Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4627
Peer-review started: October 9, 2014
First decision: November 14, 2014
Revised: December 10, 2014
Accepted: January 16, 2015
Article in press: January 16, 2015
Published online: April 21, 2015
Processing time: 193 Days and 5.6 Hours
AIM: To compare survival and recurrence in hepatocellular carcinoma (HCC) patients who did or did not receive adjuvant transarterial chemoembolization (TACE).
METHODS: A consecutive sample of 229 patients who underwent curative resection between March 2007 and March 2010 in our hospital was included. Of these 229 patients, 91 (39.7%) underwent curative resection followed by adjuvant TACE and 138 (60.3%) underwent curative resection alone. In order to minimize confounds due to baseline differences between the two patient groups, comparisons were conducted between propensity score-matched patients. Survival data and recurrence rates were compared using the Kaplan-Meier method. Independent predictors of overall survival and recurrence were identified using Cox proportional hazard regression.
RESULTS: Among 61 pairs of propensity score-matched patients, the 1-, 2-, and 3-year overall survival rates were 95.1%, 86.7%, and 76.4% in the TACE group and 86.9%, 78.5%, and 73.2% in the control group, respectively. At the same time, the TACE and control groups also showed similar recurrence rates at 1 year (13.4% vs 24.8%), 2 years (30.6% vs 32.1%), and 3 years (40.1% vs 34.0%). Multivariate Cox regression identified serum alpha-fetoprotein level ≥ 400 ng/mL and tumor size > 5 cm as independent risk factors of mortality (P < 0.05).
CONCLUSION: As postoperative adjuvant TACE does not improve overall survival or reduce recurrence in HCC patients, further study is needed to clarify its clinical benefit.
Core tip: This study examined survival and recurrence in hepatocellular carcinoma patients who did or did not undergo transarterial chemoembolization (TACE). Analyses were performed with all patients and with propensity score-matched pairs. Both analyses suggest adjuvant TACE does not reduce recurrence. Furthermore, adjuvant TACE does not improve overall survival among propensity score-matched patients. These findings raise important questions about the efficacy of postoperative adjuvant TACE that future work should address in order to justify the use of this technique as well as identify the patient populations most likely to benefit from it.
- Citation: Jiang JH, Guo Z, Lu HF, Wang XB, Yang HJ, Yang FQ, Bao SY, Zhong JH, Li LQ, Yang RR, Xiang BD. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: Propensity score analysis. World J Gastroenterol 2015; 21(15): 4627-4634
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4627.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4627
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh most common in women, and it ranks as the third leading cause of cancer-related death worldwide[1,2]. HCC is difficult to diagnose at an early stage and is associated with poor survival. Surgical resection remains the most effective curative treatment for HCC[3,4], but the high incidence of tumor recurrence makes long-term survival after resection unsatisfactory[5,6]. Various post-resection neoadjuvant and adjuvant therapies have been developed to reduce recurrence and improve overall survival[7-11].
Transarterial chemoembolization (TACE) has recently been reported as an adjuvant therapy for HCC patients after curative resection. Originally developed as a palliative treatment to improve overall survival and reduce recurrence in patients with unresectable HCC[12,13], this treatment takes advantage of the high arterial blood supply in HCC; chemotherapy and occlusion of the feeding arteries cause tumor necrosis and delay tumor progression. TACE has been shown to provide survival benefit as a postoperative adjuvant therapy in some studies, but not in others[14-19].
To gain a clearer picture of the influence of postoperative adjuvant TACE on survival and recurrence after curative resection for HCC, we carried out a retrospective study on propensity score-matched patients without risk factors who were treated by resection followed by TACE or by resection alone.
This study was approved by the Ethics Committee of the Tumor Hospital of Guangxi Medical University, and written informed consent was obtained from patients or from their parents or legal guardians if the patients were under 18 years of age.
This retrospective analysis was carried out on medical records of patients diagnosed with HCC between March 2007 and March 2010 at the Tumor Hospital of Guangxi Medical University, Nanning, China. A total of 1035 patients with HCC were enrolled in our hospital, but 432 (41.7%) were excluded because they had received treatment at other hospitals. Among the remaining 603 patients, 257 (42.6%) were excluded because they were given various post-resection neoadjuvant and adjuvant therapies, such as local ablation therapy or ethanol injection, systematic chemotherapy or sorafenib therapy. Another eight patients (1.3%) were excluded because they had been diagnosed with other malignancy before receiving initial HCC treatment. The remaining 229 (67.8%) patients with Child-Pugh A liver function were included in the analyses in this retrospective study. Of these patients, 138 (60.3%) underwent curative resection (control group) and 91 (39.7%) underwent resection followed by postoperative adjuvant TACE (TACE group).
Patients were enrolled if they satisfied the following criteria: (1) HCC diagnosis was confirmed by pathologic examination; (2) no more than three tumor nodules were present; (3) there was no invasion of the main trunk and first-order branches of the portal vein and common hepatic duct, or the main trunk of the hepatic vein and inferior vena cava; (4) there was no intra- or extra-hepatic metastasis, and without satellite nodules; (5) the primary tumor had been completely excised with a negative resection margin; (6) no residual tumor or portal tumor thromboses were detected in postsurgical imaging; and (7) elevated preoperative levels of alpha-fetoprotein (AFP) decreased to normal within 2 mo after the procedure.
There may be substantial risk that observed differences in survival or recurrence between our groups are due at least in part to baseline differences. To minimize such confounding and isolate the effects of adjuvant TACE, we used propensity score matching to balance these baseline differences and thereby simulate random group allocation[20]. Logistic regression was used to generate a binary propensity score for each patient (0 = assignment to control group, 1 = assignment to TACE group). One-to-one matching of propensity scores was performed without replacement using a caliper with a width 0.2 of the SD to generate 61 pairs comprised one control and one TACE patient (Table 1).
| Variable | Before propensity score matching | After propensity score matching | ||||
| TACE (n = 91) | Control (n = 138) | P value | TACE (n = 61) | Control (n = 61) | P value | |
| Gender | ||||||
| Male | 79 | 120 | 0.975 | 50 | 51 | 0.810 |
| Female | 12 | 18 | 11 | 10 | ||
| Age, yr | 49.8 ± 10.0 | 49.0 ± 12.5 | 0.585 | 50.1 ± 11.0 | 50.8 ± 11.0 | 0.693 |
| BMI, kg/m2 | ||||||
| < 23 | 48 | 99 | 0.003 | 41 | 42 | 0.846 |
| ≥ 23 | 43 | 39 | 20 | 19 | ||
| HbsAg | ||||||
| Positive | 76 | 119 | 0.572 | 50 | 52 | 0.625 |
| Negative | 15 | 19 | 11 | 9 | ||
| Family history of HCC | ||||||
| Yes | 21 | 29 | 0.712 | 13 | 12 | 0.823 |
| No | 70 | 109 | 48 | 49 | ||
| Total bilirubin, μmol/L | 14.1 ± 6.9 | 14.0 ± 6.7 | 0.950 | 13.4 ± 5.9 | 14.2 ± 7.4 | 0.451 |
| Total serum protein, g/L | 69.1 ± 6.0 | 69.7 ± 6.6 | 0.510 | 69.7 ± 6.2 | 70.0 ± 6.3 | 0.783 |
| Albumin, g/L | 41.2 ± 3.9 | 39.9 ± 5.1 | 0.042 | 40.1 ± 4.2 | 40.9 ± 4.2 | 0.675 |
| ALT, U/L | 38 (10-192) | 35 (9-341) | 0.572 | 38 (24-53) | 33 (23-49) | 0.536 |
| AST, U/L | 35 (16-181) | 38 (17-410) | 0.507 | 36 (30-50) | 36 (29-51) | 0.903 |
| Prothrombin time, s | 12.5 ± 1.6 | 13.1 ± 1.7 | 0.010 | 12.6 ± 1.4 | 12.6 ± 1.5 | 0.716 |
| Platelet count, 109/L | 181.1 ± 69.2 | 173.2 ± 78.8 | 0.436 | 183.8 ± 73.1 | 174.2 ± 70.8 | 0.401 |
| AFP, ng/mL | ||||||
| < 400 | 73 | 98 | 0.117 | 42 | 45 | 0.548 |
| ≥ 400 | 18 | 40 | 19 | 16 | ||
| Tumor size, cm | 5.6 ± 3.0 | 6.2 ± 2.9 | 0.155 | 5.8 ± 2.8 | 5.8 ± 2.9 | 0.932 |
| Tumor number | ||||||
| 1 | 67 | 99 | 0.754 | 44 | 46 | 0.681 |
| 2 or 3 | 24 | 39 | 17 | 15 | ||
| Liver cirrhosis | ||||||
| Positive | 78 | 102 | 0.033 | 51 | 51 | 1.000 |
| Negative | 13 | 36 | 10 | 10 | ||
| Antiviral therapies | ||||||
| Yes | 76 | 119 | 0.572 | 50 | 52 | 0.625 |
| No | 15 | 19 | 11 | 9 | ||
| Resection | ||||||
| Anatomic | 39 | 60 | 0.926 | 26 | 25 | 0.854 |
| Nonanatomic | 52 | 78 | 35 | 36 | ||
| Vascular occlusion | ||||||
| No | 22 | 23 | 0.184 | 14 | 11 | 0.111 |
| Pringle maneuver | 42 | 80 | 29 | 40 | ||
| Hemihepatic | 27 | 35 | 18 | 10 | ||
| Surgical margins, cm | ||||||
| ≥ 1 | 65 | 106 | 0.359 | 44 | 41 | 0.691 |
| < 1 | 26 | 32 | 17 | 19 | ||
| Blood loss, mL | 300 (200-500) | 300 (200-500) | 0.119 | 300 (200-500) | 300 (175-500) | 0.726 |
| Blood transfusion | ||||||
| Yes | 17 | 38 | 0.125 | 12 | 13 | 0.823 |
| No | 74 | 100 | 49 | 48 | ||
| Tumor capsule | ||||||
| Complete | 59 | 63 | 0.004 | 36 | 35 | 0.854 |
| Incomplete | 32 | 75 | 25 | 26 | ||
| Edmondson grade | ||||||
| I or II | 37 | 60 | 0.673 | 26 | 28 | 0.715 |
| III or IV | 54 | 78 | 35 | 33 | ||
| BCLC stage | ||||||
| A | 68 | 100 | 0.705 | 46 | 47 | 0.832 |
| B | 23 | 38 | 15 | 14 | ||
All patients were treated by curative resection involving 1-2 segmentectomies (minor resection, n = 116) or > 2 segmentectomies (major resection, n = 113). Surgical techniques and perioperative management were as described[21,22].
Angiography of the celiac artery and superior mesenteric artery were performed in all patients, and TACE was performed 3-4 wk after curative resection when the tumor staining and visualization of tumor vasculature were observed by hepatic angiography[23]. If the remnant livers were stained by Lipiodol using CT or magnetic resonance imaging (MRI) after the hepatic angiography 1 mo later, TACE was performed. A highly selective 5-F catheter was used to completely occlude the feeding arteries and preserve liver function. In brief, a mixture of 50-100 mg cisplatin or oxaliplatin, 30-50 mg doxorubicin, and 5-10 mL of Lipiodol were injected, after which embolization was performed under fluoroscopic guidance using Gelfoam powder and a small amount of contrast medium.
Follow-up duration was calculated from the day of curative resection until either death or the last follow-up visit; survival time was defined as 36 mo for those who survived more than three years. All patients were followed up at 1 mo and then every 3 mo for the first two years, and then every 6 mo thereafter. Follow-up visits consisted of a physical examination, liver function tests, measurement of serum AFP, abdominal ultrasonography, and CT or MRI. Patients were diagnosed with recurrence when ultrasonography, dynamic CT, or MRI detected a new hepatic lesion.
Normally distributed clinical characteristics are reported as a single number for categorical data or as mean ± SD for continuous data. Data with a non-normal distribution are reported as median (interquartile range). Categorical data were compared between control and TACE groups using the χ2 test, while continuous data were compared using a Student’s t test. Values for the mean, median, and categorical variants were compared between the two groups using the t, Mann-Whitney U, and χ2 tests, respectively. Recurrence and survival curves were constructed using the Kaplan-Meier method and compared using Cox proportional hazard regression. Multivariate Cox proportional hazard regression was used to assess the ability of clinicopathologic variables to predict overall survival based on HRs and associated 95%CIs. All analyses were performed using SPSS 19.0 (IBM Corp., Armonk, NY, United States). All statistical tests were two-sided, and inter-group differences were considered significant when P < 0.05.
Before propensity score matching, a comparison of all 229 patients revealed no significant differences in gender, age, rates of hepatitis B antigen positivity, antiviral therapies, family history of HCC, levels of total bilirubin, total serum protein, alanine aminotransferase, aspartate aminotransferase, or AFP, tumor size, tumor number, liver resection surgery, vascular occlusion, surgical margins, blood loss, blood transfusion, Edmondson grade, or Barcelona Clinic Liver Cancer (BCLC) stage (Table 1). However, TACE patients had significantly higher levels of serum albumin (P = 0.042), higher rates of liver cirrhosis (P = 0.033), and completely encapsulated tumors (P = 0.004). On the other hand, the control group showed significantly higher rates of body mass index < 23 kg/m2 (P = 0.003) and major resection, as well as significantly longer mean prothrombin time (P = 0.010).
Propensity score matching to generate 61 patient pairs eliminated all significant differences in measured variables, confirming the effectiveness of the matching procedure.
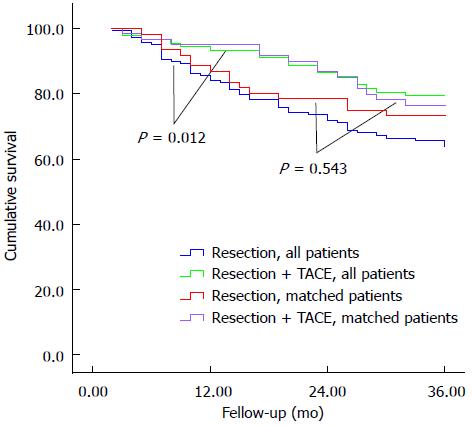
Among all patients without propensity score matching, median follow-up was 32.4 mo for the TACE group and 28.7 mo for the control group. During follow-up, 51 (56.0%) TACE patients and 79 (57.2%) control patients died of cancer or complications associated with liver disease. TACE patients showed significantly higher overall survival than controls at 1 year (93.4% vs 84.1%), 2 years (86.4% vs 71.9%), and 3 years (79.2% vs 63.7%, P = 0.012; Figure 1).
Median follow-up for the 61 pairs of propensity score-matched patients was 32.1 mo for TACE patients and 28.3 mo for controls. A total of 16 (26.2%) TACE patients died, compared to 25 (41.0%) control patients. Overall survival was higher among the propensity score-matched TACE patients compared to controls at 1 year (95.1% vs 86.9%), 2 years (86.7% vs 78.5%), and 3 years (76.4% vs 73.2%; Figure 1), though the difference was not significant.
Numerous factors were assessed for their ability to predict overall survival, including age, gender, body mass index, hepatitis B infection, antiviral therapies; family history of HCC, serum biochemistry, tumor number and size, liver cirrhosis, tumor encapsulation, liver resection surgery, vascular occlusion, surgical margins, blood loss, blood transfusion, Edmondson grade, BCLC stage, resection type, and use of adjuvant TACE. Univariate analysis identified the following significant predictors of increased risk of mortality: serum AFP level ≥ 400 ng/mL (P = 0.007), tumor size > 5 cm (P < 0.001), BCLC B stage (P = 0.020), and resection without TACE (P = 0.012) (Table 2). Multivariate Cox regression identified the following independent prognostic factors: AFP level ≥ 400 ng/mL (HR = 1.745, 95%CI: 1.008-3.019; P = 0.047) and tumor size > 5 cm (HR = 2.990, 95%CI: 1.545-5.788; P = 0.001).
| Variable | n | Overall survival (%) | Log-rankP value | ||
| 1-yr | 2-yr | 3-yr | |||
| AFP, ng/mL | |||||
| < 400 | 171 | 89.4 | 80.8 | 74.7 | 0.007 |
| ≥ 400 | 58 | 82.6 | 68.6 | 55.7 | |
| Tumor size, cm | |||||
| ≤ 5 | 93 | 96.8 | 92.1 | 86.0 | < 0.001 |
| > 5 | 136 | 81.5 | 67.8 | 58.9 | |
| BCLC stage | |||||
| A | 168 | 89.3 | 80.1 | 74.2 | 0.020 |
| B | 61 | 83.4 | 70.7 | 59.1 | |
| Blood transfusion | |||||
| Yes | 55 | 76.4 | 66.7 | 54.9 | 0.030 |
| No | 174 | 91.3 | 81.1 | 74.3 | |
| Adjuvant TACE | |||||
| Yes | 91 | 93.4 | 86.4 | 79.2 | 0.012 |
| No | 138 | 84.1 | 71.9 | 63.7 | |
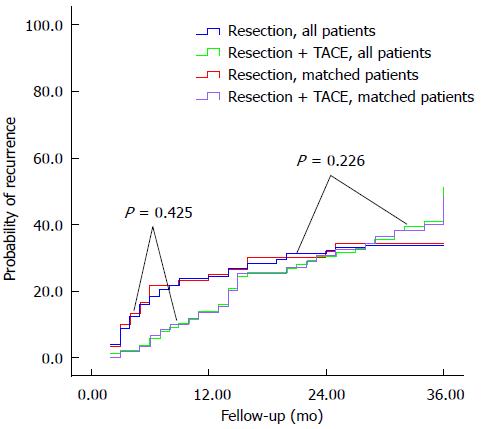
Among all patients, recurrence rates were not significantly different in the TACE group compared to controls at 1 year (13.6% vs 24.3%), 2 years (30.4% vs 32.0%), or 3 years (40.8% vs 33.9%). Similar results were obtained for the propensity score-matched patients at 1 year (13.4% vs 24.8%), 2 years (30.6% vs 32.1%), and 3 years (40.1% vs 34.0%) (Figure 2).
Univariate analysis identified the following predictors of increased risk of recurrence: tumor size > 5 cm (P = 0.046), multiple tumors (P = 0.007), BCLC B stage (P = 0.008), and resection without TACE (P = 0.030). Multivariate Cox regression identified tumor size > 5 cm (HR = 1.684, 95%CI: 1.018-2.786; P = 0.042) as an independent prognostic factor.
The rationale for postoperative TACE is to eliminate tumor cells that may have been shed from tumor masses removed during liver surgery, as well as destroy small intrahepatic metastases that may not have been detected before or during surgery. Studies have given conflicting results concerning whether postoperative adjuvant TACE can improve patient prognosis. Our retrospective study of a reasonably large patient cohort, some of whom received resection followed by TACE and others who received resection alone, suggests that the technique does not improve overall survival or reduce recurrence.
We performed analyses not only on the entire patient cohort, but also on propensity-score matched patient pairs, as the control and TACE patients showed significant differences in some baseline characteristics. These differences reflect the criteria used in our clinic to decide whether to perform TACE on given patients; such differences have been associated with differences in overall survival in HCC[24]. Although higher one-, two-, and three-year overall survival rates were observed when all TACE patients were compared with all controls, similar to those of several studies[10,14,15], this difference was not significant when compared between score-matched patient pairs.
Our results were obtained with a cohort of HCC patients who did not have complications known to increase risk of postoperative recurrence and reduce overall survival, such as vascular invasion, satellite nodules, distant and lymphovascular metastases, and positive resection margins. One meta-analysis and two randomized controlled trials (RCTs) provided strong evidence that postoperative TACE can decrease tumor recurrence and improve overall survival in patients with such risk factors for recurrence[16,17,19,23]. It may be that postoperative TACE is more appropriate for HCC patients with numerous risk factors than for those with less complicated HCC, such as the ones in our study.
Our finding that postoperative TACE does not reduce recurrence may have several explanations. TACE reduces remnant liver function and immune function, resulting in poor overall or disease-free survival[15]. Tumors in recurrence usually have different clonal origins from the primary tumors, and liver cirrhosis and liver inflammation, both of which occurred at similar rates in our propensity score-matched patients, are independently associated with recurrence[25]. Our results suggest that applying strict selection criteria when initially deciding on HCC treatments may be more effective than postoperative adjuvant TACE at improving overall survival and reducing recurrence.
Using multivariate Cox regression, we found that a serum AFP level ≥ 400 ng/mL independently predicted poor overall survival, consistent with a previous study[26]. AFP is widely used to predict intra- and extrahepatic recurrence, with extremely high levels regarded as a highly specific and sensitive biomarker. Lower AFP levels are associated with slower tumor growth and, therefore, with less-advanced tumor stage and lower risk of mortality[27-29]. Lower AFP is also associated with a more highly differentiated HCC, and with lower risk of microscopic vascular involvement. In fact, AFP serves as an adjunct diagnostic tool in many guidelines[30].
We also identified tumor size > 5 cm as an independent predictor of survival in HCC patients, again consistent with many studies[5,6,26]. Tumors > 5 cm are associated with increased invasiveness, reflected in higher incidence of intrahepatic metastasis and portal venous invasion[31,32]. Large tumors are also associated with the presence of satellite lesions and vascular invasion, which favor tumor recurrence and poor survival[29,33,34].
In conclusion, postoperative adjuvant TACE does not appear to improve overall survival or reduce recurrence in patients lacking many of the risk factors associated with poor prognosis. However, this is a retrospective analysis that is likely subject to subtle selection biases, even after propensity score matching. Larger RCTs and RCTs involving patients with more complicated HCC should examine in more detail the effects of postoperative TACE on HCC prognosis.
The authors thank Armando Chapin Rodríguez, PhD, for his language editing, which substantially improved the quality of the manuscript.
Clinicians already use postoperative adjuvant transarterial chemoembolization (TACE) to prevent recurrence of hepatocellular carcinoma (HCC), but its efficacy remains controversial.
The high incidence of tumor recurrence makes long-term survival after resection unsatisfactory. It is important for surgeons to find post-resection neoadjuvant and adjuvant therapies in order to hinder the development of HCC after curative resection.
In this study, the authors used propensity score matching to balance baseline differences between groups, and thereby simulate random group allocation. We then analyzed the overall survival and recurrence in HCC patients who did or did not receive adjuvant TACE after curative resection. This study will provide more evidence for TACE after curative resection of HCC in the future.
The study results suggest that postoperative adjuvant TACE is not useful for HCC patients without the risk factors, such as vascular invasion, satellite nodules, distant and lymphovascular metastases, and positive resection margins.
TACE is used to improve overall survival and reduce recurrence in patients with unresectable HCC. However, it was reported as an adjuvant therapy for HCC patients after curative resection.
This is an interesting study in which the authors show that postoperative adjuvant TACE does not improve overall survival or reduce recurrence in these patients lacking most of the risk factors associated with poor prognosis.
P- Reviewer: Chen YJ, Dang SS, Ramos S, Sazci A S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
| 1. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2508] [Article Influence: 192.9] [Reference Citation Analysis (2)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25542] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 3. | Moriguchi M, Takayama T, Higaki T, Kimura Y, Yamazaki S, Nakayama H, Ohkubo T, Aramaki O. Early cancer-related death after resection of hepatocellular carcinoma. Surgery. 2012;151:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 5. | Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, Weiss M, Wolfgang CL, Choti MA, Pawlik TM. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. 2014;21:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Chiche L, Menahem B, Bazille C, Bouvier V, Plard L, Saguet V, Alves A, Salame E. Recurrence of hepatocellular carcinoma in noncirrhotic liver after hepatectomy. World J Surg. 2013;37:2410-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Ueno M, Uchiyama K, Ozawa S, Hayami S, Shigekawa Y, Tani M, Yamaue H. Adjuvant chemolipiodolization reduces early recurrence derived from intrahepatic metastasis of hepatocellular carcinoma after hepatectomy. Ann Surg Oncol. 2011;18:3624-3631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Xia Y, Qiu Y, Li J, Shi L, Wang K, Xi T, Shen F, Yan Z, Wu M. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: a randomized controlled trial. Ann Surg Oncol. 2010;17:3137-3144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Kim do Y, Ahn SH, Kim SU, Choi SB, Lee KH, Park MS, Park JY, Lee do Y, Han KH, Kim KS. Adjuvant hepatic arterial infusional chemotherapy with 5-fluorouracil and cisplatin after curative resection of hepatocellular carcinoma. Oncology. 2011;81:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Nitta H, Beppu T, Imai K, Hayashi H, Chikamoto A, Baba H. Adjuvant hepatic arterial infusion chemotherapy after hepatic resection of hepatocellular carcinoma with macroscopic vascular invasion. World J Surg. 2013;37:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Kim JH, Yoon HK, Kim SY, Kim KM, Ko GY, Gwon DI, Sung KB. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 14. | Chen X, Zhang B, Yin X, Ren Z, Qiu S, Zhou J. Lipiodolized transarterial chemoembolization in hepatocellular carcinoma patients after curative resection. J Cancer Res Clin Oncol. 2013;139:773-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Li Q, Wang J, Sun Y, Cui YL, Juzi JT, Li HX, Qian BY, Hao XS. Efficacy of postoperative transarterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma complicated by portal vein tumor thrombosis--a randomized study. World J Surg. 2006;30:2004-2011; discussion 2012-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2010;40:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Ren ZG, Lin ZY, Xia JL, Ye SL, Ma ZC, Ye QH, Qin LX, Wu ZQ, Fan J, Tang ZY. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol. 2004;10:2791-2794. [PubMed] |
| 19. | Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, Zhang YQ. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1437-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Hsu CY, Hsia CY, Huang YH, Su CW, Lin HC, Pai JT, Loong CC, Chiou YY, Lee RC, Lee FY. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol. 2012;19:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 22. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Li JQ, Zhang YQ, Zhang WZ, Yuan YF, Li GH. Randomized study of chemoembolization as an adjuvant therapy for primary liver carcinoma after hepatectomy. J Cancer Res Clin Oncol. 1995;121:364-366. [PubMed] |
| 24. | Ye SL, Takayama T, Geschwind J, Marrero JA, Bronowicki JP. Current approaches to the treatment of early hepatocellular carcinoma. Oncologist. 2010;15 Suppl 4:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 26. | Nouso K, Ito Y, Kuwaki K, Kobayashi Y, Nakamura S, Ohashi Y, Yamamoto K. Prognostic factors and treatment effects for hepatocellular carcinoma in Child C cirrhosis. Br J Cancer. 2008;98:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Li P, Wang SS, Liu H, Li N, McNutt MA, Li G, Ding HG. Elevated serum alpha fetoprotein levels promote pathological progression of hepatocellular carcinoma. World J Gastroenterol. 2011;17:4563-4571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Tanaka J, Kagebayashi C, Satomura S. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol. 2014;49:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Roayaie S, Jibara G, Taouli B, Schwartz M. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol. 2013;20:3754-3760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 31. | Truant S, Boleslawski E, Duhamel A, Bouras AF, Louvet A, Febvay C, Leteurtre E, Huet G, Zerbib P, Dharancy S. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol. 2012;38:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Zhang Q, Chen H, Li Q, Zang Y, Chen X, Zou W, Wang L, Shen ZY. Combination adjuvant chemotherapy with oxaliplatin, 5-fluorouracil and leucovorin after liver transplantation for hepatocellular carcinoma: a preliminary open-label study. Invest New Drugs. 2011;29:1360-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ, Ye QH, Wang L, Tang ZY. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18:1955-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |