Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4680
Peer-review started: November 21, 2014
First decision: December 11, 2014
Revised: December 29, 2014
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: April 21, 2015
Processing time: 151 Days and 3.8 Hours
AIM: To investigate human epidermal growth factor receptor 2 (HER2) amplification and protein expression in mixed gastric carcinoma.
METHODS: Fluorescence in situ hybridization and immunohistochemistry were used to detect HER2 amplification and protein expression in 277 cases of mixed gastric carcinoma. Protein staining intensity was rate as 1+, 2+, or 3+.
RESULTS: Of the 277 cases, 114 (41.2%) expressed HER2 protein. HER2 3+ staining was observed in 28/277 (10.1%) cases, 2+ in 37/277 (13.4%) cases, and 1+ in 49/277 (17.7%) cases. A HER2 amplification rate of 17% was detected, of which 25/28 (89.3%) were observed in the HER2 3+ staining group, 17/37 (45.9%) in 2+, and 5/49 (10.2%) in 1+. Of the 47 patients with HER2 amplification who received chemotherapy plus trastuzumab, 22 demonstrated median progression-free and overall survivals of 9.1 mo and 16.7 mo, respectively, which were significantly better than those achieved with chemotherapy alone (5.6 mo and 12.1 mo, respectively) in 19 previously treated patients (Ps < 0.05).
CONCLUSION: HER2 detection in mixed gastric carcinoma displays high heterogeneity. Relatively quantitative parameters are needed for assessing the level of HER2 amplification and protein expression.
Core tip: Human epidermal growth factor receptor 2 (HER2) detection in mixed gastric carcinoma displays high heterogeneity; a standard detection of HER2 in mixed gastric cancer is needed to better guide therapeutic interventions. In this study, immunohistochemistry and fluorescence in situ hybridization were used to detect HER2 protein expression and amplification, respectively. HER2 staining was classified as extensive, partial, and focal, whereas amplification patterns were classified into cluster, large granule, dot, and HH-17 categories. Clinical therapeutic findings from the patients validated these approaches for assessing the levels of HER2, and demonstrate their clinical applicability.
- Citation: Wang YK, Chen Z, Yun T, Li CY, Jiang B, Lv XX, Chu GH, Wang SN, Yan H, Shi LF. Human epidermal growth factor receptor 2 expression in mixed gastric carcinoma. World J Gastroenterol 2015; 21(15): 4680-4687
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4680.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4680
Targeted therapy is pathologic and physiologic by targeting a key receptor in tumor development and correcting the pathologic process. In contrast to cytotoxic drugs, targeted therapy has no cellular toxicity, and mainly regulates the function and stabilization of tumor cells. In recent years, several new drugs have been developed targeting signal transduction pathways as well as growth factors and their receptors, which have led to important therapeutic achievements. Overexpression of human epidermal growth factor receptor 2 (HER2) activates cellular signal transduction systems that result in cell transformation and proliferation[1,2]. HER2 is an important therapeutic target for breast cancer treatment[3,4]. Increasing evidence suggests that HER2 is an important therapeutic target for the treatment of gastric cancer. Gastric cancer patients expressing high levels of HER2 may benefit most from HER2-targeted therapy[5].
A previous study showed unique characteristics and high heterogeneous expression of HER2 in mixed gastric cancer by an immunostaining method[6]. Mixed gastric cancer in the gastroesophageal junction is composed of mixed adenocarcinoma and non-adenocarcinoma, including squamous cell carcinoma and neuroendocrine carcinoma. Therefore, a standard detection of HER2 in mixed gastric cancer is needed to correctly guide therapeutic interventions. To provide guidance for the targeted treatment of mixed gastric cancer, we utilized immunohistochemistry (IHC) to detect HER2 protein expression and fluorescence in situ hybridization (FISH) to detect HER2 amplification in this study.
Tissue specimens from 277 cases of mixed gastric carcinoma were collected from Luoyang 150th Central Hospital, Pingdingshan 152nd Central Hospital, and Zhumadian 159th Central Hospital between July 2009 and September 2011. The histologic diagnostic criteria are in accordance with the literature of the pathologic classification of gastric tumors[7] and the 2010 edition of the World Health Organization digestive system cancer guidelines[8]. Patients were from 25-76 years-old, and the average age was 64.7 years. The specimens were fixed in 10% neutral buffered formalin for 8-48 h before IHC and/or FISH analysis.
HER2 protein was detected using a HercepTest kit (Dako of Agilent Technologies, Glostrup, Denmark). Four to six sections from each case were. For interpretation of positive staining intensity, a sample scored 0 when the membranes of tumor cells showed no staining, 1+ when the tumor cells demonstrated weak membrane staining, 2+ when the tumor cell basement membrane, side mask, or the integrity of the membrane displayed weak to moderate staining, and 3+ when the tumor cell basement membrane, the side mask, or the integrity of the membrane exhibited a strong positive staining. For interpretation of positive staining range, extensive staining referred to samples where ≥ 80% of the cells were stained positive, partial staining when 79%-21% of the cells stained positive, and focal staining when ≤ 20% of the cells stained positive.
Paraffin Pretreatment Kit II kits (including pretreatment solution and protease solution) and Path Vysion HER2 Probe kits (Vysis of Abbott Laboratories, Abbott Park, IL, United States) were used in this study. Paraffin pretreatment and FISH procedures in embedded gastric cancer tissue sections were performed according to previously published methods[9]. First, a hematoxylin and eosin (HE)-stained section confirmed the presence of positive regional cancer cells. Next, a 10 × objective lens was used to find the same cellular field as that evaluated by HE staining. The entire section was observed using a 40 × objective, and if > 75% of the cells exhibited hybridization signals the hybridization result would be considered satisfactory. A 100 × objective lens was used to count at least 30 full boundaries with no overlapping cancer cells. The ratio of chromosome 17 and the signal numbers from 30 randomly selected cells was used for HER2 gene copy number evaluation. Cases were considered positive if the ratio was > 2.2, suggesting that the HER2 gene was amplified. Cases were considered negative if the ratio was < 1.8, suggesting no HER2 amplification. However, if the ratio was 1.8-2.2, additional tumor tissue areas and 30 additional cells were counted to determine the final result. In addition, if the ratio was approximately 1, and ≥ 6 cells showed two different color signals in each cell, the sample was determined as hyperhexasomy 17 (HH-17).
In total, 22 cases with HER2 amplification were treated with trastuzumab combined with cisplatin, oxaliplatin, irinotecan, or taxane, which were compared with 19 previously treated cases with HER2 amplification who received cisplatin, oxaliplatin, irinotecan, or taxane, but no trastuzumab, for treatment. Evaluation of treatment effectiveness was based on clinical examination, B-ultrasound examination, and CT scans. Overall survival (OS) was calculated by time of chemotherapy (or plus trastuzumab) treatment to the time of death. Progression-free survival (PFS) was calculated by time of chemotherapy (or plus trastuzumab) treatment until disease progression.
Statistical analysis was performed using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, United States), and the median PFS and OS were compared using a log-rank test. P < 0.05 was considered significant.
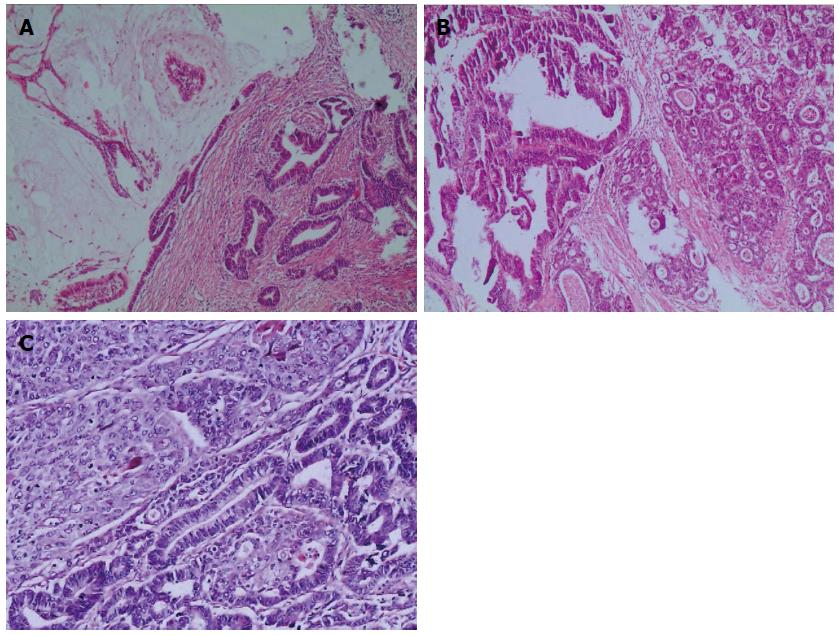
In this study, there were 216 cases of mixed gastric adenocarcinoma with a mixture of different organizational structures (including papillary adenocarcinoma, tubular adenocarcinoma, mucinous adenocarcinoma, and low adhesion adenocarcinoma) (Figure 1), 34 cases of adenosquamous carcinoma, 22 cases of gastric adenocarcinoma mixed with neuroendocrine carcinoma (including at least one type of adenocarcinoma mixed with neuroendocrine tumors), and 5 cases of gastric tumors mixed with adenocarcinoma, squamous cell carcinoma, and neuroendocrine carcinoma. All cases were at the stages of pT 2-4.
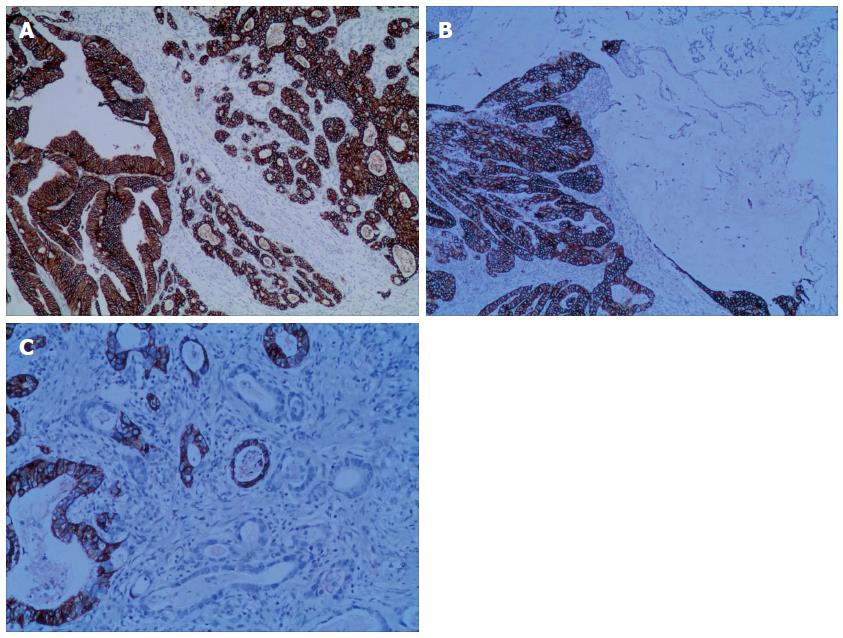
The relationships between HER2 protein expression and cell organization types in mixed gastric carcinoma are shown in Table 1. In total, 41.2% (114/277) of the mixed gastric tumors expressed HER2 protein within the cellular membrane. HER2 3+ staining was observed in 28/277 (10.1%) cases (Figure 2A), including 8 cases of the extensive type, 15 cases of the partial type (Figure 2B), and 5 cases of the focal type (Figure 2C). HER2 2+ staining was detected in 37/277 (13.4%) cases, including 11 cases of the extensive type, 19 cases of the partial type, and 7 cases of the focal type. HER2 1+ staining was demonstrated in 49/277 (17.7%) cases, including 13 cases of the extensive type, 26 cases of the partial type, and 10 cases of the focal type. 163 cases were observed to have negative HER2 staining results.
| Type | No. of cases | Immunohistochemistry | |||||
| - | 1+ | 2+ | 3+ | Positive, n (%) | |||
| Mixed adenocarcinoma | 216 | Extensive | 13 | 10 | 8 | 31 (14.4) | |
| Partial | 21 | 15 | 14 | 50 (23.1) | |||
| Focal | 7 | 5 | 3 | 15 (6.9) | |||
| Negative | 120 | ||||||
| Adenosquamous carcinoma | 34 | Extensive | 0 | 1 | 0 | 1 (2.9) | |
| Partial | 2 | 2 | 1 | 5 (14.7) | |||
| Focal | 2 | 0 | 1 | 3 (8.8) | |||
| Negative | 25 | ||||||
| Mixed adenocarcinoma and neuroendocrine carcinoma | 22 | Extensive | 0 | 0 | 0 | 0 (0.0) | |
| Partial | 2 | 2 | 0 | 4 (18.2) | |||
| Focal | 1 | 1 | 1 | 3 (13.6) | |||
| Negative | 15 | ||||||
| Mixed adenocarcinoma, squamous cell carcinoma and neuroendocrine carcinoma | 5 | Extensive | 0 | 0 | 0 | 0 (0.0) | |
| Partial | 1 | 0 | 0 | 1 (20.0) | |||
| Focal | 0 | 1 | 0 | 1 (20.0) | |||
| Negative | 3 | ||||||
| Total | 277 | 163 (58.8) | 49 (17.7) | 37 (13.4) | 28 (10.1) | 114 (41.2) | |
Significant heterogeneous expression of HER2 protein was observed in the present mixed gastric cancer tissues. In this study, we designated a case as a single-staining-range type if only one of the three (i.e., the extensive, partial, and focal types) was detected in 4-6 tissue sections examined. We only identified 36.8% (42/114) of our cases with the single-staining-range type (Table 2). If more than one type of HER2 expression patterns (i.e., HER2 3+, HER2 2+, and HER2 1+) were simultaneously detected in 4-6 tissue sections of a case, a multi-staining-intensity type was defined. In this study, 63.2% (72/114) of the cases were identified with the multi-staining-intensity type (Table 2). Notably, of the 34 cases of adenosquamous carcinoma, 10 had positive HER2 protein expression, including 2 cases with the positive staining region located in the squamous cell carcinoma and 8 cases in the adenocarcinoma. Of the 22 cases of mixed adenocarcinoma and neuroendocrine carcinoma, as well as 5 cases of mixed adenocarcinoma, squamous cell carcinoma and neuroendocrine carcinoma, the positive staining regions were all observed within the adenocarcinoma parts of these tumors.
| Extensive | Partial | Focal | Value | |
| Single-staining range | 10 | 23 | 9 | 42 (36.8) |
| Multi-staining intensity | 22 | 37 | 13 | 72 (63.2) |
| Total | 32 (11.6) | 60 (21.7) | 22 (7.9) | 114 (41.2) |
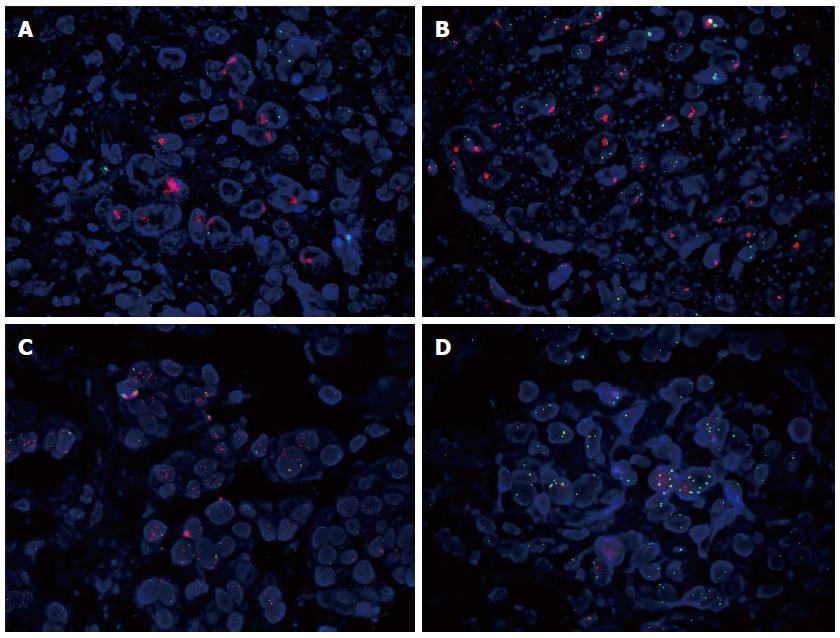
We observed a HER2 amplification rate of 17% (47/277) (Table 3). Of the 47 HER2 amplified cases, 25/28 (89.3%) were observed in the HER2 3+ staining group, 17/37 (45.9%) in the HER2 2+ staining group, and 5/49 (10.2%) in the HER2 1+ staining group. With regard to amplification patterns, these 47 HER2 amplified cases included 17 cases showing cluster amplification (Figure 3A), 12 cases with large granule amplification (Figure 3B), 11 cases with dot amplification (Figure 3C), and 7 cases with HH-17 (Figure 3D). Except one case in which the HER2-amplified region was located in the squamous cell carcinoma part of a mixed gastric carcinoma, the remaining 46 cases were all observed with the HER2-amplified regions in the adenocarcinoma parts of these mixed gastric tumors.
| Staining intensity (No. of cases) | HER2 protein expression | HER2 amplification | ||||
| Staining Range | No. of cases | Cluster | Large granule | Dot | HH-17 | |
| 3+ (28) | Extensive | 8 | 3 | 2 | 2 | 1 |
| Partial | 15 | 4 | 3 | 5 | 2 | |
| Focal | 5 | 2 | 1 | 0 | 0 | |
| 2+ (37) | Extensive | 11 | 3 | 2 | 2 | 1 |
| Partial | 19 | 3 | 2 | 1 | 1 | |
| Focal | 7 | 1 | 1 | 0 | 0 | |
| 1+ (49) | Extensive | 13 | 1 | 1 | 0 | 1 |
| Partial | 26 | 0 | 0 | 1 | 1 | |
| Focal | 10 | 0 | 0 | 0 | 0 | |
| Total | 114 | 17 | 12 | 11 | 7 | |
Twenty-two of the 47 patients with HER2 amplification who received chemotherapy plus trastuzumab (i.e., trastuzumab combined with cisplatin, oxaliplatin, irinotecan, or taxane) for treatment demonstrated a median PFS and a median OS of 9.1 and 16.7 mo, respectively, which was significantly better than those achieved with chemotherapy alone (i.e., cisplatin, oxaliplatin, Iraq irinotecan, or taxane) (5.6 and 12.1 mo, respectively) in the series of 19 previously treated patients (P < 0.05) (Tables 4 and 5). In addition, it appeared that the type of HER2 amplification was positively related with PFS and OS (Table 4); a similar finding was also observed when HER2 positive staining intensity and staining range by IHC were used to evaluate the prognosis of these patients (data not shown).
| Treatment | HER2 amplification | No. of cases | Survival analysis (mo) | |
| Median PFS | Median OS | |||
| Chemotherapy alone | Cluster | 7 | 6.3 | 11.9 |
| Large granule | 6 | 5.8 | 12.2 | |
| Dot | 4 | 5.3 | 11.6 | |
| HH-17 | 2 | 5.1 | 12.7 | |
| Trastuzumab combined with chemotherapy | Cluster | 9 | 10.7 | 18.1 |
| Large granule | 6 | 9.9 | 17.7 | |
| Dot | 5 | 8.3 | 16.5 | |
| HH-17 | 2 | 7.6 | 14.3 | |
| Treatment | No. of cases | Survival analysis | |||
| Median PFS (mo) | P value | Median OS (mo) | P value | ||
| Chemotherapy alone | 19 | 5.6 | 0.01 | 12.1 | 0.01 |
| Trastuzumab combined with chemotherapy | 22 | 9.1 | 16.7 | ||
In recent years, extensive research has been conducted to identify new targets for molecular therapeutic drugs. Targeted therapeutics act selectively at the molecular, biochemical, and genetic level to target cancer cells and specifically block abnormal cellular behavior, unlike conventional cytotoxic drugs. Trastuzumab (Herceptin) is a humanized monoclonal antibody that selectively binds to the HER2 extracellular domain that was approved by the United States Food and Drug Administration in 1998 for the treatment of patients with advanced HER2-overexpressed breast cancer. Currently, trastuzumab has already become a first-line drug for the treatment of HER2-overexpressed breast cancer[6,7]. HER2 is an important biologic marker for gastric cancer as well[9]. Gastric cancer patients with recurrence or metastasis who exhibited HER2 overexpression were treated with trastuzumab, which significantly increased their survival rates[10]. In this study, we utilized IHC and FISH techniques to analyze HER2 protein expression and gene amplification in 277 cases of mixed gastric carcinoma, which likely represents the largest population of Chinese cases studied for this purpose to date. Our results indicate that trastuzumab combined with chemotherapy offers better therapeutic effects on cases of mixed gastric carcinoma with HER2 amplification or overexpression than chemotherapy alone, and the trastuzumab-related therapeutic effects are positively associated with the level of HER2 amplification or protein expression, which confirms previous findings[11]. Therefore, from a clinical point of view, in order to better employ HER2-targeted treatment strategy, relatively quantitative parameters should be established to assess the level of HER2 amplification or protein expression. In the present study, in order to better illustrate the level of HER2 protein expression and gene amplification, for IHC analysis we used the standard scoring system (i.e., HER2 3+, HER2 2+, and HER2 1+) to define HER2-positive staining intensity and utilized the extensive, partial, and focal types to classify HER2-positive staining range; for FISH analysis, we classified HER2 amplification patterns into cluster, large granule, dot, and HH-17 categories. Clinical therapeutic findings of our patients validated our approaches for assessing the level of HER2 amplification and protein expression, and proved these parameters to be clinically useful.
Technically, we found that HER2 detection in gastric cancer is different from that in breast cancer, and can be influenced by many factors, including preoperative treatment on specimens, fixative reagents and methods used, laboratory quality control, and interpretation standards on IHC staining results[12]. HER2 detection results sometimes can vary considerably or even lack repeatability, which often leads to treatment confusion, incorrect treatment plans, or mistreatment. Therefore, more detailed and accurate HER2 detections are needed to better guide clinical practice in obtaining the greatest antitumor benefit from HER2-targeted therapy[13]. We would summarize some of our experiences in detection of HER2 protein expression by IHC in gastric cancer as follows: a tissue specimen must be fixed in fresh 10% neutral buffered formalin solution within 30 min after being removed from a patient’s body, and fixed with the formalin solution five times the volume of the specimen for 24-36 h (no more than 48 h). Due to the heterogeneity of gastric carcinoma in addition to conventional sampling materials (e.g., proximal and distal margins, tumor and adjacent gastric mucosa), other regions of a gastric carcinoma (such as the deepest point and adjacent serosal invasion) also should be sampled to make 4-6 blocks for analysis. For papillary or tubular adenocarcinoma the basement membrane and side mask of a tumor cell should be primarily analyzed for HER2 staining; for non-papillary and non-tubular gastric carcinoma with solid nests and low adhesion, intact basement membranes of tumor cells should be primarily analyzed; a tumor cell with positive membrane (but not cytoplasm and nucleus) staining is interpreted as a HER2-positive cell. The fact that 3/28 (10.7%) of HER2 3+ staining cases did not have HER2 amplification by FISH, and 17/37 (45.9%) of HER2 2+ staining cases as well as 5/49 (10.2%) of HER2 1+ staining cases had HER2 amplification demonstrates that FISH offers more accurate analyses than IHC for HER2 detection in gastric cancer, which is consistent with what is reported in breast cancer. Technically, FISH can provide relatively quantitative analyses and can effectively reduce analyzers’ impression-interference and variations among different laboratories[14].
Therefore, FISH should be considered as the gold-standard method for HER2 detection in mixed gastric carcinoma. Notably, in this study we included HH-17 as a type of anomaly showing gain of multiple HER2 copies, and our clinical data support this classification. In summary, HER2 detection in mixed gastric carcinoma displays high heterogeneity. Relatively quantitative parameters for interpretation of both IHC and FISH results are needed for assessing the level of HER2 amplification and protein expression, which can better guide HER2-targeted therapy for patients with gastric cancer.
Overexpression of human epidermal growth factor receptor 2 (HER2) activates cellular signal transduction systems that result in cell transformation and proliferation. HER2 is an important therapeutic target for breast cancer treatment. Increasing evidence suggests that HER2 is an important therapeutic target for the treatment of gastric cancer. Gastric cancer patients expressing high levels of HER2 may benefit most from HER2-targeted therapy.
Previous studies showed unique characteristics and high heterogeneous expression of HER2 in mixed gastric cancer by immunostaining methods.
The authors established a standard detection of HER2 in mixed gastric cancer to correctly guide therapeutic interventions.
To provide guidance for the targeted treatment on mixed gastric cancer, we utilized immunohistochemistry to detect HER2 protein expression and fluorescence in situ hybridization (FISH) to detect HER2 amplification in this study, which likely represents the largest such study in a Chinese population.
Mixed gastric cancer in the gastroesophageal junction is composed of mixed adenocarcinoma and non-adenocarcinoma, including squamous cell carcinoma and neuroendocrine carcinoma.
This is a good descriptive study in which the authors analyzed the HER2 protein and amplification in mixed gastric cancer in the gastroesophageal junction. The results are interesting and showed that HER2 detection in mixed gastric carcinoma displays high heterogeneity. Relatively quantitative parameters for interpretation of both immunohistochemistry and FISH results are needed for assessing the level of HER2 amplification and protein expression, which can better guide HER2-targeted therapy for patients with gastric cancer. FISH should be considered as the gold-standard method for HER2 detection in mixed gastric carcinoma.
P- Reviewer: Aurello P, Kupcinskas L S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Ma S
| 1. | García-García E, Gómez-Martín C, Angulo B, Conde E, Suárez-Gauthier A, Adrados M, Perna C, Rodríguez-Peralto JL, Hidalgo M, López-Ríos F. Hybridization for human epidermal growth factor receptor 2 testing in gastric carcinoma: a comparison of fluorescence in-situ hybridization with a novel fully automated dual-colour silver in-situ hybridization method. Histopathology. 2011;59:8-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Isinger-Ekstrand A, Johansson J, Ohlsson M, Francis P, Staaf J, Jönsson M, Borg A, Nilbert M. Genetic profiles of gastroesophageal cancer: combined analysis using expression array and tiling array--comparative genomic hybridization. Cancer Genet Cytogenet. 2010;200:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Rondón-Lagos M, Verdun Di Cantogno L, Marchiò C, Rangel N, Payan-Gomez C, Gugliotta P, Botta C, Bussolati G, Ramírez-Clavijo SR, Pasini B. Differences and homologies of chromosomal alterations within and between breast cancer cell lines: a clustering analysis. Mol Cytogenet. 2014;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Gumuskaya B, Alper M, Hucumenoglu S, Altundag K, Uner A, Guler G. EGFR expression and gene copy number in triple-negative breast carcinoma. Cancer Genet Cytogenet. 2010;203:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Preusser M, Berghoff AS, Ilhan-Mutlu A, Dinhof C, Magerle M, Marosi C, Hejna M, Capper D, VON Deimling A, Schoppmann SF. Brain metastases of gastro-oesophageal cancer: evaluation of molecules with relevance for targeted therapies. Anticancer Res. 2013;33:1065-1071. [PubMed] |
| 6. | Gordon MA, Gundacker HM, Benedetti J, Macdonald JS, Baranda JC, Levin WJ, Blanke CD, Elatre W, Weng P, Zhou JY. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann Oncol. 2013;24:1754-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Miyamae M, Komatsu S, Ichikawa D, Kubota T, Okamoto K, Konishi H, Morimura R, Murayama Y, Shiozaki A, Kuriu Y. [Discrepancies in the histological grade of submucosal gastric carcinoma between biopsy and postoperative resected specimens]. Gan To Kagaku Ryoho. 2013;40:2292-2294. [PubMed] |
| 8. | Tanabe S, Aoyagi K, Yokozaki H, Sasaki H. Gene expression signatures for identifying diffuse-type gastric cancer associated with epithelial-mesenchymal transition. Int J Oncol. 2014;44:1955-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Yk W, Cf G, T Y, Z C, Xw Z, Xx L, Nl M, Wz Z. Assessment of ERBB2 and EGFR gene amplification and protein expression in gastric carcinoma by immunohistochemistry and fluorescence in situ hybridization. Mol Cytogenet. 2011;4:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Sanaat Z, Halimi M, Ghojezadeh M, Pirovi AH, Gharamaleki JV, Ziae AE, Kermani IA. Immunohistochemical Analysis of p53, Ki-67, CD44, HER-2/neu Expression Patterns in Gastric Cancer, and Their Association with One Year Survival in North-West of Iran. Int J Hematol Oncol Stem Cell Res. 2013;7:15-20. [PubMed] |
| 11. | Tajiri R, Ooi A, Fujimura T, Dobashi Y, Oyama T, Nakamura R, Ikeda H. Intratumoral heterogeneous amplification of ERBB2 and subclonal genetic diversity in gastric cancers revealed by multiple ligation-dependent probe amplification and fluorescence in situ hybridization. Hum Pathol. 2014;45:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Kim MA, Jung EJ, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum Pathol. 2007;38:1386-1393. [PubMed] |
| 13. | Brabender J, Danenberg KD, Metzger R, Schneider PM, Park J, Salonga D, Hölscher AH, Danenberg PV. Epidermal growth factor receptor and HER2-neu mRNA expression in non-small cell lung cancer Is correlated with survival. Clin Cancer Res. 2001;7:1850-1855. [PubMed] |
| 14. | Pala EE, Bayol U, Ozguzer A, Akman O. HER2 status in gastric cancer: a comparison of two novel in situ hybridization methods (IQ FISH and dual color SISH) and two immunohistochemistry methods (A0485 and HercepTest™). Pathol Res Pract. 2013;209:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |