CAPSULE ENDOSCOPY
The swallowable-capsule concept first appeared in 1957 in Jacobson and Mackay’s[1] groundbreaking paper on radiofrequency (RF) transmission of temperature and pressure from within the human body[2]. Nowadays, capsule endoscopy (CE) is the prime mode of non-invasive and discomfort-free endoscopic exploration of the small-bowel[3,4]. It became available to clinicians at the dawn of the millennium[5]. The fact that its realization was the brainchild of Garviel Iddan D.Sc., an Israeli electro-optical engineer - initially working at the RAFAEL Armament Development Authority on guided missile technology, is to an extent multi-semantic. Parallelly, Professor Paul Swain, a British gastroenterologist, led a research group in London and published the first conceptual studies on a wireless capsule in 1994[6,7]. Since then, a great number of clinical and scientific papers have studied the clinical use of this fascinating new technology[8,9] in the investigation of small-bowel diseases. Nowadays, capsule platforms are also available for the non-invasive exploration of the oesophagus and the colon[10,11]. The full spectrum of the commercial capsule models is available elsewhere and remains beyond the scope of this editorial[8,12].
Currently, the major problem of all existing commercial capsule devices is the lack of control of movement; the latter is achieved by the peristaltic contractions of the small bowel. Therefore, as bowel peristalsis is a complex event of five contractile patterns, i.e., peristaltic waves, stationary contractions, clusters of contractions (Phase III), giant contractions and anti-peristalsis waves[13], its impact on capsule locomotion (speed, position and orientation) is unpredictable[14]. Consequently, the first significant step for a forward leap in wireless technology is achieving sustainable, active movement and control of the device’s locomotion[4,15]. However, when extrapolating from the advancements made in conventional (flexible) endoscopy since its introduction -more than 60 years ago- in regular clinical practice, the prospect for wireless devices should be considered as anything but optimistic. Manual push, perhaps the most primitive actuation method, is still used as the mainstream advancement option of conventional/flexible endoscopes[15]. Hence, embarrassment, discomfort and/or frank pain during the advancement phase of a flexible endoscope is the major drawback of this technique[15,16].
Nevertheless, the absence of wire in capsule-like endoscopy systems requires drastic measures and has pushed research forward in a more radical manner. Sliker and Ciuti[15] argue that in the next few years, an integrated, magnetically actuated, automated locomotion system will be available for regular clinical use. Furthermore, possibly with an interface application, the clinician will be able to stop and direct the device into points of interest for detailed inspection/diagnosis, and therapy delivery. Hence, passive locomotion will be replaced by externally (magnetically) controlled actuation or a combination of a miniaturization, new battery type or complete battery elimination[17]. Especially the battery-less and/or miniaturization version will eliminate any concerns about the chemicals in the batteries, can produce potential hazards and contaminate the environment when the capsules are improperly disposed of[18,19].
One of the major problems of the capsule platform for digestive endoscopy, especially when there is no locomotion control is the adoption of the single-pole lens model; however, integrating multiple cameras has still problems with size and power consumption[20]. Therefore, some ideas around this have led to few experimental developmental platforms that, at this point, should receive particular mention.
NORIKA AND SAYAKA
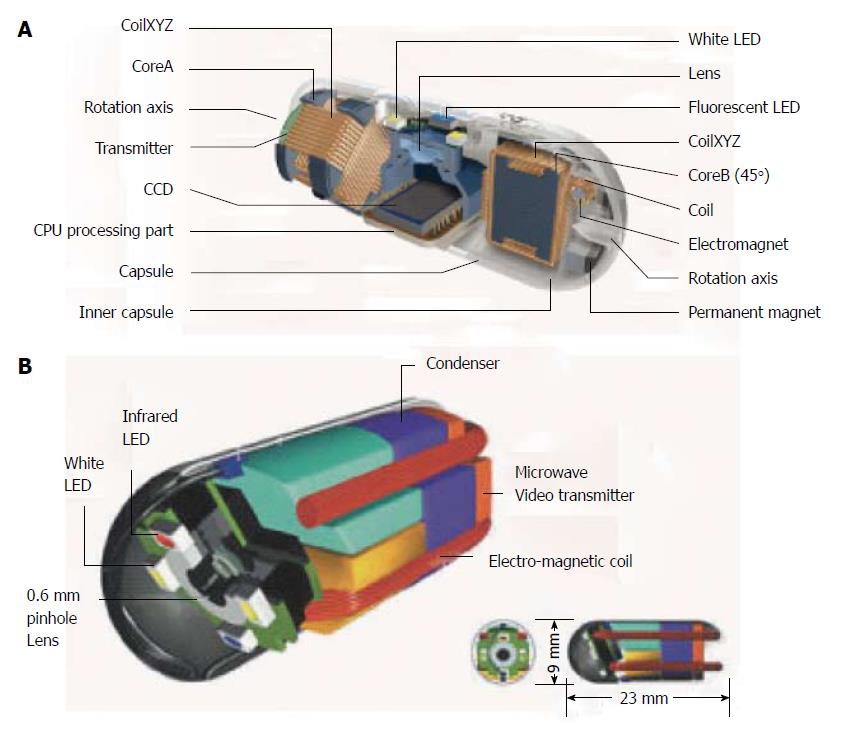
RF SYSTEM lab. (RF Co., Ltd., Nagano, Japan) was established in 1993 in Nagano. The deep-sea submarine-like capsule, named NORIKA 3 (Figure 1A), was developed in 1997 when RF Co. joined the Japanese Experimental Module Project[21]; the latter was a venture operated by the National Space Development Agency of Japan to monitor the growth of plants in a manned spacecraft[21]. SAYAKA, the first “battery-less capsule”, superseding the basic technology of NORIKA 3, was announced to the international market on December 2001; however, it remains still under development (Figure 1B)[22]. The dimension of a capsule camera is 9 mm × 23 mm. A 0.6 mm color lens and a 410000-pixel charge-coupled device camera can obtain up to 30 frames per sec (fps), as long as the patient is wearing a vest that transmits microwaves to the capsule[23]. Two tanks with valves are positioned in the center and a there is a capacitor to store electric power and a microwave video signal transmitter[22,23]. Around the camera lens, four white LEDs and magnetic coils for focus adjustment are placed[23]. More importantly, 40% of the volume is free space, which can be used for surgical purposes such as medication spray, laser treatment, pH sensing and more[24]. Mosaicing technology was developed for use with the experimental model Norika 3[25]. Mosaicing is a process by which the final image on the computer display is made by combining multiple images taken from various angles[23,25]. However, no human studies have been conducted with this capsule, which has been described on the web for almost a decade now[12].
Figure 1 NORIKA and SAYAKA capsules.
A: Sayaka capsule; B: Norika capsule, with permission.
SELF-STABILIZING CAPSULE ENDOSCOPE
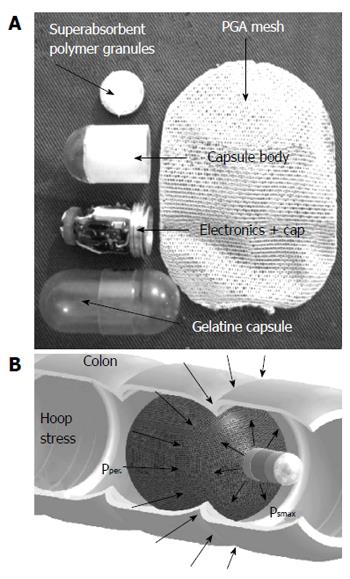
An innovative method of imaging of the GI tract by using a self-stabilizing capsule endoscope (SsCE) was first proposed in 2006 (Figure 2A)[26]. SsCE allows the use of a single-pole imager devise for visualizing the wider diameter segments of the digestive tract such as the colon without tumbling and with the ability to passively distend colon walls[27,28]. The capsule coating is designed to dissolve in the colon, which exposes a semipermeable, expandable container attached to the back of the capsule endoscope, while simultaneously turning on the camera (Figure 2B)[27,28]. In a recent experimental study with live canine models, 4 mongrel dogs underwent laparotomy and the implantation of 5-8 suture markers to approximate colon lesions. Each dog had both single-dome CE and ScCE in random order. The average percentages of the marker detection rate for unmodified capsule endoscopy, self-stabilizing capsule endoscopy, and colonoscopy, respectively, were 31.1%, 86%, and 100% (P < 0.01), with both self-stabilizing capsule endoscopy and colonoscopy performing significantly better than the unmodified capsule endoscopy[28].
Figure 2 Self-stabilising capsule endoscope.
A: Components; B: Impression of movement while in the colon, with permission.
ODOCAPSULE
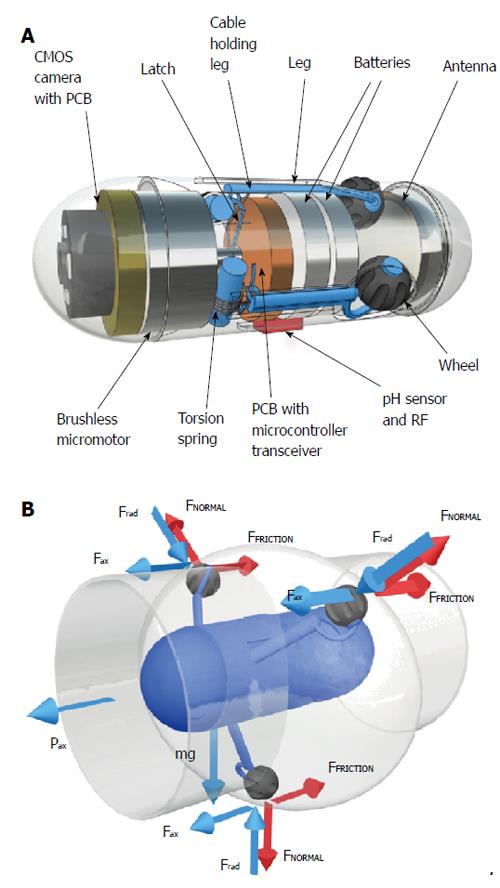
Odocapsule, a capsule device to achieve accurate localization of small-bowel lesions and endoscopic video stabilization in capsule endoscopy was proposed in 2010[29] (Figure 3A). It is equipped with 3 miniature legs, each carrying a wheel. These legs are extendable and retractable thanks to a micro-motor and three custom-made torsion springs[30,31]. The wheels are specifically designed to function as micro-odometers by registering each rotation they perform. As the Odocapsule traverses through the small bowel, the wheels turn and their rotations are translated to distance covered by the device from the point of duodenal entry to each area/point of interest. As the legs are expandable in a tripod formation, they allow the device to stabilize itself without obstructing its locomotion and thus offering smooth video capture without missing any pathologies (Figure 3B).
Figure 3 Odocapsule.
A: Componets; B: Impression of movement inside the bowel, with permission.
This editorial does not aim to be exhaustive or indeed cover areas presented in details in previous excellent reviews[2,14,15,17,22]. Its main aim is to present the authors’ views on interesting current projects in CE and the vision for potential convergence/integration of various diagnostic/therapeutic platforms.
DRUG DELIVERY CAPSULE PLATFORMS
Coagulation capsule
Various chitosan- and mineral-based haemostatic granules or powders are used for the control of compressible, external haemorrhage in combat casualties and are incorporated in first-aid kits used by the military[32]. Hemospray (TC-325) is a novel hemostatic agent (COOK Medical, Winston-Salem, NC), CE-marked for use in the endoscopic treatment of high-risk, non-variceal upper gastrointestinal (GI) bleeding in Europe and Canada. It is a proprietary inorganic mineral, absorbent powder that has no known allergens and rapidly concentrates clotting factors at the target site, thereby forming an adherent coagulum[32,33]. Currently, its hand-held container - consisting a pressurized CO2 canister, a through-the-scope delivery catheter, and a reservoir for the 21-g powder cartridge- is operated push button in 1- to 2-s bursts[32]. A prototype coagulation capsule (CoCap) has been built and tested which employs an exothermic chemical reaction to generate heat using the interaction of calcium oxide and water[34]. Professor Swain[35] pointed out that it is possible for other thermal/therapeutic applications to be added in the future. Recently, the feasibility of a novel method of controlled colonic insufflation via an untethered capsule in vivo was demonstrated (Figure 4)[36]. It is truly almost self-intuitive to combine the capabilities of various capsule designs (powder carrier, insufflator and imaging/controllability) in one CoCap for use in cases of obscure GI bleeding. These authors believe that the aforementioned ideas will be transformative on how the deal with OGIB in 2020 and allow CE to claim, once more, the role of “disruptive” technology in the medical field. Indeed, there are few capsule products nowadays that can serve as medication carriers to sites in the small-bowel.
Figure 4 Capsule that provides insufflation, with permission.
Enterion
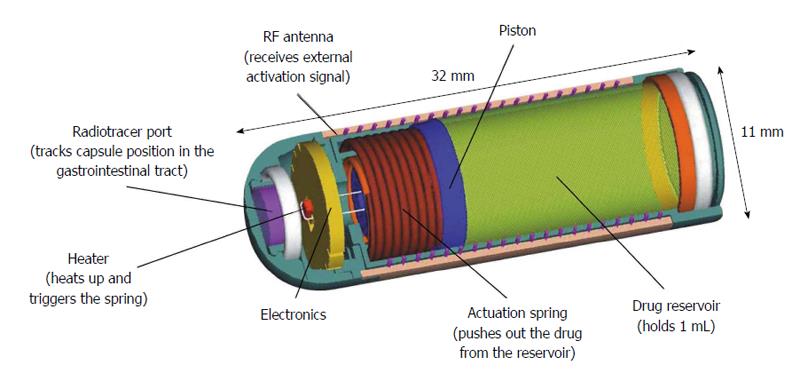
The Enterion capsule was developed by Phaeton Research, Nottingham, United Kingdom (in association with the Nottingham University spin-off Pharmaceutical Profiles) for targeted delivery of a wide range of different drug formulations into any region of the gut[37,38]. It is a 32-mm long capsule device that contains a drug reservoir with a volume capacity of approximately 1 mL (Figure 5). Enterion has the capability to deliver, through a 9-mm opening, any type of drug formulation (including dry powder, semisolid, suspension or solution formulations) when the spring is released, forcing a piston to move along the shaft of the capsule, thus, emptying the reservoir to specific locations of the GI tract[2,39]. A radioactive marker is placed inside a separate sealed tracer port to allow real-time visualization of the capsule location using gamma scintigraphy[37]. The movement of the piston also operates a switch, which transmits a weak radio signal at a precise frequency. Detection of this signal externally confirms that the capsule has opened successfully[37,38]. The piston motion is stopped near the end of the capsule, which maintains a seal and prevents contact of the internal electronic components with the GI fluids.
Figure 5 Enterion drug delivery capsule, with permission.
InteliSite® capsule
The InteliSite capsule (Innovative Devices, LLC, Raleigh, North Carolina, USA) is a 10 mm × 35 mm, radiofrequency activated, non-disintegrating drug delivery device[37]. The use of radiomarkers (gamma scintigraphy) allows determination of the capsule location[2,37]. The capsule packaging consists of inner and outer sleeves, which have portholes; when the holes align under a magnetic field straighten shape-memory alloy wires, there is release of the drugs[2,37]. To date, limited information is available in the literature on the use of the capsule[2,37].
INTELLICAP SYSTEM
Philips Research in Eindhoven, Netherlands, has developed a prototype called intelliCap[2,37,38]. IntelliCap has a drug reservoir, a drug pump, a pH sensor, a wireless transceiver, and a microcontroller. The combination allows us to measure the local pH, report the data in real time, and have full control over drug delivery, based on a combination of data such as time, pH, or operator intervention[2,37,38].
MICROROBOT FOR TARGETED DRUG DELIVERY
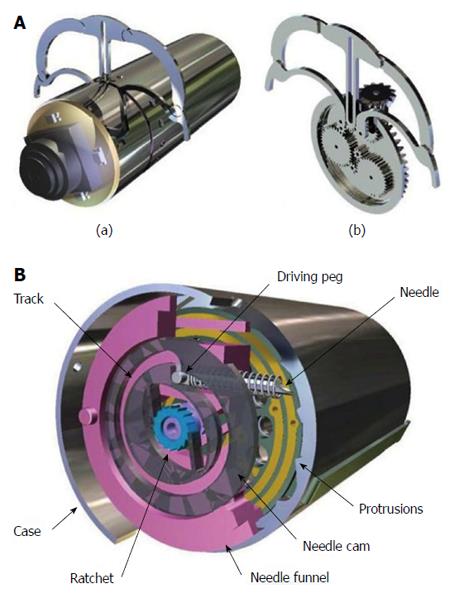
With a stabilizing/holding mechanism that resembles daVinci-like drawings, the micro-robot platform developed by Woods and Constandinou[40,41], allows targeted drug delivery in the next-generation wireless CE. The micro-positioning mechanism allows a needle to be positioned within a 22.5° segment of a cylindrical capsule and be extendible by up to 1.5 mm outside the capsule body (Figure 6B). This is controlled by a single micro-motor and occupying a total volume of just 200 mm3. The holding mechanism is depicted in Figure 6A. Perhaps adrenaline injection directly into a bleeding small bowel angioectasia is not a bream after all.
Figure 6 The legged,anchoring capsule for Stabilization of positioning (A) and Drug delivery, with permission (B).
Another micro-robot that could be used for drug or other therapy delivery employs actuated legs with compliant feet lined with micro-pillar adhesives - inspired by gecko and beetle foot hairs - to be pressed into the intestine wall to anchor the device at a fixed location[41]. The investigators envisage the use of this capsule as second-line procedure, “after images of the conventional capsule examination are analyzed by a clinician to map areas of interest in the intestine and identify any problematic regions that may require a closer look”[41].
MAGNETICALLY ACTUATED SOFT CAPSULE ENDOSCOPE
A magnetically actuated soft capsule endoscope (MASCE) has been developed by Yim and Sitti[42]. Yim and Sitti[43] design and rolling locomotion of a magnetically actuated soft capsule endoscope, for diagnostic and therapeutic medical applications in the stomach. It has a characteristic ability to deform both passively and actively. Passive deformation is possible because its cover is made from elastomer-based compliant structures. This prevents any possible tissue damage during swallowing and/or the peristaltic movement. Active deformation is possible on the axial direction by external magnetic actuation. This provides an extra degree of freedom that enables various advanced functions such as axial position control, drug releasing, drug injection, or biopsy. For example in the developed prototype the axial magnetic attraction compresses a drug chamber between two internal magnets so as to release a drug through holes at a critical pressure.
Furthermore, the magnetic actuation enables the soft capsule endoscope to roll on the surface of the stomach. Advantages of the rolling ability include smooth and continuous locomotion and stable steering because the capsule is always anchored to the tissue surface while moving, it allows improved tissue surface diagnosis and tissue targeting capability for therapeutic procedures, and it provides a more complete view of the 3-D stomach tissue wall; thus reducing the possibility of missing problematic tissue areas of the stomach wall.
EUROPEAN PROJECTS
Nemo
A consortium funded by EU 6th Framework Programme (FP) during 2006-2010, worked on NEMO (Nano based capsule-Endoscopy with Molecular Imaging and Optical biopsy) project[44] with main aim to develop an advance cancer screening method friendly enough to significantly increase compliance, simplify the diagnostic pathway and increase the sensitivity and specificity of early detection[35,45]. The concept of the NEMO approach was to combine capsule endoscopy with nano-based molecular recognition that would highlight cancerous and precancerous lesions in the GI tract, thus considerably increasing the accuracy and ease of diagnosis[46]. The consortium aimed to merge few technological platforms: nanotechnology for targeting and marking possible lesion; capsule endoscopy for detecting the marked disorder; capsule manoeuvring technologies to move the autonomous NEMO capsule backwards and forwards in the gastrointestinal tract as well as miniaturization and low power technologies[20].
A major task of the project was to develop nanocontainers, labeled with targeting agents and filled with dyeing material. The administered nanocontainers will be tailored to react with the target and mark the intestinal lesion. Another task was to develop a capsule based on narrow band imaging, combining specific optical filters with light emitting diodes (LEDs). The overall clinical outcomes from the NEMO project are summarized[43].
Versatile Endoscopy Capsule for gastrointestinal TumOr Recognitions and therapy
Versatile Endoscopy Capsule for gastrointestinal TumOr Recognitions and therapy (VECTOR)[47] was another project funded by EU FP6 during 2006-2010, aiming to develop a miniaturized robotic wireless endoscope for both diagnosis and therapy in the human digestive tract, with particular focus on the diagnosis and treatment of gastrointestinal cancer and its precursors[48]. VECTOR delivered different versions of prototype capsule endoscopes, for different clinical needs. An impressive prototype was a tele-operated robotic capsule with eight legs, capable of walking within the colon at a speed of about 3 cm per minute against peristalsis[49]. Main limitation of this capsule was the difficulty to include a power source, because most of the space within the capsule was covered by the electro-mechanic components used for moving the robotic legs. The fabrication of such a robotic spider-like capsule is described in a meso-scale engineering case study[50].
Another prototype included a submersible capsule prototype equipped with four propellers for locomotion. This capsule was intended mainly for stomach inspection. Reliable locomotion and steering within the stomach was possible after ingestion of clear water[51]. A wirelessly powered version of this prototype, based on wireless energy harvesting techniques, was later proposed for longer lasting operation. Another alternative investigated for in-vivo capsule navigation was based on externally applied magnetic fields. The magnetic field was generated by a permanent magnet attached to a robotic arm operated above the body[52]. In the context of VECTOR project magnetic control was also applied in the context of therapeutic capsules, designed to apply a customized haemostatic endoscopic clip (OTSC® clip, Ovesco Endoscopy AG, Germany) to the bowel wall[53].
TROY
Another project funded by EU FP6 during 2006-2009 was TROY (endoscope capsule using ultrasound technology)[54]. Its objective was to prove the concept of a diagnostic system for prevention and early warning of superficial cancer and pre-malignant precursor lesions in gastrointestinal tract using ultrasound technology. The outcome of the project was a WCE platform that instead of a camera with an optical imaging sensor, uses miniaturized ultrasound probes to create 3D computer generated images. The validation of this platform was performed with a synthetic bowel simulator with realistic tissue response.
SUPCAM
The SUPCAM project[55] was funded by EU FP7 during 2012-2014 for the development of a spherical endoscopic capsule which can be safely - and accurately - guided along the colonic lumen from the outside, through an electromagnet, in completely wireless mode[56]. Being designed for the evaluation of the colon, this capsule is not for ingestion, but to be administered as a suppository (Figure 7). The endoscopic device will have the appearance of a small (about 2 cm in diameter) spherical capsule (there are in fact 2 layers, the inner sphere and the outer sphere) with a compact external control system, easily manageable by the medical operator in order to support him in the diagnosis of colon rectal diseases[57]. The external device will be compact and adapted to be transported and made suitable the room of the majority of common outpatient settings, similarly to an ultrasound scanner. So far, this new system has been presented in international scientific meetings and there is data on the effectiveness of the magnetic control system with laboratory simulations[57] as well as with a phantom colon model[58]. To the best of our knowledge, no data on animal models are available yet.
Figure 7 SupCam with (A) and without (B) transparent shell,courtesy of Dr A Tozzi.
OTHER CAPSULE ENDOSCOPE DESIGNS
Mermaid capsule
A magnetically propelled fish-inspired capsule endoscope has been proposed as another navigable solution[59]. This capsule is called Mini Mermaid it has a size of 12 mm × 45 mm and a flexible silicone fin at its back with a small magnet attached at its end. It can change velocity and direction by adjusting the waveform of the electric current running in magnetic coils controlling the motion of the fin. Its velocity can reach dozens of cm per second, while its battery can last for eight to 10 h. It has been applied safely for the examination of the whole digestive tract of a human, after providing him with doses of polyethylene glycol and water.
Multiple cameras endoscopy capsule
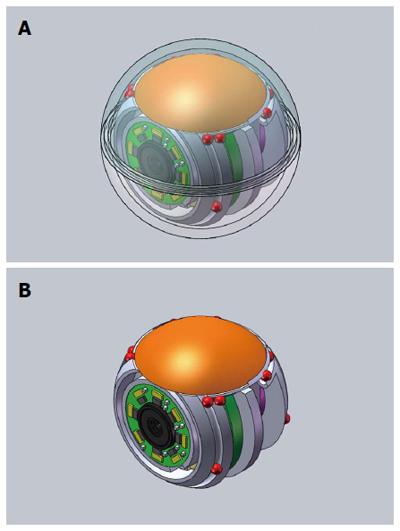
In order to maximize the diagnostic yield of CE, Gu et al[60] proposed recently an ingenious spherical device - called the MicroBall - with multiple (six) cameras (Figure 8). Although this platform allows no controllability of the capsule movement, it promises a more accurate revision of the GI tract and reduction of the false negatives by multiple cameras with ‘‘smart’’ image capture strategy, employing not only movements sensitive control but camera selection as well.
Figure 8 Spherical capsule endoscopy with multiple cameras.
Robotic biopsy
The concept of a robotic biopsy device was certainly influenced by the Crosby capsule. To date, the proposed devices consist of modules for the complete process of biopsy, which includes monitoring the intestinal wall by a tissue monitoring module, aligning onto a polyp by an anchor, and sampling of the polyp tissue by a biopsy module. The latter is constructed to remember the guillotine opening of the Crosby capsule and its control/actuation -in the prototypes and conceptual models- is left to the control of the physician[14,15,35,61].
Motility tracking system
Motility Tracking System (MTS) is swallowable, small magnetic pill (6 mm × 18 mm, density 1.8 g/cm3) coated with a plastic capsule[62]. The small electronic pill, when ingested by the patient, emits a signal during its entire transit through the GI tract. Its movements are tracked by a detector plate of 16 magnetic field sensors (4 × 4) with a frequency of 10 Hz. Data from each sensor with an iterative algorithm are used to calculate the position and orientation of the magnetic pill[62,63]. The first generation, radiation-free pill (MTS-1), has been introduced for description of gastrointestinal motility in adults. The current version, Motility Tracking System-2 (MTS2), is also known as 3D-Transit (Figure 9) and is able to track multiple pills simultaneously[63]. This signal is recorded and subsequently downloaded to a data management station.
Figure 9 3D-Transit" by Motilis Medica SA, Lausanne, Switzerland; courtesy of Mr Vincent Schlageter.
Wireless motility/pH capsule - SmartPill
The wireless motility/pH capsule (WMC or SmartPill) is data recording device that enables the simultaneous assessment of regional and whole gut transit. It has been approved by the FDA for the evaluation of patients with suspected gastroparesis and chronic idiopathic constipation[64,65]. SmartPill measures the temperature, pH, and pressure while traveling through the GI until exiting the body through the anus. Validated patterns in pH and temperature recordings allow for accurate measurement of gastric emptying, small bowel transit, colonic transit, and whole gut transit times[66]. Clinical trials showed it to be a suitable alternative to scintigraphy and radiopaque marker studies in measuring gastric emptying, small bowel, colonic, and whole gut transit times[65,67]. The WMC should be considered the transit study of choice for individuals suspected of having altered transit in more than one region of the gastrointestinal tract.
Lab-in-a-pill
This capsule has resulted by a collaboration between the Universities of Glasgow, Edinburgh and Strathclyde, and the Institute for System Level Integration in Scotland[2,37]. The capsule device, called Lab-on-a-Pill, measures 16 mm × 55 mm and weighs 13.5 g[37,68], with temperature, pH, conductivity, and dissolved oxygen sensors (Figure 10). The initial aim is to develop new tools to help the Government in its objective for screening colon cancers in all people aged over 50 years. The eventual device will allow wireless detection of markers for bowel cancer, a disease that is common in many countries. However, there have been no trials carried out using this capsule with the exception of artificial gut trials[37].
Figure 10 Lab-in-a-Pill, with permission.
Tethered capsule endomicroscopy
The system developed by Gora et al[69,70] involves a capsule containing optical frequency domain imaging (OFDI) technology - a rapidly rotating laser tip emitting a beam of near-infrared light - and sensors that record light reflected back from the oesophageal mucosa. The capsule is attached to a string-like tether that connects to the imaging console and allows a physician or other health professional to control the system[71]. Following ingestion, the capsule is carried down the oesophagus by natural peristalsis. OFDI images are taken throughout the capsule’s transit down and up the esophagus.
Check-Cap
Check-Cap (Check-Cap, Mount Carmel, Israel) is a new technology in development for colon imaging[72]. It is proposed that tiny X-ray Radar device (the exposure to radiation is described as minimal, i.e., total dose equivalent to a single plain abdominal radiograph) creates a 3-D reconstructed image of the colon[73]. The major advantage of this platform is that no prior colon cleansing is needed, although the patient is still required to drink an oral contrast solution to label faecal material[73,74]. Eventually, the capsule transmits data to a wrist-worn recorder[72]. It is understood that the clinical performance of Check-Cap device is under investigation.
Sonopill
A £5-million grant by the Engineering and Physical Sciences Research Council went to Sonopill, an ambitious Dundee University-led project aim to incorporate diagnostic and therapeutic ultrasound along with optical imaging, all deployed in capsule format[75]. It is aspired that this will complemented by other sensors, e.g., for pH measurement and auto-fluorescence imaging for multimodal diagnosis[76]. In the longer term, the investigators aim to produce a family of diagnostic and therapeutic capsules, with the patient pathway defined according to diagnostic information assembled. Essentially, the Sonopill aims to push that technology further by proving in practice the complementary role of ultrasound and visual imaging with studies of multimodal diagnosis and therapy.
Endoscopic capsule robots using reconfigurable modular assembly
In order to enhance the locomotion capabilities and the functionalities of current capsule endoscopy platforms, Yoo et al[77] proposed a modular system of robotic capsules containing steerable locomotive elements that are capable of assembling into a larger and more complex robot via mutual docking. Docking is based on permanent magnets for energy efficiency purposes. Such robots could provide abilities that are lacking from current capsule endoscopes. A prototype was developed to demonstrate how a camera-enabled wireless capsule endoscope can be enhanced with navigation capabilities by docking on it three capsules with angular and rotational servos acting like legs. Such a modular robot can be used for thorough inspection of regions of the GI tract. Issues to be addressed before such a capsule is applicable in clinical practice, include the use of biocompatible polymer materials for enhanced friction in the slippery environment of the GI tract, energy conservation, e.g., using wireless power transmission, water proofing, and miniaturization.
Inchworm-like micro-robot
A prototype miniaturized navigational capsule endoscope capable of sliding like an inchworm was developed by Kim et al[78], and validated in vitro. The capsule is equipped with four actuators and clampers that mimic insect claws[79]. It clamps the contact surface and the body moves forward during the contraction of a rear linear actuator. The clamper releases the contact surface and slides forward as the front linear actuator is contracting. In this spirit, another locomotion mechanism inspired by earthworms, is based on repeated elongations and retractions of a clamping device[80].
NANOTECHNOLOGY
Microrobots are 10 μm - 1 mm scale robots offering great new potential in medicine and nanotechnology is defined as any process performed at nanoscale. Electronics are constantly miniaturized and increase performance. Inevitably capsule endoscopy devices will follow the same trend as they did in the past leading to integration of multiple sensors (i.e., temperature, pH, iron detector, etc.) while maintaining power efficiency. Furthermore, such miniature endoscopy devices could attach to the intestinal mucosa while getting power support from human body heat allowing them to operate for long monitoring periods. Finally with the advent of nanotechnology medicine in recent years it will slowly be incorporated to capsule endoscopy devices allowing them to deliver smart drugs (i.e., targeted drug therapies), diagnosis and treatment to specific parts of the digestive tract.
CONCLUSION
In conclusion, several analogues could be drawn for the field of medicine/gastroenterology and other sciences as well. Therefore, we believe that the mainstream small bowel endoscopy in the third decade of the new millennium should be provided by an enhanced version of the capsule-based based platform, such as the one proposed by Iakovidis et al[81]. Just like aeronautical engineering - for more than a century now - has not significantly moved away from the conceptual design/idea of its most famous aviation pioneers, the Wright brothers, these authors believe that the shell of the capsule device - with some optimizations, e.g., spherical capsules would go easier through stenotic parts - will prevail over time. Furthermore, the speed (and control of the device), the functional characteristics (definition, 3-D application and therapy) and the indications for obtaining it will change over time.
Bio-mimetic/bio-inspired approaches, such as the spider and fish -like capsules[50,59], are promising approaches not only with respect to navigation within the digestive tract, but also for treatment, not only with application of haemostatic clips or drug delivery[2,37,38,40,41], but also for small operations, including biopsies or small dissections.
In conclusion, we should relish the challenges that lie ahead; in an environment like the small-bowel, the requirement is not to make micro-capsules; instead the attention should focus on how to utilize the existing carrier shape and size, but with miniaturized components such as microscopic batteries, that will leave internal space and provide enough power for internal lens rotation (just like the deep sea submarines), space for microscopic labs (lab-in-a-pill) and other sensing capacities and -hopefully- deliver powder medication for bleeding.
P- Reviewer: Higaki S, Redondo-Cerezo E S- Editor: Yu J L- Editor: A E- Editor: Wang CH