Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4607
Peer-review started: September 11, 2014
First decision: October 29, 2014
Revised: November 24, 2014
Accepted: December 13, 2014
Article in press: December 16, 2014
Published online: April 21, 2015
Processing time: 221 Days and 14 Hours
AIM: To describe a three-dimensional model (3DM) to accurately reconstruct anatomic relationships of centrally located hepatocellular carcinomas (HCCs).
METHODS: From March 2013 to July 2014, reconstructions and visual simulations of centrally located HCCs were performed in 39 patients using a 3D subject-based computed tomography (CT) model with custom-developed software. CT images were used for the 3D reconstruction of Couinaud’s pedicles and hepatic veins, and the calculation of corresponding tumor territories and hepatic segments was performed using Yorktal DMIT software. The respective volume, surgical margin, and simulated virtual resection of tumors were also estimated by this model preoperatively. All patients were treated surgically and the results were retrospectively assessed. Clinical characteristics, imaging data, procedure variables, pathologic features, and postoperative data were recorded and compared to determine the reliability of the model.
RESULTS: 3D reconstruction allowed stereoscopic identification of the spatial relationships between physiologic and pathologic structures, and offered quantifiable liver resection proposals based on individualized liver anatomy. The predicted values were consistent with the actual values for tumor mass volume (82.4 ± 109.1 mL vs 84.1 ± 108.9 mL, P = 0.910), surgical margin (10.1 ± 6.2 mm vs 9.1 ± 5.9 mm, P = 0.488), and maximum tumor diameter (4.61 ± 2.16 cm vs 4.53 ± 2.14 cm, P = 0.871). In addition, the number and extent of portal venous ramifications, as well as their relation to hepatic veins, were visualized. Preoperative planning based on simulated resection facilitated complete resection of large tumors located in the confluence of major vessels. And most of the predicted data were correlated with intraoperative findings.
CONCLUSION: This 3DM provides quantitative morphometry of tumor masses and a stereo-relationship with adjacent structures, thus providing a promising technique for the management of centrally located HCCs.
Core tip: Relatively accurate and convenient measurements of morphometric parameters of tumor masses using radiology is important for planning surgical strategy, especially for centrally located hepatic tumors, which should be individualized in each patient with the aim of preserving major vascular branches. In this study, we describe a three-dimensional model to accurately reconstruct the relationships between the tumor, hepatic veins, and Glissonian pedicles for centrally located hepatocellular carcinomas, which is essential for correctly defining the hepatic segments and the limits of tumors with wide variations in anatomy.
- Citation: Tian F, Wu JX, Rong WQ, Wang LM, Wu F, Yu WB, An SL, Liu FQ, Feng L, Bi C, Liu YH. Three-dimensional morphometric analysis for hepatectomy of centrally located hepatocellular carcinoma: A pilot study. World J Gastroenterol 2015; 21(15): 4607-4619
- URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4607.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4607
Centrally located hepatocellular carcinomas (HCCs) are traditionally characterized as tumors in Couinaud’s segments IV, V, or VIII of the liver[1]. Extensive major hepatectomy or mesohepatectomy often offers the best chance of cure[2-4]. However, extensive hepatectomy may sacrifice a large amount of functioning liver and result in suboptimal residual liver volume, causing hepatic failure or death. The en bloc resection of Goldsmith and Woodburne’s[5] left medial and right anterior segments or Couinaud’s segments IV, V, and VIII (mesohepatectomy) have always been satisfactory, but are technically demanding, and often can not be performed safely or are associated with a high risk of tumor recurrence[6]. Therefore, a mathematic finite element model which can demonstrate the structural relationship and provide relatively accurate staging of hepatic tumor masses is urgently required. In addition, such a model is also crucial for determining appropriate treatment, guiding operative strategy, and predicting the prognosis of patients with centrally located HCCs.
The diagnosis of intrahepatic masses has significantly changed over the past decade from the use of invasive procedures such as angiography or biopsy to noninvasive imaging including contrast-enhanced ultrasound (US), multidetector-row computed tomography (MDCT), and magnetic resonance imaging (MRI). CT is the most commonly used imaging modality in diagnosing intrahepatic masses due to its widespread availability and short examination time, and it was estimated that the overall accuracy of MDCT for detection and characterization of HCC was 89% and 43%, respectively, compared with pathologic examination[7]. In addition, MDCT provides detailed mapping and assessment of hepatic arteries, portal veins, and hepatic veins, which can be reconstructed to provide angiographic pictures and is able to replace direct angiograms. However, the anatomic and vascular pathologic details provided by the MDCT scanners do not completely meet the requirements for preoperative planning.
Considering the known difficulties of virtual simulation of centrally located tumor masses by radiologic imaging, we constructed a three-dimensional morphometric analysis model (3DM) of tumor reconstruction with custom-made software, as only a few studies have explored the accuracy and reliability of data-processing using 3D models[8-10]. A risk analysis was also included in the preoperative planning by calculating the volume of tumor and peritumor territories and visualizing the relationships between the masses, portal veins, hepatic veins, and bile ducts, which is essential for correctly defining the hepatic segments, as well as the limits of a tumor with wide variations in anatomy. Therefore, the aims of this study were to describe a customized application framework using Yorktal DMIT software for the 3DM reconstruction and assessment of the morphology of centrally located HCCs on the basis of micro-CT data, and to analyze its reliability in pre- and intraoperative assessment.
Fifty-three patients with complex centrally located HCC masses referred to our institution for surgical treatment from March 2013 to July 2014 were retrospectively investigated in this study. We used a revised definition for centrally located HCC, previously described as “carcinoma adjoined to the porta hepatis, < 1 cm from major vascular structures [including the inferior vena cava (IVC), main portal branches, and main trunks of the hepatic veins] and usually located in Couinaud’s segment I, IV, V, VIII, or at the junction of the central segments”[11]. Patients were preoperatively selected as suitable for hepatectomy by a multidisciplinary team. All patients had undergone a preoperative indocyanine green (ICG) test and surgical resection. Patients with any of the following characteristics were excluded: (1) evidence of extra- or intrahepatic metastasis, satellitosis, multifocal tumors, and other concurrent malignancy; (2) previous radiation, transarterial chemoembolization, or surgery; (3) palliative resection with residual tumor and non-HCC confirmed by postoperative pathology; and (4) postoperative comorbidities due to anesthetic and cardiovascular complications or technical factors. We used the Barcelona Clinic Liver Cancer (BCLC) staging classification for tumor staging and Child-Pugh criteria for liver function evaluation. Tumors with vascular adhesion, but not severe vascular invasion, were not classified as BCLC stage C. The fibrosis score system (0-6 scale) as defined by Ishak et al[12] was used to assess liver fibrosis. A total of 39 patients met these criteria.
All eligible patients were provided written informed consent. This study was approved by the Ethical Committee of the Cancer Institute and Hospital of the Chinese Academy of Medical Science and was performed in accordance with principles of Good Clinical Practice and Declaration of Helsinki guidelines (1975, revised in 1983).
All patients underwent three-phase dynamic contrast-enhanced CT at initial diagnosis. Contrast-enhanced multiphase CT scans were obtained using a 64-section scanner (Light Speed VCT; General Electric Corp., Fairfield, CT, United States). Arterial, portal venous, and delayed phases were obtained by adding 18 s to the time of peak aortic enhancement, 30 s to the end of arterial phase, and 150 s to the end of the portal venous phase, respectively. Scanning parameters were as follows: collimation, 64 rows × 0.5 mm; gantry rotation speed, 0.6 s; section thickness, 5 mm; image reconstruction increment, 1 mm; 120 kVp; and effective tube current-time charge, 450 mAs.
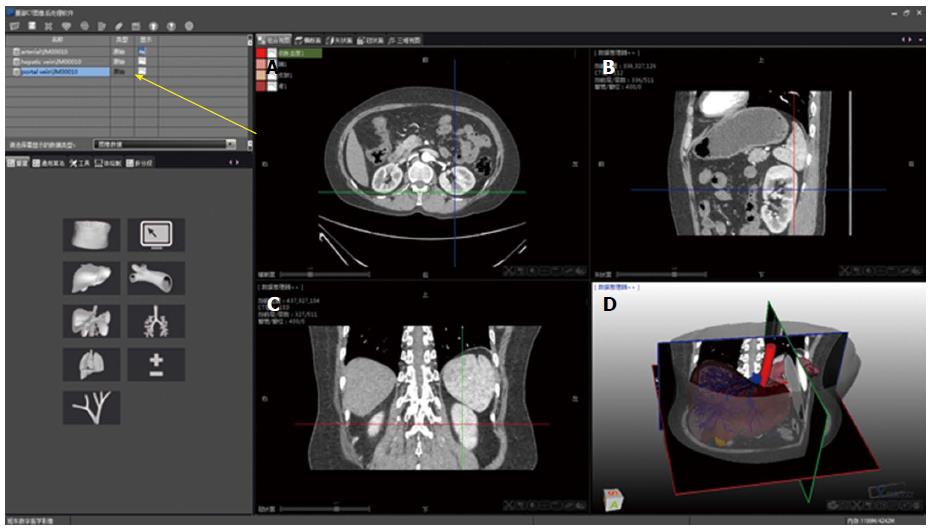
DMIT is software specifically developed by the Yorktal Corporation (Shenzhen, China) for medical image processing. It is used for the segmentation of two-dimensional CT images to generate highly accurate 3D models of the hepatic anatomy. The DMIT package provides a visual data processing environment on its graphic user interface (Figure 1). The boxes on the operation interface are called modules, and each module encapsulates a specific function. Parameters measured by CT were imported and included in the customized application framework, and then the data format was transferred from Dicom to Bitmap. In addition, different functional application frameworks can be established with different image modules (Figure 1), which can be combined with complex image processing networks. Taken as a whole, the various modules comprise a data-flow framework.
The 3D simulation model was composed of Couinaud’s segments I-VIII, portal and hepatic veins, inferior and superior vena cava, abdominal aorta, splenic artery and vein, and adjacent organs. The process consisted of the following steps: (1) extracting the CT data from each organ and accurate segmenting; (2) geometrical measurement of the tumor, portal vein, hepatic vein, and liver parenchyma and individual reconstruction; (3) subtraction and calculation of the intrahepatic vessels to separate the portal system from the hepatic venous system; (4) reconstruction of relationships between the tumor, hepatic veins, and Glissonian pedicles, and geometrical quantification of the distances between them; (5) volumetric measurements of the liver, tumor, and imaged vessels; (6) calculation of the vascular perfusion area in each area based on direction and diameter of the hepatic vessels; and (7) editing multiple resection patterns and performing 3D visualization of the results. The 3D images of the above segments were then overlapped to create integrated 3D images that showed tumor localization and provided detailed hepatic vascular anatomy and macrovascular invasion. Couinaud’s eight segments, hepatic tumor masses, inflow and outflow vascular trees, and adjacent organs were edited using different colors, with multi-faceted visualization acquired accordingly. Rendering views of the specialized structures were created according to the original change, and coronal, sagittal, and transverse sections were thus demonstrated.
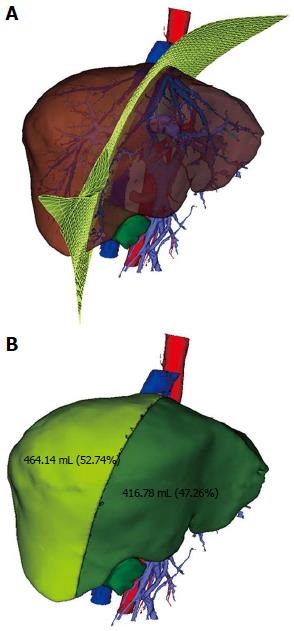
Quantitative information on the volumes of the eight Couinaud’s segments, removed tumor masses, nontumorous parenchyma of the resected liver, and the untouched surface during canal preparation was obtained. The volume of the corresponding sub-territory at risk of an impaired hepatic venous outflow or portal vein inflow was also calculated and formed the basis of the ratio analysis. The volume of large vessels, including the inferior vena cava and the extrahepatic portal vein, the major fissures, and the gallbladder fossa were excluded. The functional liver mass was calculated by subtracting the volume of the potentially resected and blood supply affected zone from the mass of the remnant liver. Thresholds for the ratio of functional liver mass to nontumorous parenchymal volume of the whole liver were set at 50% and 40%, respectively, for patients with chronic liver diseases and without underlying liver disease to predict the resectability of hepatic tumor mass. When the ratio exceeded the threshold, the resection was considered safe. If the calculated functional liver mass of the remnant was below the currently accepted limits, the planned procedure carries a high risk for the development of a small-for-size syndrome.
A specialist in mathematics was invited to assist this study in assessing the corresponding volumes in order to monitor software calculation deviation. The verification formula was as follows:
V = M/∑i = 1 d Ni/∑j = 1 e Lij = de M/∑i = 1 Ni/∑j = 1 Lij
where d is distance between the two layers, e is distance between the two lines, M is the number of layers, Ni is the number of lines in layer i, and Lij is the length of line j in layer i. The data were then compared with those obtained from the software workstation to monitor reliability.
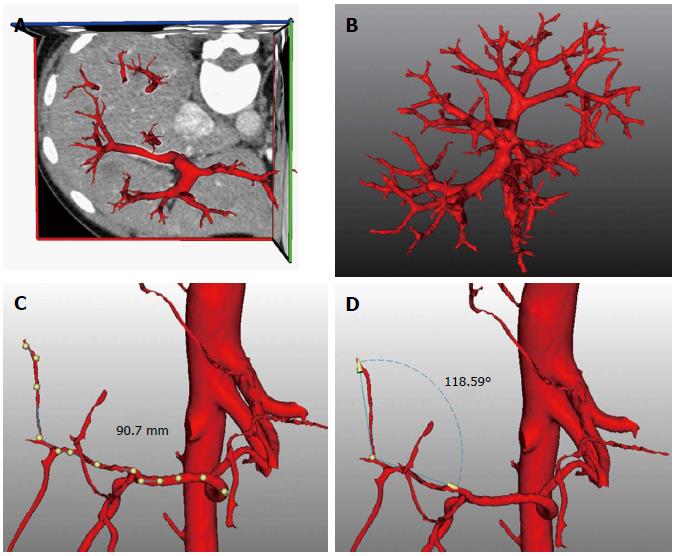
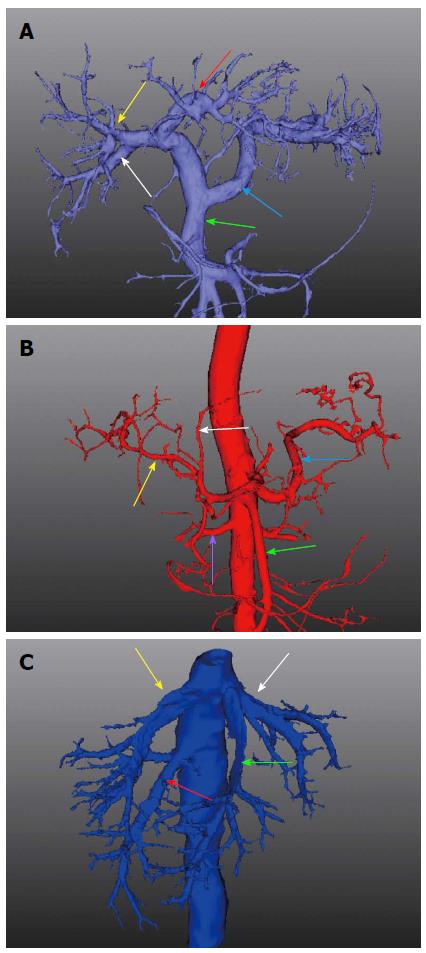
The enhanced CT data of the vessels were extracted automatically using a standard threshold technique due to the use of a contrast agent. An algorithm based on the fractal approach recognized the vessels starting from a seed point near the major branches and gradually searched for all ramification structures with a diameter > 1 mm. The transectional diameter and bending angle of vessel canals were also calculated with individualized wide variations, allowing intuitive interpretation and measurement (Figure 2). The resulting vessel tree was visualized using different colors for the hepatic artery, portal vein and hepatic vein systems. Calculation of the portal venous supply and the hepatic venous drainage area in each area based on the direction and diameter of the hepatic vessels was also carried out. By using this method for data analysis, individualized territories that were supplied or drained by a certain vascular branch were fully identified automatically.
Preoperative resection margin was estimated by measuring the minimal distance between the remnant parenchymal border and tumor rim in the predicted resection plane. We calculated the distance in a preinstalled algorithm where the predicted margin equaled the distance between slice thresholds. Postoperative actual resection margin was measured by calculating minimal vertical parenchymal thickness in the resected tumor specimen surface. The preoperative estimated and postoperative actual resection margins were then compared to assess the accuracy of the 3DM.
The operative procedure for liver resection was then imitated in this complex image-processing network. Environments for anatomic curative resections defined as mesohepatectomy (removal of segments IV, V, and VIII), right anterior sectionectomy (removal of segments V and VIII), segment IV resection, caudate lobe resection, or other non-anatomic irregular hepatectomies were established according to tumor size, location, degree of hepatic cirrhosis, and individual resection ranges in individual patients. The established 3DM of the liver was then imported to the intraoperative computer workstation to guide the surgical resection of hepatic tumor masses, which was viewed from the visceral and diaphragmatic surfaces, and simulation of the whole process was also performed with a virtual irregular dissection plane in the system. Prior to division of the parenchyma, a selective and dynamic region-specific vascular occlusion (SDRVO) technique[11] was also used and appropriate areas were selected for inflow hepatic blood occlusion depending on tumor location and the resection area. In cases where tumors were adherent to major vascular structures, we carefully dissected and resected lesions away from the vascular surface using a cavitational ultrasonic surgical aspirator (CUSA). When vascular wall injury or rupture occurred, stay sutures were applied for control of hemostasis and bile leaks. From the workstation, we also investigated the cross-sectional images before and after canal preparation and calculated the untouched surface in the canal preparation three-dimensionally. The viewing direction and dissection plane were presented in the graphic interface and could be chosen arbitrarily. The relationship between the dissection plane and the tumor edge was followed in real time. With the direction of the dissection plane modified when needed, the remnant functional liver volume, venous territories, and affected vascular tree were automatically calculated (Figure 3). The ratio of pre- and postoperative volumes of sound liver tissue was described and the optimal threshold was suggested. The combination of the information obtained from the analysis of volume, associated vessel trees, and simulation of different resection strategies resulted in an efficient computer-assisted preoperative risk analysis system. All the operations were completed by the same surgical team with the aim of standardizing operative quality and safety. For synchronous surgical guidance, dynamic computerized 3DMs were directly transferred and displayed on flat screen monitors near the operation field. Following tumor removal, several silver marks were sutured onto the two resection planes to set marks for postoperative radiotherapy.
All values are expressed as a median or mean ± standard deviation. Student’s t-tests were used to evaluate the differences between comparisons. Correlations were presented as scatter plots and used to assess the predictive accuracy of the 3DM in preoperative evaluation of volume, resection margin, and tumor diameter. A P < 0.05 was considered statistically significant. All analyses were performed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, United States). The statistical methods of this study were reviewed by a biostatistician from the medical statistical department in our hospital.
The demographic, clinical, and pathologic characteristics of the 39 patients with a mean age of 54.3 ± 12.1 years (range: 32-80 years) are shown in Table 1. All patients had complete simulation data and subsequently underwent liver resection. Cirrhosis was found in 24 (61.6%) patients in the background liver on histological examination, with hepatitis B as the most common cause of chronic liver disease. All patients in this study had a liver function of Child-Pugh class A and the median 15 min retention rate of ICG (ICG-R15) was 5.2%. Three patients had an ICG-R15 > 20% and were high-risk patients for surgical treatment, but underwent successful hepatectomy. There was no 30-day operative mortality or serious complications that required additional surgery. Eight complication events occurred in six (6/39; 15.4%) patients. All these postoperative complications resolved after conservative treatment. Child-Pugh C status occurred transiently on postoperative day 7 in 4/39 (10.3%) patients, which was managed successfully when patients were discharged. The mean duration of postoperative hospitalization was 9.1 ± 2.3 d (range: 5-16 d).
| Variables | Value |
| Gender | |
| Male | 34 (87.1) |
| Female | 5 (12.9) |
| Hepatitis B carrier | 37 (94.8) |
| Hepatitis C carrier | 2 (5.1) |
| ICG-R15 | 7.1% ± 5.9% |
| Pathologic characteristics | |
| Fibrosis score F0 | 15 (38.4) |
| Fibrosis score F1 | 24 (61.5) |
| Tumor diameter (cm) | 4.5 ± 2.1 |
| Resection margin (mm) | 9.1 ± 5.9 |
| Vascular adhesion | 11 (28.2) |
| Liver capsule invasion | 24 (61.5) |
| Major vascular invasion | 3 (7.7) |
| BCLC staging | |
| Stage A | 25 (64.1) |
| Stage B | 11 (28.2) |
| Stage C | 3 (7.7) |
| Surgical procedure | |
| Hemi-hepatectomy | 7 (17.9) |
| Mesohepatectomy | 5 (12.8) |
| Segmentectomy | 13 (33.3) |
| Irregular resection | 14 (35.9) |
| Postoperative complications | |
| Massive hemorrhage | 2 (5.1) |
| Bile leakage | 2 (5.1) |
| Massive ascites | 3 (7.7) |
| Pleural effusion | 1 (2.6) |
| Hospital mortality | 0 (0.0) |
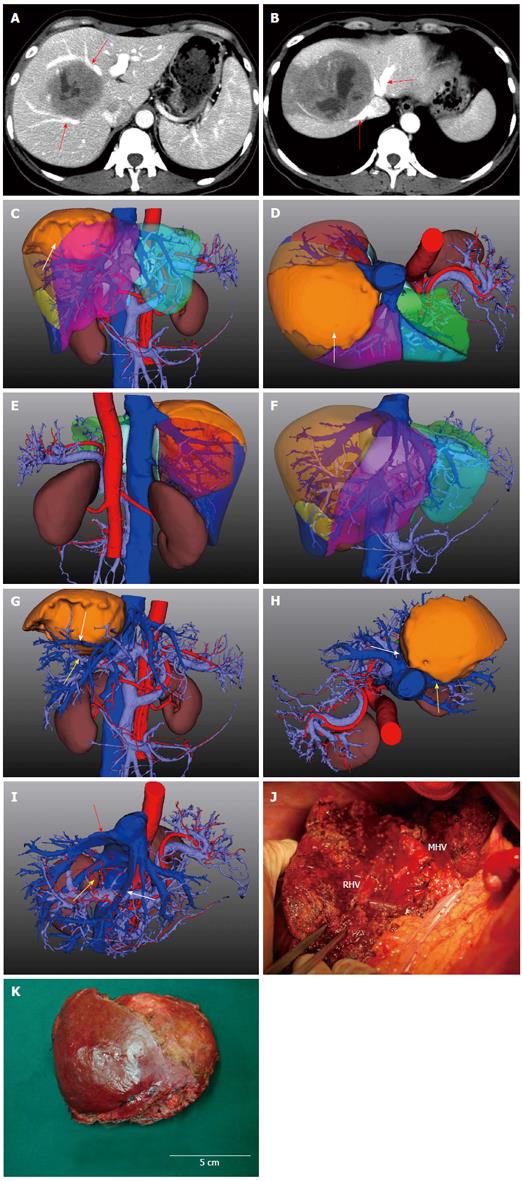
3D images of the liver, vascular system, bile duct system, and adjacent organs were reconstructed and the manipulation environment presented the model with high-quality visualization (Figure 4). The framework provided 3D quantitative information with a color-coded map of the five lobes and eight segments using Couinaud’s method, which quantified the characteristics of the internal and external anatomy of the tumor masses. Tumor infiltration of vessels, liver capsule, or adjacent tissues were identified and demonstrated more precisely compared with the original CT data. The hepatic artery, portal vein, and hepatic vein system were stained red, light blue, and dark blue, respectively. Individual segments or organs could be added or concealed arbitrarily to clearly represent the target structure. In cases where tumors were adherent to major vascular structures, the tumor masses were concealed to demonstrate whether intravascular invasion had occurred. In addition, the nutritional arteries for tumor tissue were also viewed to guide surgical operation and postoperative transarterial chemoembolization.
3D images of the abdominal aortic, portal vein and hepatic vein systems were anatomically reconstructed to view the patient’s individual vessel stratifications (Figure 5). Of the 39 livers investigated, a conventional right-left branching pattern of the main portal vein was seen in 27 cases. In seven cases, a right-middle-left trifurcation of the portal vein was observed. In addition, a relatively large right posterior branch in five cases originated from the portal vein. At the second level ramification, the mean number of branches originating from the left and the right portal vein was 18 (range: 11-38). For the classification of hepatic veins, three types of veins were found. The most common type (approximately 56%) belonged to the left-middle and right form, in which the left and middle hepatic veins flowed into a confluence and then joined the IVC, but the right vein joined it alone. Second, the left-middle-right type accounted for 37%, in which three hepatic veins joined the IVC separately. Third, the left-middle-right-right posterior type accounted for approximately 7%, in which the right posterior supramarginal vein and the main right vein joined the IVC separately.
Vascular variations were also demonstrated and evaluated; right posterior inferior hepatic veins were identified in 31/39 (79.4%) cases, caudate hepatic veins were confirmed by a mean of six branches in each patient with a median diameter of 3.7 mm, and the superior suprarenal vein was also found to converge into the right posterior hepatic veins in 23.1% (9/39) cases.
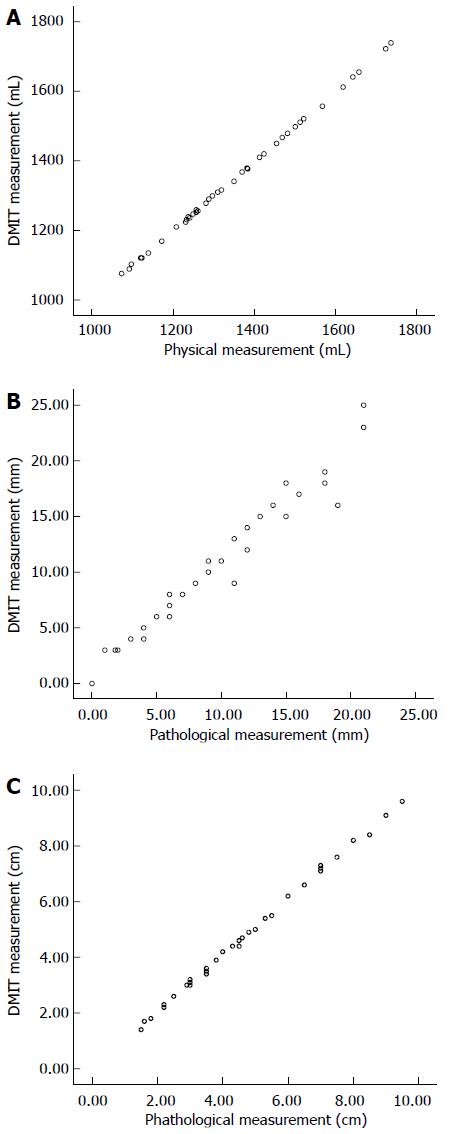
Quantitative information regarding the volume of tumor masses, peritumor parenchyma of the resected liver, and the functional liver mass was obtained. The mean and median whole liver volume calculated by DMIT software were 1348.9 ± 179.2 and 1310 mL, respectively (n = 39; range: 1076-1739 mL), which were less than those by physical measurement (1351.2 ± 185.1 and 1330 mL, respectively). However, the difference was not statistically significant (P = 0.954) (Figure 6A). The mean volume of tumor mass and functional liver mass measured using the software workstation calculation were 82.4 ± 109.1 mL and 1022.1 ± 153.5 mL, respectively, similar results regarding the volume of tumor mass were also measured by the mathematics specialist (84.1 ± 108.9 mL) (P = 0.910). The average κ between the 3D software calculation and manual counting was 0.93 ± 0.12. The functional liver mass could not be computed by conventional planimetric MDCT data. The ratio of functional liver mass to nontumorous parenchymal volume of the whole liver ranged from 55.7 to 88.1%, and no cases developed a small-for-size syndrome.
In 36/39 resections, the actual margin achieved from the resected specimen ranged from 1 to 21 mm, with a median of 9 mm. The predicted and actual margins did not differ significantly (10.1 ± 6.2 mm vs 9.1 ± 5.9 mm, P = 0.488) (Figure 6B). The resection margin could not be determined by planimetric MDCT data. Three patients with centrally located tumors adherent to or compressing major vessels who demonstrated a 0 mm resection margin in the simulation system ultimately underwent successful hepatectomy with exposure of the tumor surface.
The predictive accuracy of the 3DM software for preoperative calculation of maximum tumor diameter was assessed by comparing results with postoperative pathologic examinations. Simulated maximum tumor diameter was consistent with the pathologic maximum diameter (4.61 ± 2.16 cm vs 4.53 ± 2.14 cm, P = 0.871) (Figure 6C).
All cases withstood radical hepatectomy using DMIT for prediction. And the high predictive accuracy for risk analysis was remarkable. The environments for mesohepatectomy, right and left hepatectomies, segmentectomy, or irregular hepatectomies were simulated and conducted by different methods for the various circumstances. During parenchymal dissection using the SDRVO maneuver, the origin of the right-left portal vein and three dorsal hepatic veins were divided and identified as landmark structures, along which the tumor masses deeply located were detected precisely and the dissection lines were marked. The line of dissection was adjusted when the safety margin was guaranteed, and the vessels or bile ducts located in the security margin were also detected. By combining this information, major pedicles were well preserved during real-time surgical guiding on the computer-assisted workstation. Clipping the first- or second-order level branches of the portal vein and hepatic vein resulted in a prompt calculation of the affected area during curative resection. To secure maximal residual liver volume and minimal vascular tree injury, the tumor masses adherent to or compressing major vessels were meticulously dissected from the vascular walls.
Simulated 3D resection in one case showed that a huge HCC which was fixed at the confluence of the right and middle hepatic veins was feasible for complete resection. However, it could not be judged definitively by the conventional data (Figure 4). In another case, hepatectomy of the left lobe appeared possible after conventional ultrasound imaging and CT scan assessment. However, 3D reconstruction revealed an infiltration into the middle hepatic vein and ultimately mesohepatectomy with middle hepatic vein ligation at the root was performed.
Surgery remains the most effective treatment and provides a chance of cure for patients with centrally located HCCs. Conventional extensive hepatectomy carries a considerable risk of liver failure due to compromised liver functional reserve, particularly in patients with chronic liver disease. The nomenclature for mesohepatectomy, defined according to the Brisbane 2000 system[13], is also referred to as middle hepatic lobectomy, central hepatectomy, and central bisectionectomy in the literature. This procedure often preserves more liver parenchyma and is theoretically associated with a better recovery in the short term[4]. However, mesohepatectomy is also difficult, time-consuming, and a technically demanding procedure[14]. Lee et al[2] retrospectively reviewed 436 patients with HCCs operated on with curative intent and reported mesohepatectomy in only 6.2% of patients. The presence of two hepatic parenchymal transections with proximity to important vascular structures makes it technically more complex than hemi-hepatectomy. Moreover, in clinical practice, we found that a large number (> 60%) of patients with centrally located HCCs had tumors adherent to or compressing major vascular structures. Therefore, the operation fields are often bordered by two cut surfaces and with exposure of important structures, such as the great vessels and main branches of the bile duct. Improper ligation of these important branches may result in ischemia or necrosis of the residual liver, and may lead to liver failure and death[15].
Due to the great variability of Glissonian pedicles and hepatic veins, preoperative understanding of the relationship between the tumor and these structures is of great importance. Recently, with the wide usage of intraoperative ultrasonography and a CUSA, major vessels and intrahepatic bile ducts can be localized, visualized, skeletonized, and meticulously controlled. Lee et al[2] recommended the use of cholangiography during preoperative MRI or intraoperative exploration to characterize the HCC, which can provide information on bile duct anatomy, including variations and the relationship with the tumor, which might help prevent bile duct injury. They demonstrated that after completion of the resection, any bile leak from the resection planes could be detected by infusion of water and air simultaneously via the cystic duct. In their study, preoperative angiography and reconstruction of computed tomograms were used to assess tumor resectability and to determine the resection line. Fang et al[16] described an abdominal image processing system to reconstruct a 3DM of the liver based on CT scan data for volume calculation and identification of vascular variations. The reconstructed model of the abdominal blood vessels and the liver was digitally consistent with the anatomy, different methods were available for selection, and liver transplantation was subsequently simulated. However, to the best of our knowledge, reports of 3D geometric visualization specialized for centrally located HCCs are rare.
It is well known that hepatic functional reserve is highly related to the quantity and quality of liver cells. Previous work has shown that the larger the resected normal liver volume, the greater the risk of liver failure[17]. Hence, estimating the percentage of the resected nontumorous parenchymal volume and the ratio of functional liver mass is necessary. In the present study, we designed a morphometric analysis model of tumor reconstruction with DMIT software based on MDCT scans, which included all anatomic structures that were relevant for surgical resections. Computerized 3D CT-based visualization of the liver is a fast, standardized, and noninvasive procedure that may replace multiple and invasive diagnostic approaches. This system demonstrated accuracy in predicting the liver resection volume as well as the surgical resection margin. Yamanaka et al[18] used a simulation system to validate volumetric accuracy, and compared the predicted liver resection volume with actual weight of the resected specimen, assuming that 1 g of liver tissue had a volume of 1 mL. They concluded that in patients undergoing major hepatectomy, a strong positive correlation was evident between simulation-predicted liver resection volume and actual weight of the resected specimen. Nevertheless, the density of liver tissue is not completely equal to that of water (1 g/mL) and always depends on the degree of cirrhosis. When the degree of cirrhosis is higher, the density of liver tissue is often greater (> 1 g/mL). In our study, we invited a mathematics specialist to assist in assessing the volume of functional liver mass and tumor mass in order to monitor software calculation deviation. Using an algebraic and geometric algorithm, the volume can be verified in a more objective way. The mean whole liver volume and tumor mass volume calculated by DMIT software demonstrated high similarity to physical measurement (κ = 0.93 ± 0.12).
Some centrally located HCCs are often adherent to or compress major vessels. The presence of two cut surfaces with proximity to important vascular structures makes it technically more complex than hemi-hepatectomy. Moreover, large tumors located in the central portion of the liver are usually associated with an increased risk of intraoperative bleeding[4]. In the present series, preoperative 3D simulation facilitated visualization of the crucial extra- and intrahepatic portal vein anatomy, recognition of hepatic vein variants, and determination of the optimal point of surgical division. Computer supported visualizations also enable virtual display of the divided Couinaud’s pedicle branches at the site of the resection surface. In addition, we applied the SDRVO technique, which was reported to control intraoperative bleeding, to avoid ischemia and ischemia-reperfusion injury of the entire liver. Maintaining portal vein blood flow reduces intraoperative visceral congestion and facilitates a more rapid postoperative recovery. The longer inflow occlusion time also allows surgeons to dissect, skeletonize, and manage major vessels and bile ducts in a more relaxed manner, allowing removal of more complex centrally located HCCs. In this study, the digitized reconstruction of the hepatic portal vein and proper hepatic artery converged in the porta hepatis, which made it possible to distinguish all the variations clearly so that the surgeons were able to avoid injury to the variant hepatic vessels during the SDRVO technique.
Although three patients with centrally located tumors adherent to or compressing major vessels demonstrated a 0 mm resection margin in the simulation system, resection with exposure of the tumor surface was ultimately carried out safely. In the software interface, the safety margin was marked by setting a threshold calculated from the minimum safety distance. By combining this information with the image of the segmented vessels, the vessels located in the safety margin were detected. During the operation, tumors were meticulously dissected from the exposed surface of the main vascular structures, and CUSA and sutures were applied for vascular surface dissection and vascular wall injury repair. The result of a preoperatively evaluated 0 mm resection margin obtained by the simulation system was not considered a contraindication for resection of centrally located HCCs that were adherent to or compressed major vessels. This technique provides an opportunity for hepatectomy with exposure of the tumor surface, and although technically demanding, it can be performed safely.
Yu et al[19] reported that adjuvant radiotherapy for centrally located HCCs after narrow-margin hepatectomy was technically feasible and relatively safe, and a post-hoc subgroup comparison showed that adjuvant radiotherapy considerably improved recurrence-free survival. As the growth pattern of HCCs is usually pushing rather than invading, and most intrahepatic recurrences arise from multicentric carcinogenesis or are distant from the resection margin, obtaining a wide negative surgical margin is usually not necessary[4,20-22]. The predictive accuracy of the 3DM software for preoperative BCLC staging in this study was also significantly higher than that by MDCT assessment. As the maximum tumor diameter can only be measured in the transectional plane by conventional CT imaging, our simulation allowed not only a more accurate, but also a more detailed stereoscopic measurement of the maximum tumor diameter, including whether there was vascular invasion or portal hypertension. Therefore, the application of virtual 3DM software was useful in preoperative staging and in making appropriate treatment decisions.
All the patients in this study had a liver function of Child-Pugh class A, and the median ICG-R15 was 5.2%. Three patients had an ICG-R15 > 20%, which was too high for major hepatic resection to be performed in cirrhotic patients. However, when using DMIT for prediction, the decision to perform hepatectomy was made. Through virtual tumor resection, the surgeon can judge the parenchyma of organs, identify areas at risk of insufficient blood supply or blood drainage, and estimate the postoperative liver function. Depending on the exploration of various strategies and the results of different risk-analysis calculations, the optimal surgical strategy can be chosen and documented, and the potential benefit from preserving as much functional liver mass as possible can also be predicted. Therefore, morbidity and mortality rates in the present series were more advantageous than those in the literature[1-3,23]. Biliary complications developed in two patients and one patient had pleural effusion, which is lower than the published rates of 5%-11%. In a previous report, the incidence of complications after mesohepatectomy ranged from 17.0% to 26.3%, and the reported surgical mortality was between 0% and 6.25%[1]. The incidence of Child-Pugh C status on postoperative day 7 and postoperative hospitalization were also favorable compared with previous studies[3,4,24]. This may be attributed to a small amount of necrosis in the blood supply affected zone, more preserved functional liver mass, and sufficient hepatic venous outflow, as well as successful protection of portal and arterial flow.
From our experience, computer-assisted simulation and a risk analysis system specifically for centrally located HCCs provide a reliable preoperative 3D view of the spatial relationship between the tumor mass and adjacent structures. With this, surgeons are able to optimize the operation strategy and are assisted by direct intraoperative surgical guidance. Previous literature on computerized CT-based 3D visualization of liver resection has mainly focused on living-related liver transplantation or huge HCC resection[4,9,10,25,26], and no virtual simulation customized for centrally located HCCs have been introduced. In our single HCC center, hepatectomy guided by the 3DM was associated with lower rates of complications, shorter postoperative hospitalization, and faster recovery. Therefore, our virtual hepatectomy should be implemented routinely for centrally located HCCs, which are more complicated and technically demanding. More prospective cohort studies are necessary to investigate the advantages and convenience of 3D interactive visualization and quantitative evaluation that is ready for routine use during perioperative treatment of centrally located HCCs.
The treatment of patients with hepatic tumors lies a considerable portion of the workload of abdominal surgeons. Relatively accurate and convenient measurements of morphometric parameters of tumor masses using radiology are important for planning the surgical strategy, especially for resection of centrally located hepatic tumors, which should be individualized in each patient with the aim of preserving major vascular branches. To the best of our knowledge, three-dimensional geometric visualization specifically for centrally located hepatocellular carcinomas (HCCs) has rarely been reported, as literature values are mainly based on two-dimensional imaging data.
This three-dimensional model, which is a computer-assisted simulation and risk analysis system specifically for centrally located HCCs, can provide a reliable preoperative three-dimensional view of the spatial relationship between the tumor mass and adjacent structures. Surgeons are able to optimize the operation strategy and are assisted by direct intraoperative surgical guidance. In the surgical resection of centrally located HCCs, the research hotspot is how to correctly define the hepatic segments, understand the relationships between the tumor masses, portal veins, hepatic veins, and bile ducts, and determine appropriate treatment, guide operative strategy, and simultaneously predict prognosis.
The diagnosis of intrahepatic masses has significantly changed over the past decade from the use of invasive procedures such as angiography or biopsy, to noninvasive imaging including contrast-enhanced ultrasound, multidetector-row computed tomography (MDCT), and magnetic resonance imaging. CT is the most commonly used imaging modality for diagnosing intrahepatic masses due to its widespread availability and short examination time, and it was estimated that the overall accuracy of MDCT for the detection and characterization of hepatocellular carcinoma is 89% and 43%, respectively, compared with pathologic examination. However, the anatomic and vascular pathologic details provided by MDCT scanners do not completely meet the requirements for preoperative planning. Therefore, a mathematical finite element model that can demonstrate the structural relationship and provide relatively accurate staging of hepatic tumor masses is necessary. In addition, this model is also crucial for determining appropriate treatment, guiding operative strategy, and predicting the prognosis of patients with centrally located HCCs. To overcome these disadvantages, the authors constructed a morphometric analysis model of tumor reconstruction using DMIT software based on MDCT scans, which included all anatomic structures that were relevant for surgical resections. This computerized three-dimensional model is a fast, standardized, and noninvasive procedure that may replace multiple and invasive diagnostic approaches. It can accurately reconstruct the relationships between the tumor, hepatic veins, and Glissonian pedicles in centrally located HCCs, which is essential for correctly defining the hepatic segments and the limits of a tumor with wide variations in anatomy.
The study results suggest that the three-dimensional model is potentially advantageous and could be implemented routinely for centrally located HCCs, which are more complicated and technically demanding. In these single liver-tumor treatment center, hepatectomy guided by the three-dimensional model was associated with lower rates of complications, shorter postoperative hospitalization, and faster recovery.
The authors detailed the use of computational three-dimensional modeling based on CT liver scans of patients with HCC and determined if this model can improve treatment. The manuscript represents an interesting technologic advance and asserts that such a model improves patient care. While the number of patients is likely not large enough to prove an improvement in care, it is a rational assertion that the more information that the surgeon has before and during excision of a hepatic tumor, the better.
P- Reviewer: Felmlee DJ S- Editor: Qi Y L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Stratopoulos C, Soonawalla Z, Brockmann J, Hoffmann K, Friend PJ. Central hepatectomy: the golden mean for treating central liver tumors? Surg Oncol. 2007;16:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Lee JG, Choi SB, Kim KS, Choi JS, Lee WJ, Kim BR. Central bisectionectomy for centrally located hepatocellular carcinoma. Br J Surg. 2008;95:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Mehrabi A, Mood ZA, Roshanaei N, Fonouni H, Müller SA, Schmied BM, Hinz U, Weitz J, Büchler MW, Schmidt J. Mesohepatectomy as an option for the treatment of central liver tumors. J Am Coll Surg. 2008;207:499-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Cheng CH, Yu MC, Wu TH, Lee CF, Chan KM, Chou HS, Lee WC. Surgical resection of centrally located large hepatocellular carcinoma. Chang Gung Med J. 2012;35:178-191. [PubMed] |
| 5. | Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet. 1957;105:310-318. [PubMed] |
| 6. | DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178-184. [PubMed] |
| 7. | Luca A, Caruso S, Milazzo M, Mamone G, Marrone G, Miraglia R, Maruzzelli L, Carollo V, Minervini MI, Vizzini G. Multidetector-row computed tomography (MDCT) for the diagnosis of hepatocellular carcinoma in cirrhotic candidates for liver transplantation: prevalence of radiological vascular patterns and histological correlation with liver explants. Eur Radiol. 2010;20:898-907. [PubMed] |
| 8. | Lang H, Radtke A, Liu C, Frühauf NR, Peitgen HO, Broelsch CE. Extended left hepatectomy--modified operation planning based on three-dimensional visualization of liver anatomy. Langenbecks Arch Surg. 2004;389:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Lang H, Radtke A, Hindennach M, Schroeder T, Frühauf NR, Malagó M, Bourquain H, Peitgen HO, Oldhafer KJ, Broelsch CE. Impact of virtual tumor resection and computer-assisted risk analysis on operation planning and intraoperative strategy in major hepatic resection. Arch Surg. 2005;140:629-638; discussion 638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Yamanaka J, Okada T, Saito S, Kondo Y, Yoshida Y, Suzumura K, Hirano T, Iimuro Y, Fujimoto J. Minimally invasive laparoscopic liver resection: 3D MDCT simulation for preoperative planning. J Hepatobiliary Pancreat Surg. 2009;16:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Yu W, Rong W, Wang L, Wu F, Xu Q, Wu J. R1 hepatectomy with exposure of tumor surface for centrally located hepatocellular carcinoma. World J Surg. 2014;38:1777-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Ishak KG, Zimmerman HJ. Morphologic spectrum of drug-induced hepatic disease. Gastroenterol Clin North Am. 1995;24:759-786. [PubMed] |
| 13. | Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 680] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 14. | Hu RH, Lee PH, Chang YC, Ho MC, Yu SC. Treatment of centrally located hepatocellular carcinoma with central hepatectomy. Surgery. 2003;133:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Wu CC, Ho WL, Chen JT, Tang CS, Yeh DC, Liu TJ, P’eng FK. Mesohepatectomy for centrally located hepatocellular carcinoma: an appraisal of a rare procedure. J Am Coll Surg. 1999;188:508-515. [PubMed] |
| 16. | Fang CH, Li XF, Li Z, Fan YF, Lu CM, Huang YP, Peng FP. Application of a medical image processing system in liver transplantation. Hepatobiliary Pancreat Dis Int. 2010;9:370-375. [PubMed] |
| 17. | Tu R, Xia LP, Yu AL, Wu L. Assessment of hepatic functional reserve by cirrhosis grading and liver volume measurement using CT. World J Gastroenterol. 2007;13:3956-3961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Yamanaka J, Saito S, Iimuro Y, Hirano T, Okada T, Kuroda N, Sugimoto T, Fujimoto J. The impact of 3-D virtual hepatectomy simulation in living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Yu W, Wang W, Rong W, Wang L, Xu Q, Wu F, Liu L, Wu J. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (< 1 cm): a prospective randomized study. J Am Coll Surg. 2014;218:381-392. |
| 20. | Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 21. | Matsui Y, Terakawa N, Satoi S, Kaibori M, Kitade H, Takai S, Kwon AH, Kamiyama Y. Postoperative outcomes in patients with hepatocellular carcinomas resected with exposure of the tumor surface: clinical role of the no-margin resection. Arch Surg. 2007;142:596-602; discussion 603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Chouillard E, Cherqui D, Tayar C, Brunetti F, Fagniez PL. Anatomical bi- and trisegmentectomies as alternatives to extensive liver resections. Ann Surg. 2003;238:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Kim WW, Lee KW, Choi SH, Heo JS, Kim YI, Kim SJ, Lee DS, Lee HH, Paik SW, Koh KC. [Risk factors of morbidity and mortality following surgical resection for hepatocellular carcinoma]. Korean J Hepatol. 2004;10:51-61. [PubMed] |
| 24. | Giuliante F, Nuzzo G, Ardito F, Vellone M, De Cosmo G, Giovannini I. Extraparenchymal control of hepatic veins during mesohepatectomy. J Am Coll Surg. 2008;206:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Yamanaka J, Saito S, Fujimoto J. Impact of preoperative planning using virtual segmental volumetry on liver resection for hepatocellular carcinoma. World J Surg. 2007;31:1249-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Fuchs J, Warmann SW, Szavay P, Kirschner HJ, Schäfer JF, Hennemuth A, Scheel-Walter HG, Bourquain H, Peitgen HO. Three-dimensional visualization and virtual simulation of resections in pediatric solid tumors. J Pediatr Surg. 2005;40:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |