Published online Apr 21, 2015. doi: 10.3748/wjg.v21.i15.4555
Peer-review started: July 31, 2014
First decision: August 27, 2014
Revised: September 20, 2014
Accepted: December 5, 2014
Article in press: December 8, 2014
Published online: April 21, 2015
Processing time: 264 Days and 16.3 Hours
AIM: To study the effect of hydrogen sulfide (H2S) on severe acute pancreatitis (SAP) in a rat model.
METHODS: Sprague-Dawley (SD) rats were administered an intraperitoneal injection of saline containing 20% L-Arg (250 mg/100 g) hourly for over 2 h to induce SAP. The rats were treated with DL-propargylglycine (PAG, 50 mg/kg) or different dosages of NaHS (5 mg/kg, 10 mg/kg, 20 mg/kg or 100 mg/kg). PAG or NaHS was administered 1 h before induction of pancreatitis. Rats were sacrificed 24 h after the last L-Arg injection. Blood and pancreas tissues were collected.
RESULTS: The H2S and cystathionine-γ-lyase mRNA levels in SAP rats were significantly lower than those in the control group, and treatment with PAG further reduced the H2S level. Nevertheless, H2S was significantly increased after NaHS administration compared with the SAP group, and the degree of upregulation was associated with the NaHS dosage. NaHS reduced the levels of plasma amylase, interleukin-6 and myeloperoxidase in pancreatic tissue. NaHS suppressed the degradation of IκBα and the activity of nuclear factor-κB, as well as the phosphorylation of PI3K/AKT.
CONCLUSION: H2S plays an anti-inflammatory role in SAP in vivo.
Core tip: In this research, we provide wide-ranging dosages of exogenous H2S (NaHS), from 5 mg/kg to 100 mg/kg, to study the role of H2S in severe acute pancreatitis (SAP) in vivo, and finally found that the level of H2S was reduced in rat SAP models, suggesting that H2S donor NaHS reduced the inflammatory indices of SAP, by inhibiting PI3K/AKT phosphorylation and down-regulating IκBα degradation and nuclear factor-κB activity.
-
Citation: Rao CY, Fu LY, Hu CL, Chen DX, Gan T, Wang YC, Zhao XY. H2S mitigates severe acute pancreatitis through the PI3K/AKT-NF-κB pathway
in vivo . World J Gastroenterol 2015; 21(15): 4555-4563 - URL: https://www.wjgnet.com/1007-9327/full/v21/i15/4555.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i15.4555
Severe acute pancreatitis (SAP) is an inflammatory condition of the pancreas that is characterized by elevated pancreatic enzymes in the blood, systemic inflammatory response syndrome (SIRS), and multiple organ dysfunctions syndrome (MODS), which is ultimately responsible for most pancreatitis-associated mortality and morbidity[1]. Since the study by Rindernecht in 1988[2] implicated cytokines and stress-activated pathways in SAP, cytokines have received much attention in SAP. These inflammatory mediators interact with microcirculation, thereby increasing vascular permeability and inducing thrombosis and hemorrhage and ultimately leading to tissue necrosis[1]. Thus, blocking inflammatory cytokine expression at the time of initial injury might obstruct the development of inflammation. However, various cytokines participate in the inflammatory response in SAP, and the efficient regulation of the production and interaction of cytokines is important for preventing and treating SAP. Therefore, it is reasonable to speculate that regulating the key cytokines and signal transduction pathways in inflammatory responses will help develop an effective anti-inflammatory therapy for SAP.
Nuclear factor kappa B (NF-κB) comprises a family of transcriptional activators, which include homodimers or heterodimers of RelA (p65), p50, p52, c-Rel, and RelB[3]. In resting cells, NF-κB is sequestered in the cytoplasm via interaction with the inhibitor of κBα (IκBα) proteins[4]. The phosphorylation of IκBα induces ubiquitination and proteasomal degradation upon stimulation, and the subsequent release of NF-κB transcription factors, which translocate to the nucleus and bind to cognate DNA binding sites to initiate the transcription of a wide array of genes, including cytokines[4]. IκBα phosphorylation is triggered by activating the IκB kinase (IKK) complex, through serine/threonine kinase (AKT). AKT is a major downstream target of phosphatidylinositol-3-kinase (PI3K) and is rigorously modulated by PI3K. PI3K inhibition reduces the LPS-induced expression of interleukin (IL)-1β in mouse macrophages[5].
Hydrogen sulfide (H2S) is a small molecular weight liposoluble gas, which permeates through the cell membrane freely to exert important physiological functions. H2S is predominantly produced through the action of three enzymes: cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE), which generate H2S using L-cysteine as a substrate and pyridoxal 5’-phosphate (PLP) as a cofactor[6], and 3-mercaptopyruvate sulfurtransferase (3-MST), which produces H2S using 3-mercaptopyruvate, a metabolite of L-cysteine, as a substrate[7]. CSE is the major H2S-producing enzyme in the peripheral tissue. Numerous studies have shown the beneficial effects of H2S, particularly in cardiovascular and neurological disorders[8]. However, the role of H2S in inflammation is only recently beginning to emerge, and the exact role of this gas is still not clearly understood.
In this study, we aimed to investigate the hypothesis that the exogenous H2S vehicle (NaHS) could be beneficial against SAP induced by intraperitoneal administration of L-Arg in Sprague-Dawley (SD) rats in vivo. We observed that H2S donor NaHS protects against experimental SAP in vivo by suppressing NF-κB activation via the down-regulation of PI3K/AKT phosphorylation.
Animal care and experimental protocols were performed in compliance with the animal management guidelines of the Government of the People’s Republic of China. Male SD rats (223-265 g) were obtained from the Animal Center, Daping Hospital, Third Military Medical University. The rats were randomly assigned to either a control (n = 7) or an experimental (n = 10) group.
The control group animals were given hourly intraperitoneal injections of normal saline.
The SAP group animals were given hourly intraperitoneal injections of saline containing 20% L-Arg (250 mg/100 g) for over 2 h[9].
The PAG + SAP group animals were treated as previously described for the SAP group above, except that PAG (50 mg/kg, Sigma), a CSE inhibitor, was dissolved in saline and administered intraperitoneally 1 h before the first L-Arg injection.
In the 5, 10, 20 and 100 mg/kg NaHS + SAP groups, different dosages of NaHS (Sigma) were diluted in saline and administered 1 h before the first L-Arg injection.
In the wortmannin (W) + SAP, 5 mg/kg NaHS + W + SAP, and 100 mg/kg NaHS + W + SAP groups, the animals were treated as described for the SAP group, with 5 mg/kg NaHS + SAP and 100 mg/kg NaHS + SAP, except that wortmannin (1.4 mg/kg, Sigma)[10], a PI3K inhibitor, was dissolved in dimethyl sulfoxide (DMSO) and administered 30 min before the first L-Arg injection.
Twenty-four hours after the first L-Arg injection, the animals were sacrificed using an intraperitoneal injection of a lethal dose of pentobarbital (50 mg/kg). Blood and pancreas samples were collected.
Total RNA was collected from pancreatic tissues using TRIzol Reagent (Sigma) according to the manufacturer’s instructions. Total RNA (2 μg) was reverse transcribed using a ReverTra Ace-α-cDNA Synthesis Kit (Toyobo) at 42 °C for 10 min, 30 °C for 20 min, and 99 °C for 5 min, followed by 4 °C for 5 min. The cDNA was used as a template for detecting CSE and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. The optimal annealing temperature, optimal cycles, and desired product sizes are shown in Table 1. Real-time PCR was performed on an iCycler iQ apparatus (Fermentas) using the iCycler optical system software (version 3.1) and the SYBR Green PCR Master Mix (ABI). The ∆CT value was determined, and the formula 2-∆∆CT was used to determine the relative quantity of the amplified fragments.
| Gene | Primer sequence | Size |
| CSE | Forward: 5’GCGCTGCTCCAACTGCTGTATAA 3’ | 253 bp |
| Reverse: 5’GGGTCCGGTTTCAGCATGTTT 3’ | ||
| GAPDH | Forward: 5'CTCATGACCACAGTCCATGC3’ | 155 bp |
| Reverse: 5'TTCAGCTCTGGGATGACCTT3’ |
Aliquots (120 μL) of plasma were mixed with distilled water (100 μL), trichloroacetic acid (10% w/v, 120 μL), zinc acetate (1% w/v, 60 μL), N,N-dimethyl-p-phenylenediamine sulfate (NNDPP) (20 μmol/L; 40 μL) in 7.2 mol/L HCl, and FeCl3 (30 μmol/L; 40 μL) in 1.2 mol/L HCl in 96-well plates. The absorbance of the resulting solution was measured after 10 min at 670 nm[11]. All samples were assayed in duplicate, and the H2S was calculated against a calibration curve of NaHS (3.125-100 μmol/L). The results are expressed as the plasma H2S concentration in micromoles per liter. Plasma amylase was assessed using auto-biochemistry equipment.
To measure the plasma IL-6, ELISA kits from R&D Systems were used according to the manufacturer’s instructions. The ELISA results were reproducible with an interassay variability of < 9.5% and an intraassay variability of < 6.5%. The results were corrected for the DNA content of the tissue samples and expressed as picograms per microgram of DNA.
Inflammatory cell sequestration in the pancreas was quantified as a measurement of the tissue myeloperoxidase (MPO) activity[12]. The tissue samples were thawed, homogenized in 20 mmol/L phosphate buffer (pH 7.4) and centrifuged at 13000 rpm for 10 min at 4 °C, and the resulting pellet was resuspended in 50 mmol/L phosphate buffer (pH 6.0) (Sigma). The suspension was subjected to four cycles of freezing and thawing and further disruption though sonication (40 s). The sample was subsequently centrifuged at 13000 rpm for 5 min at 4 °C, and the supernatant was used for the MPO assay. This mixture was incubated at 37 °C for 110 s; the reaction was terminated after adding 50 μL of 0.18 mol/L H2SO4, and the absorbance was measured at 405 nm.
The frozen pancreatic tissue samples were cut into small pieces, homogenized in 0.5 mL of RIPA buffer, and incubated at 4 °C overnight. The dissolved proteins were collected after centrifugation at 10000 g for 30 min, and the supernatant was collected. The pancreatic tissue proteins were subsequently separated through SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane (Millipore). The membrane was incubated with primary antibodies against p-AKT (1:300, Santa), IκBα (1:500, Abcam), and GAPDH (1:8000, ProMab), followed by incubation with the appropriate secondary horseradish peroxidase conjugated anti-rabbit IgG antibodies (1:3000, Santa; 1:3000, Abcam; and 1:8000, ProMab, respectively).
Nuclear extracts from pancreas (100 mg) tissues were prepared using a nuclear extraction kit, according to the manufacturer’s instructions (Kaiji Biotech). Briefly, the NF-κB oligonucleotide probe (5′-TCAACTCCCCTGAAA GGGTCCG-3′) was labeled with biotin and purified on a NICK column (Yuantai Biotech). The binding reaction was performed in a 20 μL reaction mixture containing 6 μg of incubation buffer, 8 μg of nuclear extract, and 20 fm of biotech labeled oligonucleotides. After incubating for 20 min at room temperature, 2 μL of 0.1% bromophenol blue was added, and the samples were electrophoresed on a 6% non-denaturing polyacrylamide gel at 100 V for 30 min.
To observe the therapeutic effect of NaHS, the rats were randomly assigned to normal saline + SAP (n = 10), SAP (n = 10), 5 mg/kg NaHS + SAP (n = 10), and 100 mg/kg NaHS + SAP (n = 10) groups. NaHS and L-Arg were administered as previously described, but the rats were allowed to drink water freely and fast after the procedure.
The data are expressed as mean ± SE. Differences between the mean values of multiple groups were analyzed using one-way analysis of variance (ANOVA) followed by the LSD test and survival analysis through Kaplan-Meier using SPSS 13.0 software, and P﹤0.05 was considered statistically significant.
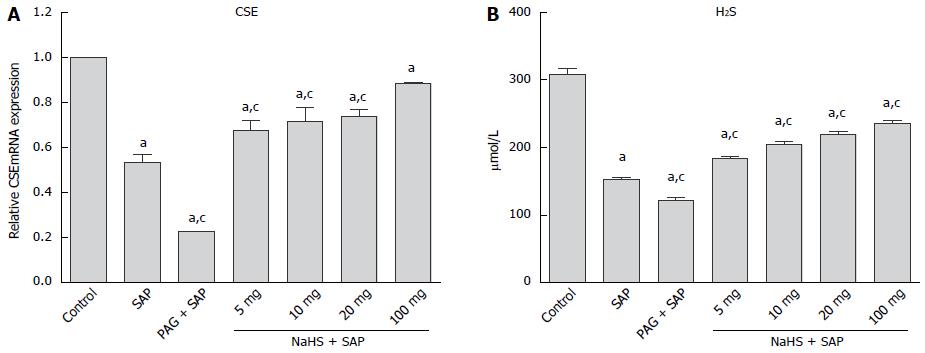
As shown in Figure 1A, CSE mRNA expression in the pancreatic tissue was significantly suppressed in the SAP group compared with the control group (P < 0.05), and the CSE mRNA expression was further down-regulated after the PAG (P < 0.05) treatment. Compared with the control group, plasma H2S levels were significantly lower (P < 0.05) in the SAP rats, and administering PAG further reduced the H2S level. However, after treatment with different dosages of NaHS, plasma H2S levels in these groups were all significantly increased compared with the SAP group (P < 0.05), and within the NaHS groups, the H2S levels were gradually upgraded with increasing NaHS dosage (Figure 1B).
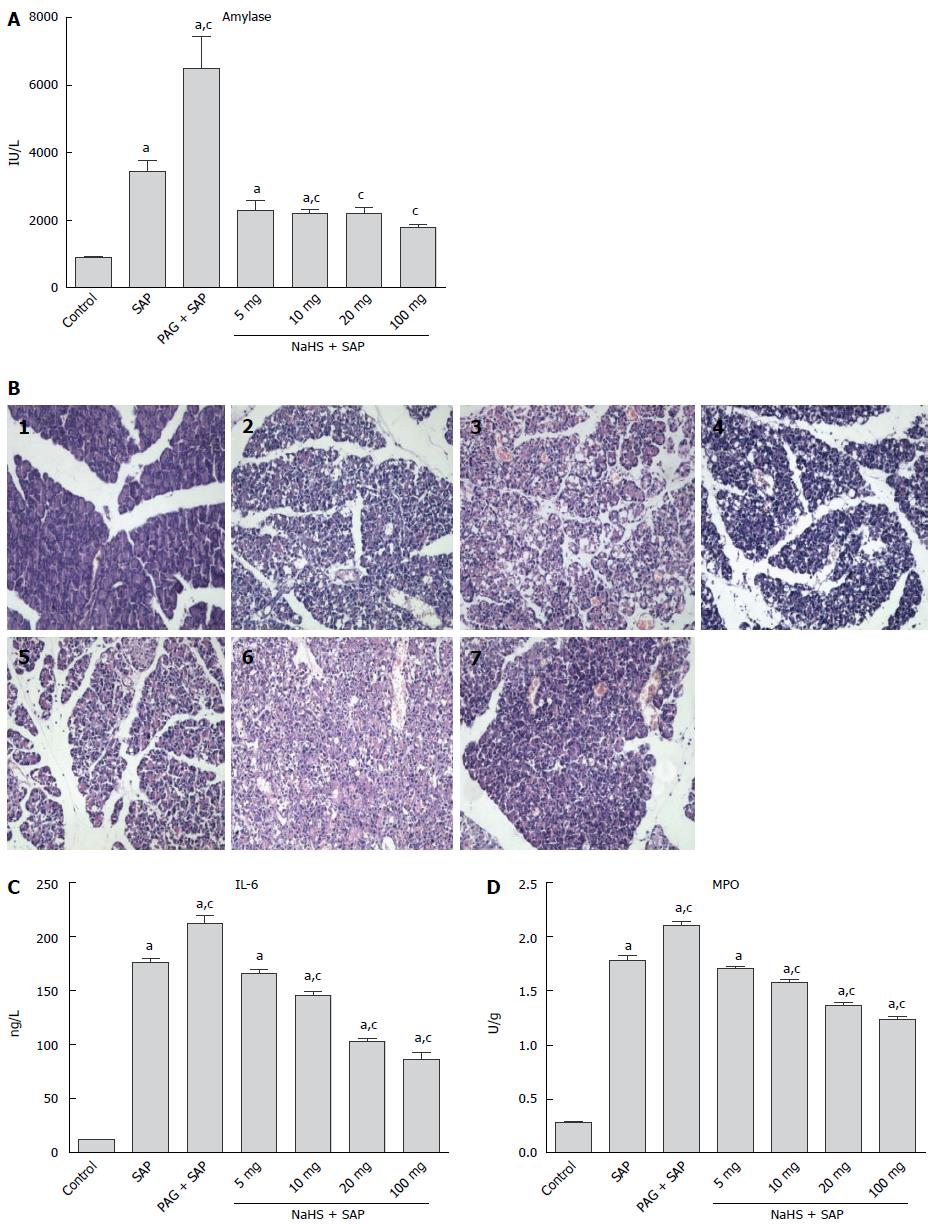
As shown in Figure 2A, plasma amylase was significantly increased in the SAP group compared with the control group (P < 0.05), and the level in the PAG + SAP group was further increased (P < 0.05). However, this increase could be abolished after administering different doses of NaHS, and no significant differences between the SAP group and 5 mg/kg NaHS + SAP group was observed; however, a significant reduction in the plasma amylase activity was observed in the rats receiving 10, 20, or 100 mg/kg NaHS (P < 0.05). Within these NaHS groups, the levels of plasma amylase were reduced with decreasing NaHS dosage. The histological examination of the pancreas samples obtained from the L-Arg, PAG and NaHS treated rats was also performed (Figure 2B). As shown in Figure 2C, a significant increase in interleukin (IL)-6 expression was observed in the SAP group. The pretreatment of SAP rats with PAG further increased the IL-6 levels, while treatment with different dosages of NaHS induced a significant reduction in IL-6 compared with SAP rats. There was a significant MPO increase in SAP rats compared with the controls (P < 0.05), and the rats that were administered PAG showed an even higher MPO increase than the SAP rats (P < 0.05). Nevertheless, treatment with different doses of NaHS significantly reduced the MPO activity compared with SAP rats (P < 0.05, Figure 2D).
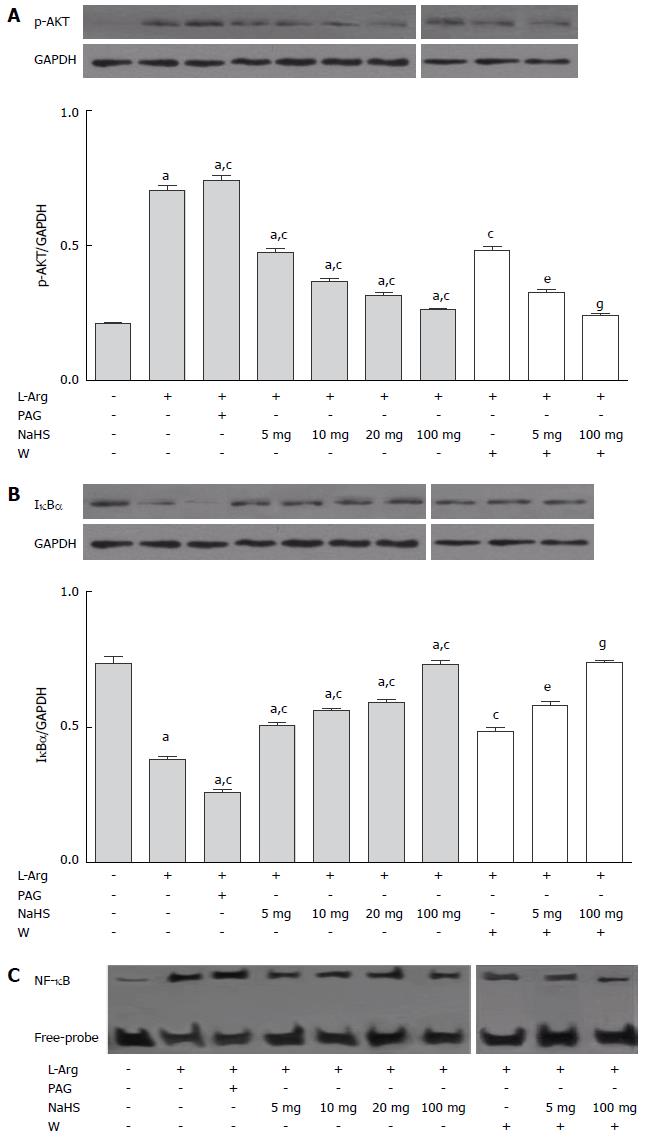
The Western blot analysis showed that the in vivo expression of p-AKT was notably increased in the SAP rats compared with the controls (P < 0.05, Figure 3A), and p-AKT expression was further augmented after the PAG administration. NaHS limited the increase in SAP-induced p-AKT levels, and the role of NaHS was enhanced gradually as the NaHS dosage increased. To determine the contribution of AKT in H2S-mediated inflammation during SAP, we treated rats with the PI3K inhibitor wortmannin for 30 min after the NaHS administration. We observed that the inactivation of PI3K with wortmannin treatment further reduced p-AKT expression. NF-κB is a downstream target of the PI3K/AKT pathway, and the release of NF-κB from the inhibitory protein IκBα is essential for activation of NF-κB. The phosphorylation of IκBα is a prerequisite for the degradation of this protein, which is primarily dependent on AKT activation. The induction of SAP resulted in significant IκBα degradation (Figure 3B) and NF-κB activation (Figure 3C), which was enhanced through PAG treatment, while the effect of H2S was suppressed through the NaHS application. We also observed that the role of NaHS was increased through the robust inhibition of PI3K activity using wortmannin.
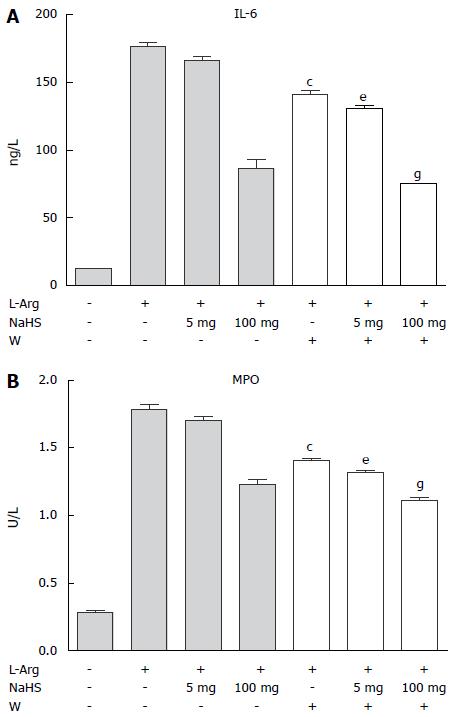
Administering the PI3K inhibitor wortmannin for 30 min after the NaHS treatment further reduced the plasma IL-6 levels and MPO activity in pancreatic tissue (Figure 4).
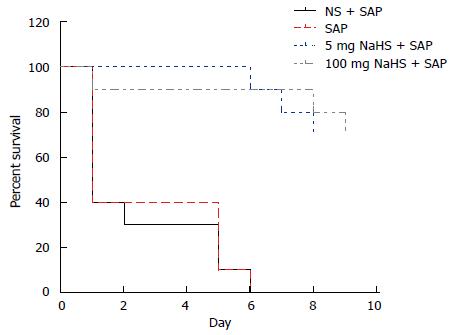
The Kaplan-Meier survival curves are shown in Figure 5 for the 7-d study period. The results showed that the normal saline + SAP and SAP rats all died within 6 d without administering the H2S donor NaHS; however, the survival rate in the groups treated with NaHS was 70%.
The role of H2S in inflammation is ambiguous. Here, we show an anti-inflammatory role for this compound in in vivo experiments involving SAP. Similarly, we observed that CSE mRNA expression was significantly reduced in the L-Arg-induced rat models, and treatment with the CSE inhibitor (PAG) further reduced the CSE mRNA levels, accompanied by increases in plasma amylase, IL-6, and MPO activity in vivo. Thus, we initially proposed that the down-regulation of the endogenous H2S/CSE system might be involved in the pathogenesis of inflammatory infiltration in SAP and that H2S/CSE alterations were associated with the activity and severity of this disease. We pre-treated the rats with different dosages of NaHS before inducing SAP in vivo. NaHS successfully reduced the levels of plasma amylase, IL-6 and leukocyte infiltration (shown by MPO activity) in the pancreas of SAP rats, accompanied by the up-regulation of CSE mRNA expression. Intriguingly, the effect of NaHS was gradually increased with increasing NaHS dosage in vivo. These results suggested that H2S could be a negative regulator in SAP.
IL-6 is produced by many cell types, including monocytes/macrophages, endothelial cells, fibroblasts, and smooth muscle cells[1,13], which indicates the profile of leukocyte recruitment during the inflammatory response via the selective regulation of inflammatory chemokines and apoptotic events[14,15]. Circulating levels of IL-6 are a sufficient indicator of the severity of acute pancreatitis[16]. We observed that the levels of plasma IL-6 were significantly up-regulated in SAP rats. The concentrations of IL-6 in the plasma were obviously attenuated after pre-administration with different dosages of NaHS. The present study suggests that the anti-inflammatory action of H2S might be mediated through inhibition of IL-6. This result is consistent with previous data, showing that the H2S donor in OA-treated rats significantly reduced IL-6 and IL-8 levels in the plasma and in the lungs[17], and the administration of NaHS attenuated the increase in plasma TNF-α and ICAM-1 levels in hepatic ischemia-reperfusion (HIR) rats[18].
There is much evidence confirming that the development and progression of SAP depend on NF-κB transcription, which plays an important role in the regulation of inflammatory mediators. Furthermore, evidence suggests that NF-κB transcription plays a role in exogenous H2S-induced cytokine expression in human leukocytes[19], confirming the close relationship between NF-κB and H2S. We observed that the role of H2S in SAP involved the suppression of IκBα degradation, NF-κB transcription, and pro-inflammatory gene expression. Moreover, the PI3K inhibitor wortmannin further decreased NF-κB transcription, plasma IL-6 levels, and MPO activity in pancreatic tissue.
AKT is a major PI3K effector and the full activation of AKT requires phosphorylation at Thr308 and Ser473, and Thr308 phosphorylation by 3’-PDK is also a PI3K effector[20]. Although AKT is not directly phosphorylated through PI3K, these post-translational modifications are strictly dependent on PI3K activity[20]. Tarassishin et al[21] suggested that activation of the PI3K/AKT pathway could play an anti-inflammatory role. Therefore, we determined whether H2S inhibits AKT phosphorylation in vivo and examined the effect of the PI3K/AKT pathway in the H2S-mediated inflammatory response. For this purpose, we administered the PI3K inhibitor wortmannin before SAP induction and observed that the effect of NaHS was enhanced by wortmannin. Therefore, we supposed that H2S regulates AKT through a node downstream of PI3K. Previous studies have demonstrated that H2S significantly improves cardiodynamic functions and attenuates the size of myocardial infarction via AKT stimulation[22]. Furthermore, Tamizhselvi et al[23] reported that the H2S-mediated down-regulation of TNF-α and IL-1β in cerulein-treated acinar cells was associated with the activation of PI3K/AKT.
H2S protects gastric mucosal epithelial cells against oxidative stress through the activation of p38 mitogen-activated protein kinases (MAPK)[24]. It has been reported that H2S significantly attenuated the phosphorylation of MAPK and activated NF-κB and expression of the anti-apoptotic protein Bcl-2[25]. The treatment of C28/I-2 cells with H2S deactivated extracellular-regulated kinase (ERK1/2), inhibited the phosphorylation of NF-κB p65 at Ser536 and reduced IL-6 and IL-8 expression[26]. Taken together, these results demonstrated that H2S possesses an extensive pathophysiological action and exerts a protective role via intra- and extra-cellular signal transduction pathways or genetic transcription in various animal models and cell culture.
Sidhapuriwala et al[27] considered that exogenous H2S (NaHS) at 10 mg/kg significantly down-regulated amylase levels in the plasma and MPO activity in acute pancreatitis; however, the role of NaHS was not enhanced at 15 mg/kg. Faller et al[28] recently showed that only inhalation of H2S at 80 ppm prevents lung injury in ventilated mice. In myocardial ischemia-reperfusion injury, treatment with different doses of H2S donor (NaHS) generated a U-shaped dose response curve[29,30]. However, we showed that the protective role of H2S in SAP was gradually increased from 5 to 100 mg/kg in vivo; thus, further studies are needed to confirm these findings.
In conclusion, in the present study, we showed that the down-regulation of the CSE/H2S system plays an important role in SAP development and progression and in preconditioning the H2S donor with NaHS-induced anti-inflammatory activity in SAP. This protective effect was associated with PI3K/AKT phosphorylation inhibition, the repressed degradation of IκBα, and NF-κB activation. We also observed that the prophylactic administration of NaHS significantly reduced the mortality of SAP rats. These data indicated that H2S/CSE plays a crucial role in SAP development, which suggests that NaHS might provide new valuable therapeutic strategies for improving the clinical course of this disease.
Severe acute pancreatitis (SAP) is an inflammatory disease with high morbidity and mortality, but the pathogenesis of SAP remains unknown. Recent studies have implicated H2S in different inflammatory reactions. However, the exact role of this gas is still not clearly understood, especially in SAP.
Cystathionine-γ-lyase (CSE) is the major H2S-producing enzyme in the peripheral tissue. Numerous studies have shown the beneficial effects of H2S, particularly in cardiovascular and neurological disorders.
The authors provide wide-ranging dosages of exogenous H2S (NaHS), from 5 mg/kg to 100 mg/kg, to study the role of H2S in SAP in vivo, and finally certified the role of H2S in SAP rats though survival analysis.
These data indicated that H2S/CSE plays a crucial role in SAP development, which suggests that NaHS might provide new valuable therapeutic strategies for improving the clinical course of this disease.
SAP is an inflammatory condition of the pancreas that is characterized by elevated pancreatic enzymes in the blood, SIRS and MODS, which is ultimately responsible for most pancreatitis-associated mortality and morbidity. H2S is predominantly produced through the action of three enzymes: CBS and CSE, and 3-mercaptopyruvate sulfurtransferase (3-MST), however, CSE is the major H2S-producing enzyme in the peripheral tissue.
The paper is important because the effects of H2S on pancreatitis have not been investigated thoroughly in the Arginine model. The conclusions are supported by the well-presented and executed experiments.
P- Reviewer: Tulassay ZJ S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343-351. [PubMed] |
| 2. | Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988;3:105-112. [PubMed] |
| 3. | Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2115] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 4. | Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597-605. [PubMed] |
| 5. | Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191-201. [PubMed] |
| 6. | Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future? Trends Pharmacol Sci. 2003;24:609-611. [PubMed] |
| 7. | Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 742] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 8. | Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917-935. [PubMed] |
| 9. | Tani S, Itoh H, Okabayashi Y, Nakamura T, Fujii M, Fujisawa T, Koide M, Otsuki M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci. 1990;35:367-374. [PubMed] |
| 10. | Singh VP, Saluja AK, Bhagat L, van Acker GJ, Song AM, Soltoff SP, Cantley LC, Steer ML. Phosphatidylinositol 3-kinase-dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest. 2001;108:1387-1395. [PubMed] |
| 11. | Chunyu Z, Junbao D, Dingfang B, Hui Y, Xiuying T, Chaoshu T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem Biophys Res Commun. 2003;302:810-816. [PubMed] |
| 12. | Yang G, Cao K, Wu L, Wang R. Cystathionine gamma-lyase overexpression inhibits cell proliferation via a H2S-dependent modulation of ERK1/2 phosphorylation and p21Cip/WAK-1. J Biol Chem. 2004;279:49199-49205. [PubMed] |
| 13. | Bhatia M, Sidhapuriwala JN, Sparatore A, Moore PK. Treatment with H2S-releasing diclofenac protects mice against acute pancreatitis-associated lung injury. Shock. 2008;29:84-88. [PubMed] |
| 14. | Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463-3468. [PubMed] |
| 15. | McLoughlin RM, Witowski J, Robson RL, Wilkinson TS, Hurst SM, Williams AS, Williams JD, Rose-John S, Jones SA, Topley N. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest. 2003;112:598-607. [PubMed] |
| 16. | Panek J, Karcz D, Pieton R, Zasada J, Tusinski M, Dolecki M, Winiarski M. Blood serum levels of proinflammatory cytokines in patients with different degrees of biliary pancreatitis. Can J Gastroenterol. 2006;20:645-648. [PubMed] |
| 17. | Li T, Zhao B, Wang C, Wang H, Liu Z, Li W, Jin H, Tang C, Du J. Regulatory effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the plasma and pulmonary tissue of rats with acute lung injury. Exp Biol Med (Maywood). 2008;233:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Kang K, Zhao M, Jiang H, Tan G, Pan S, Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 2009;15:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Ganster F, Burban M, de la Bourdonnaye M, Fizanne L, Douay O, Loufrani L, Mercat A, Calès P, Radermacher P, Henrion D. Effects of hydrogen sulfide on hemodynamics, inflammatory response and oxidative stress during resuscitated hemorrhagic shock in rats. Crit Care. 2010;14:R165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Ehrhardt C, Ludwig S. A new player in a deadly game: influenza viruses and the PI3K/Akt signalling pathway. Cell Microbiol. 2009;11:863-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Tarassishin L, Suh HS, Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. 2011;8:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Yong QC, Lee SW, Foo CS, Neo KL, Chen X, Bian JS. Endogenous hydrogen sulphide mediates the cardioprotection induced by ischemic postconditioning. Am J Physiol Heart Circ Physiol. 2008;295:H1330-H1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Tamizhselvi R, Sun J, Koh YH, Bhatia M. Effect of hydrogen sulfide on the phosphatidylinositol 3-kinase-protein kinase B pathway and on caerulein-induced cytokine production in isolated mouse pancreatic acinar cells. J Pharmacol Exp Ther. 2009;329:1166-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2007;241:11-18. [PubMed] |
| 25. | Tripatara P, Patel NS, Collino M, Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown PA, Yaqoob MM. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Invest. 2008;88:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Kloesch B, Liszt M, Steiner G, Bröll J. Inhibitors of p38 and ERK1/2 MAPkinase and hydrogen sulphide block constitutive and IL-1β-induced IL-6 and IL-8 expression in the human chondrocyte cell line C-28/I2. Rheumatol Int. 2012;32:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Sidhapuriwala JN, Ng SW, Bhatia M. Effects of hydrogen sulfide on inflammation in caerulein-induced acute pancreatitis. J Inflamm (Lond). 2009;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA. 2007;104:15560-15565. [PubMed] |
| 30. | Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |