouch was subsequently created. Next, the incised retroperitoneum was closed. Finally, an artificial anus was created on the rat dorsum by suturing the intestinal membrane of the colonic lumen, which remained open, to the dorsal skin, which subsequently reversed the mucosa (the inside of the mucosa now faces outwards) (Figure 1B and C).
Cell preparation procedures were performed as previously described[12-17]. The small intestinal epithelial cells were obtained from the C57BL/6-Tg (CAG-EGFP) mice purchased from Charles River Laboratories Japan (Tokyo, Japan). Primary cells were seeded on temperature-responsive culture dishes (35 mm in diameter, UpCell®, CellSeed Inc., Tokyo, Japan). These cells were co-cultured with mouse embryonic fibroblasts (MEF) serving as feeder cells (ReproCELL Inc., Tokyo, Japan) in Advanced Dulbecco’s Modified Eagle Medium/Ham’s F-12 (Life TechnologiesTM) supplemented with N-2 Supplement (Life TechnologiesTM), B-27® Supplement (Life TechnologiesTM), N-acetylcysteine (Sigma-Aldrich®), murine recombinant epidermal growth factor (Life TechnologiesTM), murine recombinant Noggin (PeproTech Inc.), human recombinant R-spondin1 (RD Systems), and Rho-associated protein kinase (ROCK) inhibitor (Y-27632, Sigma-Aldrich®).
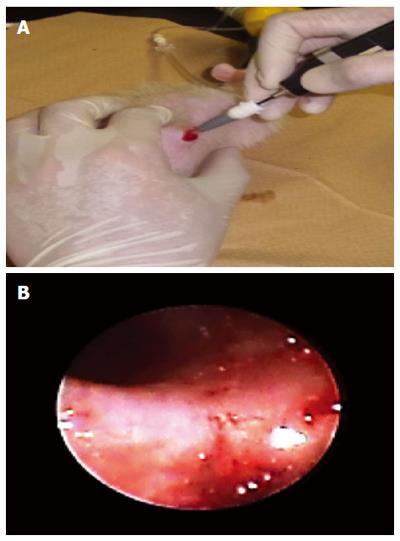
To observe the inside of the cecum, small animal endoscopy was used (AE-F16070, AVS Co., Ltd., Japan) (Figure 2).
The data are presented as the mean ± SD and frequency. A normality test was performed to evaluate sample distribution. The independent sample-t test and Fisher’s exact test were used to compare the groups. A P-value of less than 0.05 (P < 0.05) was considered significant. The statistical methods of this study were reviewed by Dr. Satoshi Iimuro from Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University (TWIns).
A new rat intestinal model was developed wherein the cecal pouch was created to isolate the cecum from an excretory passage. In this animal model, mucus and feces are excreted through the reconstructed passage. Accordingly, the cecal pouch mucosa was not obstructed or contaminated by feces, thereby facilitating observations of the luminal surface of the intestine (Figure 3A). The cecal pouch was clearly visualized via endoscopy given the absence of feces. The membrane surface of the cecum was clearly observed (Figure 3B).
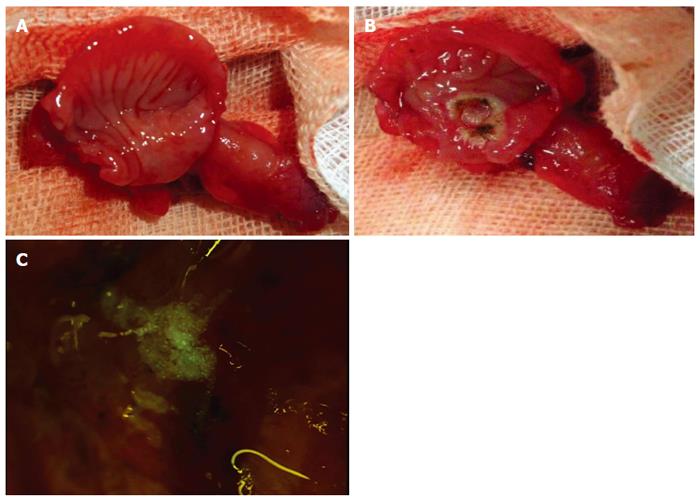
At 1 wk, during post-operative surgical re-entry of the cecal pouch, we confirmed no fecal contamination. Furthermore, we successively created an ulcerative lesion on the cecal pouch membrane (Figure 4A and B). Figure 4C depicts the administration of cultured small intestinal epithelial cells to an artificial ulcerative lesion.
To create an anastomotic intestine, we used and compared two distinct surgical techniques: “side-to-side” and “end-to-end” anastomosis techniques.
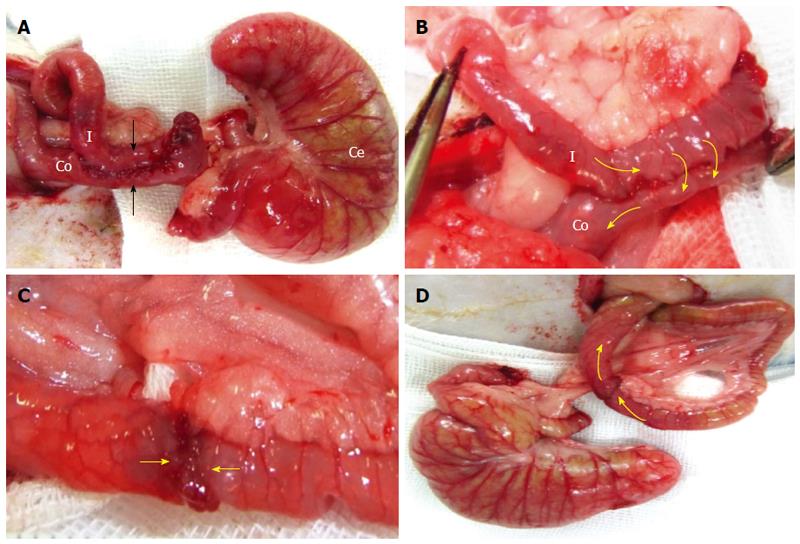
Technically, the “side-to-side” anastomosis (SSA) technique involves the creation of a 2-cm longitudinal incision into each intestinal wall. To create an anastomosis along the ileal and colonic walls, both intestines were cut, and a continuous 7-0 PDS*II suture procedure was performed that included all layers of both intestines. The serous membrane was sutured along the edge and on the anterior wall of the anastomosis (Figure 5A and B).
The EEA technique was performed and compared with the SSA technique. In the EEA technique, the frontal surfaces of both cut intestinal lumens were joined together by continuous 7-0 PDS*II sutures (Figure 5C). Additional sutures were placed at the serosa (Figure 5D).
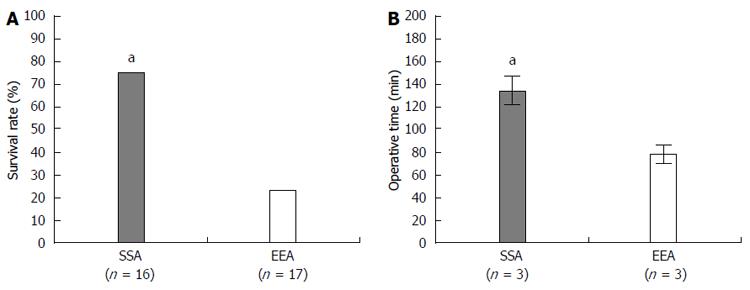
The two methods used to create an anastomotic intestine, the SSA vs EEA techniques, were compared with regard to the animal survival rate, complication rate, and operation time. The follow-up period was 7-10 d after the operation. The SSA technique resulted in a significantly increased survival rate (SSA vs EEA; 75% vs 23%, respectively) (Figure 6A) and exhibited a lower incidence of complications compared with the EEA technique (Table 1). Stenosis and leakage complications resulted in death in the EEA technique. Thus, the EEA technique exhibited a lower survival rate compared with the SSA technique. However, the SSA technique required a significantly longer operation time compared with the EEA technique (Figure 6B).
| Complication | SSA | EEA |
| Stenosis | 0 (0) | 7/13 (41) |
| Leakage | 2/4 (12.5) | 6/13 (36) |
| Local infection | 2/4 (12.5) | 0 (0) |
| Total | 4/16 (25) | 13/17 (77) |
The possibility of contamination and obstruction of the intestinal tract with animal waste products cannot be eliminated; thus, it would be difficult to interpret the obtained results regarding drug administration and cell transplantation into the intestinal lumen in a conventional bowel disease model. To overcome these problems, in the present study, we developed a new rat intestinal model by creating a cecal pouch that is not exposed to fecal contamination. Furthermore, an excretory orifice was artificially created on the rat dorsum for the excretion of waste product from the cecal pouch.
By preventing fecal contamination inside of the pouch, it is easy to perform intestinal observations and various experimental tasks. Therefore, we attempted to create an ulcerative lesion on the surface of the pouch membrane and transfer cultured cells to a lesion site; these experiments were performed with ease. Our results suggested that this novel isolated cecal pouch model is applicable to several types of animal experiments related to gastrointestinal studies.
In several animal studies, experimental animals must be sacrificed in an attempt to observe temporal changes[1,7,9,18-21]. Because the rat excretory passage is separated from the cecal pouch, the present animal model provided good visualization internally and of the luminal surfaces of the cecal pouch via endoscopy (Figure 3B). Using a small animal endoscope, the luminal surfaces of the pouch are easily observed over time via a non-invasive procedure, and repeated examinations can be performed as often as necessary in the living animal.
A stable and secured field of operation is a critical factor for a precise operation. However, in small animal operations, a microscope is often used[3], and the operation is routinely performed by a single operator. In the present study, we introduced a primary suture at the posterior wall of the each intestine to maintain the position of the operative site. This additional suturing eased intestinal anastomosis.
Compared with the EEA technique, the SSA technique offered enhanced stabilization and a wider diameter of the anastomotic intestine. This difference likely accounts for the increased survival rate and reduced morbidity observed in the SSA group. When performing the EEA method, the tissues moved easily, which complicated the suturing procedure. Consequently, the unstable suture technique caused stenosis and leakage at the anastomosed area that resulted in mortality, as indicated by the low survival numbers. Therefore, in our model, the SSA technique successfully extended animal. Although the SSA technique was more time-consuming than the EEA technique due to a complex suturing procedure, the increased time did not reduce the survival rates in the SSA group, which was most likely due to the stress-reducing protocol used during surgery. A micro warm plate and bipolar electrocoagulation were used, resulting in reduced stress and a faster operation. One week after the operation, the animals had recovered; they were stabilized and ready for use in further experiments.
In conclusion, researchers can use our new intestinal rat model with various approaches. The manipulation of drugs or cells into the isolated cecal pouch was performed via an artificial anus without any fecal contamination. Additionally, an endoscope can be employed to conveniently observe the intestinal membrane and administer drugs or cells.
This animal model enables reproducibility with regard to observing the pathological changes and enabling the time-dependent effects of the interventions on the intestinal tract. This reproducibility will be useful in understanding the morbid state of bowel disease and will help researchers to perform successful future studies of gastrointestinal diseases.
The authors thank Dr. Shinichiro Kobayashi (Department of Surgery, Nagasaki University, Graduate School of Biomedical Sciences, Nagasaki, Japan) for his valuable advice and suggestions and Dr. Supreda Suphanantachat (Department of Periodontology, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand) for English editing. This study was partially supported by the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems “Cell Sheet Tissue Engineering Center (CSTEC)” and the Global COE program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
The models of inflammatory bowel diseases (IBD) have been established in the animal intestines. Trinitrobenzenesulfonic acid-induced colitis is a conventional experimental colitis model that is widely used to understand the pathophysiology of them. These models were also recently used in stem cell transplantation studies that involve the transplantation of in vitro cultured stem cells into an ulcerative lesion in animal intestines.
In these researches, the method of delivery system is done by means of injecting drug or cell per anal. However, a limited visualization and obstruction inside the luminal surface caused by animals’ feces hamper an effectiveness of conventional drug or cell delivery system. Therefore reliable models are required for experimental colitis research.
In this study, a new rat model was created. The rats’ cecum were isolated to create the cecal pouch. The intestines were reconstructed in order to avoid a contamination by feces. These newly proposed methods allowed a fecal-free internal surface of a cecal pouch, thus, feces are not interfered with drug absorption and cell engraftment. At the same time, a newly created anastomotic intestines and artificial anus enabled the defecation of animals’ waste product.
The present rats’ cecal pouch model allowed the researchers to apply transplanted cells or drugs to an intestinal target site directly and easily. As one side of the lumen of this pouch was used as an artificial anus located at the rats’ dorsum, this facilitated an application of the small animal’s endoscope in observing a luminal surface of a cecal pouch via an artificial anus.
The IBD is caused by an abnormality of the bowel immune system. The failure of restoration of gastrointestinal mucosa and bowel barrier system is caused by chronic inflammation. Ulcerative colitis and Crohn’s disease are representative of the IBDs.
In this paper, a new suturing technique of the animal operation was proposed. This technique helped reducing complications that associated with an anastomosis. A creation of this novel isolated intestinal pouch used for a transplantation site is of which our original idea. This animal model could be applied for the study related to the bowel disease models and stem cell transplantation.
P- Reviewer: Zhu YL S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Sueyoshi R, Woods Ignatoski KM, Okawada M, Teitelbaum DH. Distraction-induced intestinal growth: the role of mechanotransduction mechanisms in a mouse model of short bowel syndrome. Tissue Eng Part A. 2014;20:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ueno A, Lazaro R, Wang PY, Higashiyama R, Machida K, Tsukamoto H. Mouse intragastric infusion (iG) model. Nat Protoc. 2012;7:771-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Wang L, Jiang Y, Lao J, Zhao X. Contralateral C7 transfer to lower trunk via the prespinal route in the repair of brachial plexus injury: an experimental study in rats. J Plast Reconstr Aesthet Surg. 2014;67:1282-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-1367. [PubMed] |
| 5. | Boismenu R, Chen Y. Insights from mouse models of colitis. J Leukoc Biol. 2000;67:267-278. [PubMed] |
| 6. | Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3101] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 7. | Adachi T, Hinoi T, Sasaki Y, Niitsu H, Saito Y, Miguchi M, Shimomura M, Ohdan H. Colonoscopy as a tool for evaluating colorectal tumor development in a mouse model. Int J Colorectal Dis. 2014;29:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Terai T, Osawa S, Tani S, Oishi S, Arai Y, Yamada T, Sugimoto M, Furuta T, Kanaoka S, Miyajima H. Induction of murine TNBS colitis is strictly controlled by a modified method using continuous inhalation anesthesia with sevoflurane. Dig Dis Sci. 2014;59:1415-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 620] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 10. | Komen N, van der Wal HC, Ditzel M, Kleinrensink GJ, Jeekel H, Lange JF. Colorectal anastomotic leakage: a new experimental model. J Surg Res. 2009;155:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Goulder F. Bowel anastomoses: The theory, the practice and the evidence base. World J Gastrointest Surg. 2012;4:208-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 12. | Sato T, Clevers H. Primary mouse small intestinal epithelial cell cultures. Methods Mol Biol. 2013;945:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2253] [Cited by in RCA: 2725] [Article Influence: 194.6] [Reference Citation Analysis (0)] |
| 14. | Jung P, Sato T, Merlos-Suárez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 546] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 15. | Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G455-G460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4394] [Cited by in RCA: 5174] [Article Influence: 323.4] [Reference Citation Analysis (0)] |
| 17. | Booth C, Patel S, Bennion GR, Potten CS. The isolation and culture of adult mouse colonic epithelium. Epithelial Cell Biol. 1995;4:76-86. [PubMed] |
| 18. | Nordentoft T, Sørensen M. Leakage of colon anastomoses: development of an experimental model in pigs. Eur Surg Res. 2007;39:14-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Sasaki R, Watanabe Y, Yamato M, Aoki S, Okano T, Ando T. Surgical anatomy of the swine face. Lab Anim. 2010;44:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Johnson RL, Fleet JC. Animal models of colorectal cancer. Cancer Metastasis Rev. 2013;32:39-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |