Published online Mar 21, 2015. doi: 10.3748/wjg.v21.i11.3232
Peer-review started: October 14, 2014
First decision: December 11, 2014
Revised: December 18, 2014
Accepted: February 5, 2015
Article in press: February 5, 2015
Published online: March 21, 2015
Overall 5-years survival of pancreatic cancer patients is nearly 5%, making this cancer type one of the most lethal neoplasia. Furthermore, the incidence rate of pancreatic cancer has a growing trend that determines a constant increase in the number of deceases caused by this pathology. The poor prognosis of pancreatic cancer is mainly caused by delayed diagnosis, early metastasis of tumor, and resistance to almost all tested cytotoxic drugs. In this respect, the identification of novel potential targets for new and efficient therapies should be strongly encouraged in order to improve the clinical management of pancreatic cancer. Some studies have shown that the mitochondrial uncoupling protein 2 (UCP2) is over-expressed in pancreatic cancer as compared to adjacent normal tissues. In addition, recent discoveries established a key role of UCP2 in protecting cancer cells from an excessive production of mitochondrial superoxide ions and in the promotion of cancer cell metabolic reprogramming, including aerobic glycolysis stimulation, promotion of cancer progression. These observations together with the demonstration that UCP2 repression can synergize with standard chemotherapy to inhibit pancreatic cancer cell growth provide the molecular rationale to consider UCP2 as a potential therapeutic target for pancreatic cancer. In this editorial, recent advances describing the relationship between cancer development and mitochondrial UCP2 activity are critically provided.
Core tip: The dramatic poor prognosis of pancreatic cancer forces towards the identification of novel efficient therapeutic targets against this neoplasia. Overexpression of uncoupling protein 2 (UCP2) and its functional involvement in cancer development, reactive oxygen species production, and cancer metabolic reprogramming may represent the rationale and the starting point for future drug design projects focused on the identification of specific UCP2 inhibitors as innovative therapeutic tool against pancreatic cancer.
- Citation: Donadelli M, Dando I, Dalla Pozza E, Palmieri M. Mitochondrial uncoupling protein 2 and pancreatic cancer: A new potential target therapy. World J Gastroenterol 2015; 21(11): 3232-3238
- URL: https://www.wjgnet.com/1007-9327/full/v21/i11/3232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i11.3232
Pancreatic cancer (PC) ranks amongst the most lethal cancers and has a mortality rate that nearly equals the incidence rate and an overall 5-years survival of approximately 5%[1,2]. Dismally, its mortality rate has been increasing in the last years with a prediction for next years having the same trend. In contrast, in the last decades, an overall reduction in cancer-related mortality in Western countries has been observed for lung, breast, colorectal, and prostate cancers[3]. The reduced cancer mortality for the latter tumors is likely the result of several strategies, including development of early detection, prevention programs, and the discovery of new therapeutic targets and drugs. In the case of PC, because of its low incidence, population-based screening has been considered not feasible[4]. Indeed, the worldwide incidence of all the types of pancreatic cancers (85% of which are adenocarcinomas) ranges from 1 to 10 cases per 100000 people and is generally higher in developed countries and among men. Furthermore, PC has been classified as the eighth leading cause of death for cancer in men and the ninth in women[5]. Pancreatic adenocarcinoma (PDAC), the most aggressive and frequent PC, possesses a variety of hallmarks that include: (1) high rate of KRAS activating mutations; (2) progression from distinct types of precursor lesions; (3) propensity for both local invasion and distant metastasis; (4) extensive stromal reaction (desmoplasia) resulting in a hypovascular and hypoxic microenvironment; (5) reprogramming of cellular metabolism; and (6) tumor immune evasion[6]. More than 90% of all grades pancreatic intraepithelial neoplasia possess KRAS mutations[7]. Instead, the mutational inactivation of the CDKN2A, p53, and SMAD4 tumor suppressors has been detected with increasing frequency in type II and type III lesions of pancreatic intraepithelial neoplasia, suggesting that they may represent rate-limiting events for tumor progression, while KRAS mutations would contribute to its inception[8]. The epidermal growth factor receptor, the nuclear factor κB, the antiapoptotic protein Bcl-xL, and mitogen-activated protein kinase pathways have also been shown to contribute to KRAS-mediated pancreatic adenocarcinoma, suggesting alternative combinatorial tumorigenic strategies[9-11].
Several efforts made to identify pancreatic tumor biomarkers[12,13] have brought to the identification of the carcinoembryonic antigen and the carbohydrate antigen 19-9, which, however, are considered low sensitive and specific for screening pancreatic cancer at early stages. Despite these advances, more than 90% of patients who have received a diagnosis of pancreatic cancer die from the disease as a result of extensive metastasis (70%) or of bulky primary tumors with limited metastatic disease (30%)[14]. Thus, delayed diagnosis, early metastasis, and resistance to almost all the classes of cytotoxic drugs are considered the main causes of the extremely poor prognosis of PC. For all these reasons, research is now focused on the identification of new prognostic and diagnostic biomarkers and efficient therapeutic targets in order to improve the clinical management of PC. In this respect, we here provide critical comments on the possible usage of the antioxidant mitochondrial uncoupling protein 2 (UCP2) as a new potential target for PC treatment. Several studies have indeed shown that UCP2 is broadly over-expressed in various cancer types and its over-expression is strictly related with the regulation of reactive oxygen species (ROS) and cell metabolism (including autophagy), both processes known to be generally altered in cancer cells.
Mitochondrial ATP production occurs by coupling the electron transport chain (ETC) with the phosphorylation of ADP into ATP, the so-called oxidative phosphorylation. These two processes are not always efficiently coupled, mainly because of the presence in the inner membrane of mitochondrial transporters, such as uncoupling proteins (UCPs). The UCPs belong to the superfamily of anion transport carriers of the mitochondrial inner membrane[15] and some of them are involved in thermogenesis and regulation of mitochondrial ROS. UCP1 was first discovered and cloned in 1986[16] and is involved in the non-shivering thermogenesis activity of brown adipose tissue (BAT)[17]. Since then, the discovery of UCPs has grown rapidly, UCP1 homologues being found across mammalian species (UCP2 and UCP3) but also in other eukaryotes from plants to animals[18,19]. UCP1, UCP2, and UCP3 are thought to differ in the nature of their uncoupling activity[20,21] and of their potential physiological roles[22]. A rapid overview of data collected on UCP1, 2 and 3 highlights how these proteins differ from each other. First, while UCP1 tissue expression is localized and abundant in BAT, UCP2 has been found in several tissues, including liver, brain, pancreas, adipose tissue, immune cells, spleen, kidney, and the central nervous system[23-25], and UCP3 is mainly present in the skeletal muscle[18]. Also, the physiological role of UCP1 is restricted to thermogenesis, which is unlikely to be the role for UCP2 and 3, as shown by their respective knock-out models[26,27]. UCP2 and 3 have been involved in a number of postulated functions in energy regulation, including regulation of insulin secretion[28] or ROS production and control of the immune response[26]. The other two members of the UCP superfamily, UCP4 and UCP5, are expressed in a tissue-specific manner and are involved in mitochondrial membrane potential reduction[29].
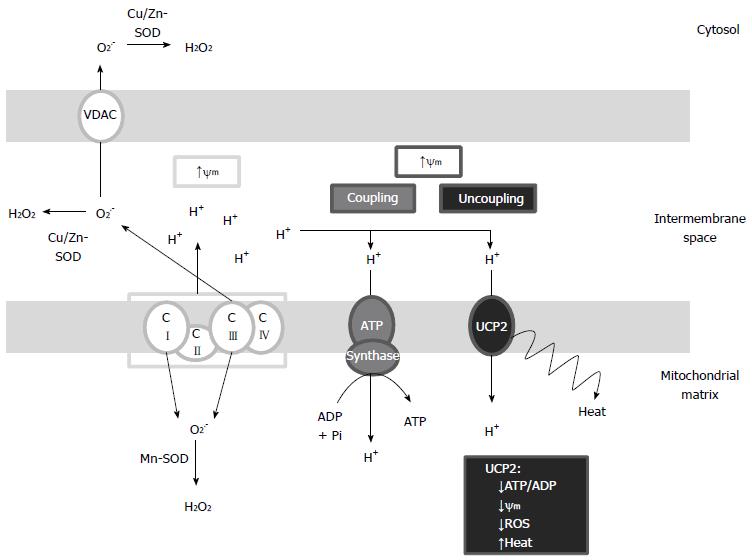
The cellular antioxidant systems include a large set of enzymes and low-molecular-weight compounds that sequester excessively generated ROS or prevent their production by aerobic respiration. Some antioxidant systems can be energetically expensive because of their dependence on both ATP and NADPH usage. The UCP system represents an acute and energetically costly mechanism to decrease ROS production in mitochondria[30]. Indeed, as shown in Figure 1, the uncoupling of oxidative phosphorylation is a short circuit in which the transport of protons from the intermembrane space to the matrix bypasses ATP synthase resulting in a decrease of: (1) mitochondrial inner membrane potential; (2) leakage of electrons from ETC; and (3) consequently, ROS generation. The existence of a strong correlation between mitochondrial membrane potential and ROS production is well known[31]. Minor increases in membrane potential induce ROS formation, whereas slight decreases can substantially diminish their production, without greatly lowering the efficiency of oxidative phosphorylation. Hence, the mild uncoupling of mitochondrial oxidative phosphorylation may represent the first line of defense against oxidative stress[32]. According to this pattern, UCP2 can dissipate the proton gradient to prevent the proton-motive force from becoming excessive, thus decreasing ROS produced by electron transport[33]. Overall, it is estimated that 0.2%-2% of the O2 consumed in mitochondria is reduced to superoxide by electron leakage. Mitochondrial superoxide ion is considered the initial and leading molecule of ROS signaling and is generally converted into hydrogen peroxide (H2O2) by superoxide dismutases. In addition, upon reaction with H2O superoxide ion can generate hydroxyl radicals (•HO) implicated in lipid damage and protein oxidation[34,35]. The electron leakage causing superoxide production can occur both at complex I (CI) and complex III (CIII) of the respiratory chain[36]: at CI, superoxide has been shown to be exclusively directed toward the mitochondrial matrix and converted into membrane-permeable H2O2 by manganese-superoxide dismutase (Mn-SOD), while at CIII, superoxide is released to both the matrix and the intermembrane space where it can be converted into H2O2 by copper/zinc-superoxide dismutase (Cu/Zn-SOD) (Figure 1)[37]. In addition, some evidence indicates that mitochondrial matrix-directed superoxide can be released from mitochondria through voltage dependent anion channels causing an increase of cytosolic ROS[38]. Therefore, UCP2 acts as a sensor of mitochondrial oxidative stress and constitutes an important component of local feedback mechanisms generally implicated in cyto-protective activities controlling the production of mitochondrial ROS and regulating redox-sensitive cytosolic signaling pathways.
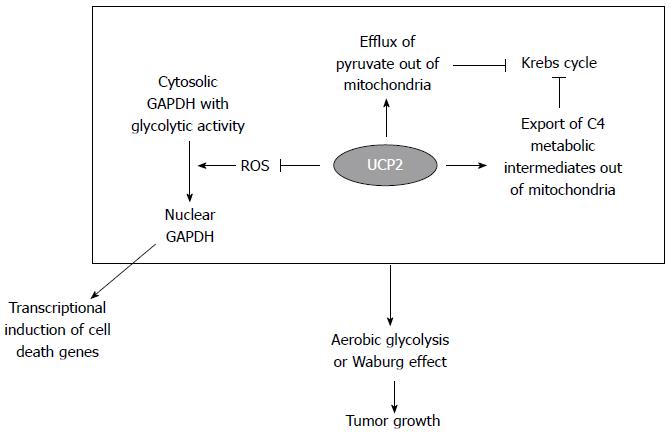
In 1956, Warburg et al[39] proposed that cancer was caused by defects in mitochondria, forcing cells to shift to energy production through glycolysis despite aerobic conditions. This characteristic of cancers is described as the ‘‘Warburg effect.’’ Warburg statement is based on the observation that the irreversible injury to mitochondrial respiration is followed by a long fight for existence in which a part of the cells perishes for lack of energy while another part succeeds in replacing the lost respiration energy by developing aerobic glycolysis. The Warburg effect, considered now a hallmark of cancer, plays an important role in the growth of tumors, including gastrointestinal cancers, by remodeling the metabolic profile in order to allow tumor cell survival under adverse conditions[40]. More recently, some scientists tried to create a cellular model of the Warburg effect by developing an epithelial cell line lacking mitochondrial DNA (rho0)[41]. Among the regulated genes, UCP2 expression was predominantly higher in rho0 cells suggesting that UCP2 may inhibit ROS accumulation and protect the cells from excessive ROS production induced by mitochondrial defects linked to Warburg effect. In this respect, UCP2 may function as a potential diagnostic marker of cancer associated with the Warburg effect[42]. In addition to its antioxidant role, UCP2 acts as a direct metabolic regulator contributing to the Warburg phenotype. Indeed, as schematically reported in Figure 2, UCP2 has been proposed to function as a uniporter for pyruvate, which promotes pyruvate efflux from mitochondria, restricts mitochondrial respiration, and increases the rate of glycolysis in cancer cells[43]. Furthermore, UCP2 catalyzes the exchange of intramitochondrial C4 metabolites for cytosolic phosphate by an H+-assisted mechanism, which is stimulated by both the electrical potential (negative inside) and pH gradient (acidic outside) existing across the inner mitochondrial membrane of respiring cells[44]. In particular, by exporting oxaloacetate and related C4 compounds from mitochondria, UCP2 negatively controls the oxidation of acetyl-CoA-producing substrates via the Krebs cycle, thus lowering the redox pressure on the mitochondrial respiratory chain, the ATP: ADP ratio, and ROS production. Notably, the mitochondrial concentration of oxaloacetate is usually very low, and its availability regulates the entry of acetyl-CoA into the Krebs cycle. Thus, UCP2 prevents mitochondrial glucose oxidation and favors a higher glucose utilization by aerobic glycolysis. In this context, our research group further confirmed the pro-glycolytic effect of UCP2 demonstrating for the first time that UCP2 can stabilize the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in the cytoplasm of cancer cells[45]. Accordingly, in response to oxidative stress, GAPDH has been demonstrated to undergo protein oxidation of redox-sensitive cysteine residues that stimulates its translocation to cell nuclei[46], where the enzyme favors transcriptional induction of cell death-related genes[47,48]. Thus, the antioxidant effect of UCP2 can inhibit both GAPDH oxidation and nuclear translocation supporting the glycolytic flux and preventing cancer cells from stimulating cell death mechanisms (Figure 2).
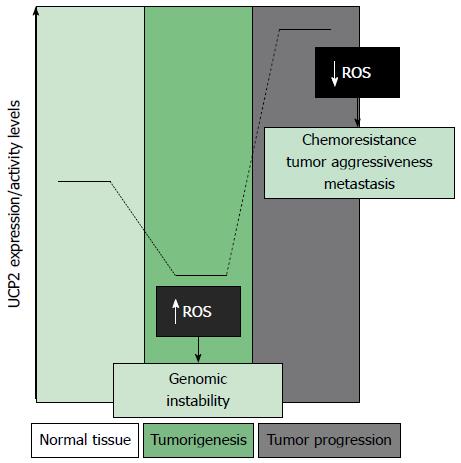
A careful analysis of the recent scientific literature concerning the role of UCP2 in tumor development reveals that UCP2 and cancer may have a double relationship. Indeed, a dual regulation of UCP2 expression, depending on the stages of cancer development, has been observed in many tumor types. A number of studies have established the key role that UCP2 has in tumorigenesis and in chemoresistance. The generally accepted thesis envisages that, during the first stages of tumorigenesis, UCP2 is repressed to allow ROS accumulation and genomic instability, while it is triggered or over-expressed in the following stages of cancer development, determining chemoresistance and tumor aggressiveness by defending cancer cells from apoptosis through the negative regulation of mitochondrial ROS production (Figure 3)[49-51]. Accordingly, UCP2-null mice have a predisposition for enhanced tumorigenesis in the proximal colon, providing the first in vivo confirmation of a link between mitochondrial uncoupling proteins and cancer[52], while highly expressed UCP2 is associated with metastatic colon cancer and tumor aggressiveness[53]. The dual and opposite regulation of UCP2 expression in various stages of tumor development has also been demonstrated in breast cancer. In this system, the repression of UCPs by estrogens, a major risk factor for breast cancer initiation, may play a key role in estrogen-induced breast carcinogenesis[54]. On the contrary, the enhanced expression of UCP2 has been correlated to breast cancer progression. Indeed, a significant correlation between UCP2 levels and tumor grade-associated functional phenotypes has been found in a large number of breast cancer patients (n = 234)[55]. Concerning PC, some studies have shown that the protein level of UCP2 is significantly higher in human PC samples than in the adjacent normal tissues, suggesting that UCP2 may promote tumor growth in this tumor type[56]. An extensive study on Oncomine data sets addressed to analyze the UCP2 expression level in a number of cancer types, including pancreatic cancer, has revealed that UCP2 is over-expressed in ovarian, bladder, esophageal, testicular, kidney, colorectal, lung, breast, leukemia, prostate, as well as pancreas cancers[42]. This study has concluded that UCP2 over-expression is a general phenomenon linked to the progression of human cancers. Along this line of evidence, our research group has demonstrated that increased expression of UCP2 mRNA directly correlates with resistance to gemcitabine treatment, in a panel of pancreatic adenocarcinoma cell lines, and that the UCP2 gene is induced by gemcitabine, demonstrating that the antioxidant effect of UCP2 plays a critical role in pancreatic cancer cell resistance to standard chemotherapy. We have also shown that UCP2 inhibition has a synergistic antiproliferative effect with gemcitabine in pancreatic adenocarcinoma cell growth[57]. Despite the availability of the above described data on the relationship between UCP2 expression/activity and PC, we believe that further studies need to be performed in order to better clarify the functional role of UCP2 in PC tumorigenesis and progression. Of crucial importance will be analyses on proteome and metabolic profiles of pancreatic cancer cells after knock-down or over-expression of UCP2 and clinical studies correlating UCP2 expression with clinicopathological factors and prognosis outcome on PC patients.
UCP2 over-expression may be considered a strategy adopted by cancer cells to protect themselves from excessive ROS production and to support the Warburg effect by reprogramming cancer cell metabolism. Thus, UCP2 inhibition can represent a therapeutic opportunity, in association to radio- or chemo-therapy, to treat tumors resistant to traditional therapy, such as PC. For this reason, we believe that UCP2 may be considered a potential target therapy for this tumor type. However, an efficient and specific UCP2 inhibitor is not yet available. The tools currently used in research studies to inhibit UCP2 are the genetic repression of UCP2 mRNA by a specific siRNA or the inhibition of UCP2 activity by genipin, a natural aglycon derived from geniposide, an iridoid glycoside extracted from the fruit of gardenia jasminoides. Genipin, however, has unspecific pharmacological properties including anti-inflammatory and antidepressant-like effects[58]. Thus, drug design research to identify or synthetize a specific and effective UCP2 inhibitor should be strongly encouraged in order to counteract progression of pancreatic cancer and of many other tumor types over-expressing this protein, which is crucial for their aggressive phenotype.
P- Reviewer: Fu DL, Giovannetti E, Stefaniak T, Su WC S- Editor: Yu J L- Editor: A E- Editor: Liu XM
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 2. | Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3032] [Cited by in F6Publishing: 3078] [Article Influence: 236.8] [Reference Citation Analysis (0)] |
| 3. | Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792-800. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 4. | Del Chiaro M, Segersvärd R, Lohr M, Verbeke C. Early detection and prevention of pancreatic cancer: is it really possible today? World J Gastroenterol. 2014;20:12118-12131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 95] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1500] [Cited by in F6Publishing: 1541] [Article Influence: 154.1] [Reference Citation Analysis (0)] |
| 6. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 841] [Cited by in F6Publishing: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 7. | Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730-733.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 487] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 8. | Hustinx SR, Leoni LM, Yeo CJ, Brown PN, Goggins M, Kern SE, Hruban RH, Maitra A. Concordant loss of MTAP and p16/CDKN2A expression in pancreatic intraepithelial neoplasia: evidence of homozygous deletion in a noninvasive precursor lesion. Mod Pathol. 2005;18:959-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 10. | Corcoran RB, Cheng KA, Hata AN, Faber AC, Ebi H, Coffee EM, Greninger P, Brown RD, Godfrey JT, Cohoon TJ. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell. 2013;23:121-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 11. | Navas C, Hernández-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 12. | Cecconi D, Palmieri M, Donadelli M. Proteomics in pancreatic cancer research. Proteomics. 2011;11:816-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | He XY, Yuan YZ. Advances in pancreatic cancer research: moving towards early detection. World J Gastroenterol. 2014;20:11241-11248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 52] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806-1813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 812] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 15. | Hughes J, Criscuolo F. Evolutionary history of the UCP gene family: gene duplication and selection. BMC Evol Biol. 2008;8:306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Bouillaud F, Weissenbach J, Ricquier D. Complete cDNA-derived amino acid sequence of rat brown fat uncoupling protein. J Biol Chem. 1986;261:1487-1490. [PubMed] [Cited in This Article: ] |
| 17. | Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1017] [Cited by in F6Publishing: 1004] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 18. | Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP. Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett. 1997;408:39-42. [PubMed] [Cited in This Article: ] |
| 19. | Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1226] [Cited by in F6Publishing: 1194] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 20. | Couplan E, del Mar Gonzalez-Barroso M, Alves-Guerra MC, Ricquier D, Goubern M, Bouillaud F. No evidence for a basal, retinoic, or superoxide-induced uncoupling activity of the uncoupling protein 2 present in spleen or lung mitochondria. J Biol Chem. 2002;277:26268-26275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Mozo J, Ferry G, Studeny A, Pecqueur C, Rodriguez M, Boutin JA, Bouillaud F. Expression of UCP3 in CHO cells does not cause uncoupling, but controls mitochondrial activity in the presence of glucose. Biochem J. 2006;393:431-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Criscuolo F, Gonzalez-Barroso Mdel M, Bouillaud F, Ricquier D, Miroux B, Sorci G. Mitochondrial uncoupling proteins: new perspectives for evolutionary ecologists. Am Nat. 2005;166:686-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Donadelli M, Dando I, Fiorini C, Palmieri M. UCP2, a mitochondrial protein regulated at multiple levels. Cell Mol Life Sci. 2014;71:1171-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Brüning JC, Lowell BB. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 830] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 27. | Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem. 2000;275:16258-16266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 501] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 28. | Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745-755. [PubMed] [Cited in This Article: ] |
| 29. | Hoang T, Smith MD, Jelokhani-Niaraki M. Toward understanding the mechanism of ion transport activity of neuronal uncoupling proteins UCP2, UCP4, and UCP5. Biochemistry. 2012;51:4004-4014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106-1115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 399] [Cited by in F6Publishing: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 31. | Mailloux RJ, Harper ME. Mitochondrial proticity and ROS signaling: lessons from the uncoupling proteins. Trends Endocrinol Metab. 2012;23:451-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 603] [Cited by in F6Publishing: 603] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 33. | Garlid KD, Jabůrek M, Jezek P, Varecha M. How do uncoupling proteins uncouple? Biochim Biophys Acta. 2000;1459:383-389. [PubMed] [Cited in This Article: ] |
| 34. | Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222-230. [PubMed] [Cited in This Article: ] |
| 35. | Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann N Y Acad Sci. 1994;738:8-14. [PubMed] [Cited in This Article: ] |
| 36. | Lanciano P, Khalfaoui-Hassani B, Selamoglu N, Ghelli A, Rugolo M, Daldal F. Molecular mechanisms of superoxide production by complex III: a bacterial versus human mitochondrial comparative case study. Biochim Biophys Acta. 2013;1827:1332-1339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Papa L, Manfredi G, Germain D. SOD1, an unexpected novel target for cancer therapy. Genes Cancer. 2014;5:15-21. [PubMed] [Cited in This Article: ] |
| 38. | Lustgarten MS, Bhattacharya A, Muller FL, Jang YC, Shimizu T, Shirasawa T, Richardson A, Van Remmen H. Complex I generated, mitochondrial matrix-directed superoxide is released from the mitochondria through voltage dependent anion channels. Biochem Biophys Res Commun. 2012;422:515-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [PubMed] [Cited in This Article: ] |
| 40. | Sawayama H, Ishimoto T, Sugihara H, Miyanari N, Miyamoto Y, Baba Y, Yoshida N, Baba H. Clinical impact of the Warburg effect in gastrointestinal cancer (review). Int J Oncol. 2014;45:1345-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Kulawiec M, Safina A, Desouki MM, Still I, Matsui S, Bakin A, Singh KK. Tumorigenic transformation of human breast epithelial cells induced by mitochondrial DNA depletion. Cancer Biol Ther. 2008;7:1732-1743. [PubMed] [Cited in This Article: ] |
| 42. | Ayyasamy V, Owens KM, Desouki MM, Liang P, Bakin A, Thangaraj K, Buchsbaum DJ, LoBuglio AF, Singh KK. Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin. PLoS One. 2011;6:e24792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Baffy G. Uncoupling protein-2 and cancer. Mitochondrion. 2010;10:243-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA. 2014;111:960-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 45. | Dando I, Fiorini C, Pozza ED, Padroni C, Costanzo C, Palmieri M, Donadelli M. UCP2 inhibition triggers ROS-dependent nuclear translocation of GAPDH and autophagic cell death in pancreatic adenocarcinoma cells. Biochim Biophys Acta. 2013;1833:672-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Dastoor Z, Dreyer JL. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J Cell Sci. 2001;114:1643-1653. [PubMed] [Cited in This Article: ] |
| 47. | Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16:1573-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 48. | Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523-540. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1031] [Cited by in F6Publishing: 1059] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 49. | Derdak Z, Mark NM, Beldi G, Robson SC, Wands JR, Baffy G. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res. 2008;68:2813-2819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 50. | Robbins D, Zhao Y. New aspects of mitochondrial Uncoupling Proteins (UCPs) and their roles in tumorigenesis. Int J Mol Sci. 2011;12:5285-5293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Su WP, Lo YC, Yan JJ, Liao IC, Tsai PJ, Wang HC, Yeh HH, Lin CC, Chen HH, Lai WW. Mitochondrial uncoupling protein 2 regulates the effects of paclitaxel on Stat3 activation and cellular survival in lung cancer cells. Carcinogenesis. 2012;33:2065-2075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Derdák Z, Fülöp P, Sabo E, Tavares R, Berthiaume EP, Resnick MB, Paragh G, Wands JR, Baffy G. Enhanced colon tumor induction in uncoupling protein-2 deficient mice is associated with NF-kappaB activation and oxidative stress. Carcinogenesis. 2006;27:956-961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Kuai XY, Ji ZY, Zhang HJ. Mitochondrial uncoupling protein 2 expression in colon cancer and its clinical significance. World J Gastroenterol. 2010;16:5773-5778. [PubMed] [Cited in This Article: ] |
| 54. | Sastre-Serra J, Valle A, Company MM, Garau I, Oliver J, Roca P. Estrogen down-regulates uncoupling proteins and increases oxidative stress in breast cancer. Free Radic Biol Med. 2010;48:506-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Sayeed A, Meng Z, Luciani G, Chen LC, Bennington JL, Dairkee SH. Negative regulation of UCP2 by TGFβ signaling characterizes low and intermediate-grade primary breast cancer. Cell Death Dis. 2010;1:e53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Li W, Nichols K, Nathan CA, Zhao Y. Mitochondrial uncoupling protein 2 is up-regulated in human head and neck, skin, pancreatic, and prostate tumors. Cancer Biomark. 2013;13:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Dalla Pozza E, Fiorini C, Dando I, Menegazzi M, Sgarbossa A, Costanzo C, Palmieri M, Donadelli M. Role of mitochondrial uncoupling protein 2 in cancer cell resistance to gemcitabine. Biochim Biophys Acta. 2012;1823:1856-1863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Araki R, Hiraki Y, Yabe T. Genipin attenuates lipopolysaccharide-induced persistent changes of emotional behaviors and neural activation in the hypothalamic paraventricular nucleus and the central amygdala nucleus. Eur J Pharmacol. 2014;741:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |