Published online Mar 14, 2015. doi: 10.3748/wjg.v21.i10.2982
Peer-review started: August 26, 2014
First decision: September 15, 2014
Revised: October 11, 2014
Accepted: November 30, 2014
Article in press: December 1, 2014
Published online: March 14, 2015
Processing time: 203 Days and 5.7 Hours
AIM: To evaluate the safety and feasibility of endoscopic resection using band ligation (EMR-B) for the diagnostic and therapeutic removal of tumors located in the esophageal subepithelial region having originated from the submucosa.
METHODS: From May 2009 to September 2014, after medical chart and endoscopic ultrasonography report review, a total of 15 esophageal tumors located in the submucosal layer were resected by EMR-B. Previous symptom, location, pathology, complete resection rate, incidence of complications, incidence of minor complication, size, length of procedures time and follow up months were evaluated. To evaluate local recurrence at the resection site, periodic follow-up endoscopic examination was undertaken in all of the patients. The first endoscopic examination was performed about 6 mo after the endoscopic resection. Thereafter, the endoscopic follow up were scheduled annually.
RESULTS: The mean age was 50.3 ± 9.67 years. The mean tumor size was 6.93 ± 3.15 mm and most of the lesions size was between 5-10 mm in diameter (10/15, 66.6%). In all patients, endoscopic en bloc resection was achieved. In one patient, the vertical margin was involved. The mean procedural time was 8.86 ± 3.66 min. In all patients, no evidence of severe complications such as perforation or bleeding occurred. Minor complications such as chest pain (2/15, 13.3%) and heartburn (3/15, 13.3%) were reported but they symptoms were controlled by proton pump inhibitors, ulcermin and/or analgesics. Histologic assessments of the removed specimens revealed 10 granular cell tumors (66.6%), 4 leiomyomas (16.6%) and one lipoma (6.6%). No recurrence was observed during the mean follow up period of 45 ± 3.5 mo (range: 5-64 mo).
CONCLUSION: EMR-B might be considered safe and effective for the diagnosis and treatment of lesions measuring less than 10 mm in diameter.
Core tip: In cases of esophageal tumors originating in the submucosal layer, we consider that endoscopic resection may be necessary if esophageal biopsy results are non-conclusive. Endoscopic resection using band ligation is effective for diagnosis and treatment of lesions measuring less than 10 mm in diameter.
- Citation: Hong JB, Choi CW, Kim HW, Kang DH, Park SB, Kim SJ, Kim DJ. Endoscopic resection using band ligation for esophageal SMT in less than 10 mm. World J Gastroenterol 2015; 21(10): 2982-2987
- URL: https://www.wjgnet.com/1007-9327/full/v21/i10/2982.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i10.2982
With the increased use of routine endoscopy for health checks in Korea, the frequency of identifying asymptomatic incidental esophageal subepithelial tumors is increasing. Although most of such small tumors are clinically insignificant, some which have originated from the submucosal layer, identified by endoscopic ultrasonography (EUS), have malignant potential[1-3]. For example, granular cell tumor, carcinoid tumor, gastrointestinal stromal tumor and lymphoma could be found in the submucosal layer[4,5]. Therefore, it is essential to distinguish between benign and malignant, or potentially malignant, tumors.
Recently, EUS has been demonstrated to apparently provide an advantage over endoscopy in the diagnosis of tumors beneath the mucosal layer, and can differentiate intramural lesions from extrinsic compression. For intramural lesions, EUS can determine the exact size, layer of origin, echogenicity and margin with surrounding structures[6]. However, the EUS only predicted the correct histologic diagnosis in 43% of cases and was dependent on the operator’s experience[6-9]. Ultimately, histologic confirmation should be obtained whenever possible. But, the diagnostic yield of simple endoscopic biopsy for subepithelial tumor is low. Where the endoscopic “bite on bite” technique is used in esophageal submucosal tumors, the diagnostic yield is 14%-42% in some reports, but there was a high risk of bleeding requiring endoscopic intervention in about 2.8% of cases[10,11].
Conventional endoscopic mucosal resection (EMR) can usually be used for superficial gastrointestinal neoplasms confined to the mucosal layer and esophageal submucosal tumors less than 10 mm in diameter. However, complete histologic resection is not always easy to achieve using EMR for tumors located in the submucosal layer, which results in frequent involvement of the resection margin. Recently, in the case of small tumors of less than 10 mm in diameter and located in the submucosal layer, EMR using a band-ligation device (EMR-B) showed a high complete resection rate[12].
This study aimed to evaluate the safety and feasibility of EMR-B for diagnostic and therapeutic removal of tumors located in the esophageal subepithelial region having originated from the submucosa.
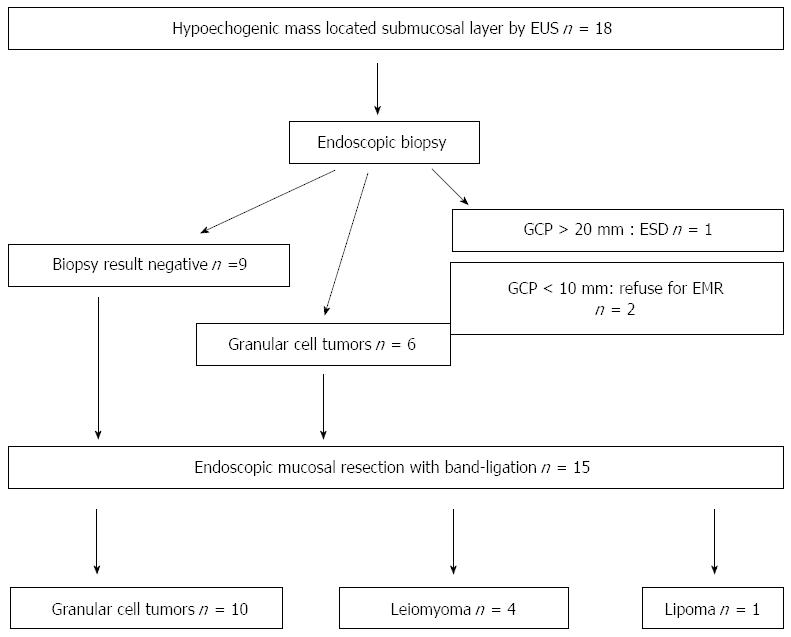
From May 2009 to September 2014, after medical chart and EUS report review, a total of 18 hypoechogenic mass lesions in the esophageal submucosa, defined by EUS, were found. After exclusion of cystic and vascular lesions by EUS, endoscopic biopsies had been performed in these patients; however in nine patients negative pathologic results were obtained. After appropriate exclusions, 15 of the 18 patients underwent EMR-B at Pusan National University Yangsan Hospital in Korea during the study period. The data was collected prospectively, but the data analysis was done retrospectively. This study was reviewed and approved by the Institutional Review Board at Pusan National University Yangsan Hospital. Written informed consent was obtained from all the patients prior to EMR-B. The procedures were performed under conscious sedation (intravenous administration of midazolam and/or meperidine) by two endoscopists (Choi CW and Kim HW) with > 5 years of experience in performing therapeutic endoscopy (including endoscopic submucosal dissection). For sedation, 2.5 mg of midazolam and 12.5 mg of meperidine were initially administered and another dose of 2.5 mg of midazolam and 12.5 mg of meperidine were injected at endoscopist’s discretion when required.
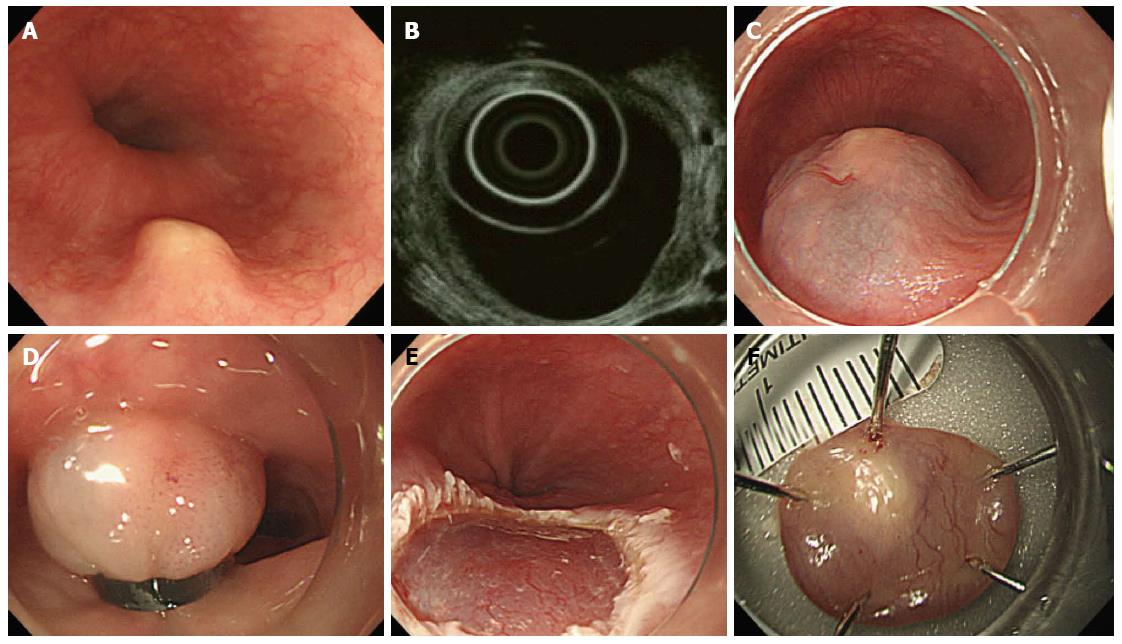
All patients were examined by endoscopy and EUS before the endoscopic resection. For EUS, the UM3R ultrasonic mini-probe (UMP, 20 MHz; Olympus, Tokyo, Japan) was used. Indications for endoscopic resection were as follows: measured tumor size < 10 mm in diameter, hypoechogenic lesions, hard mass, and confined to the submucosal layer as assessed by the EUS catheter probe (Figure 1).
There is a general consensus that solid tumors in the submucosal layer (hypoechoic lesions on EUS) are to be removed. However, EMR-B is not recommended for cystic or vascular lesions because they have a tendency to rupture during band ligation. For EMR-B, a model GIF-H260 single-channel endoscope (Olympus) was inserted into the esophagus. After careful inspection, a solution (10% glycerin plus 5% fructose in 0.9% saline diluted 1:100000 with epinephrine-normal saline solution and mixed with a small amount of indigo carmine) was injected submucosal layer around the lesion to lift it off the muscle layer. A single-channel endoscope with a band ligation device attached to its tip was reinserted into the esophagus. The lesion was then aspirated into the ligator device, followed by deployment of the elastic band. Snare resection was performed below the band by using an Endocut Q current (effect 3, cut duration 2, cut interval 5), which was generated using a VIO300D electrosurgical unit (ERBE, Tuebingen, Germany) (Figure 2).
The specimens were carefully evaluated histo-pathologically in slices at 2 mm intervals, with the microscopic evaluation including histopathologic type, depth of invasion, and lateral and vertical resection margins. En bloc resection was defined as endoscopic resection of the entire lesion in a single piece. Complete resection was defined as being unable to identify tumor cell tissue microscopically at the resection margin.
To evaluate local recurrence at the resection site, periodic follow-up endoscopic examination was undertaken in all of the patients. The first such endoscopic examination was performed about 6 mo after the endoscopic resection. Thereafter, the endoscopic checkups were scheduled annually.
During the study period, a total of 15 esophageal tumors located in the submucosal layer were resected by EMR using band ligation. Table 1 shows the details of patients’ information and endoscopic and pathologic results. The mean patient age was 50.3 ± 9.67 years. The locations of lesions were equally distributed. The mean tumor size was 6.93 ± 3.15 mm, and most of the lesions were between 5 and 10 mm in diameter (10/15, 66.6%) (Table 2).
| No. | Gender | Age (yr) | Pathology | Symptoms | Location | Procedure time (min) | Complication | Minor complication | Size (mm) | En bloc resection | Margin status | Follow-up (mo) | Outcomes |
| 1 | M | 54 | GCP | None | Middle | 10 | No | Chest pain | 8 | Yes | LM (-)/VM (+) | 64 | No recur |
| 2 | F | 52 | GCP | None | Upper | 4 | No | No | 7 | Yes | LM (-)/VM (-) | 46 | No recur |
| 3 | M | 44 | GCP | Reflux | Lower | 7 | No | No | 8 | Yes | LM (-)/VM (-) | 41 | No recur |
| 4 | M | 52 | Lipoma | None | Lower | 17 | No | No | 7 | Yes | LM (-)/VM (-) | 41 | No recur |
| 5 | F | 31 | Leiomyoma | None | Upper | 7 | No | Hot burn | 13 | Yes | LM (-)/VM (-) | 25 | No recur |
| 6 | M | 46 | GCP | Heartburn | Middle | 10 | No | Chest pain | 7 | Yes | LM (-)/VM (-) | 20 | No recur |
| 7 | M | 57 | GCP | None | Lower | 8 | No | Chest pain | 7 | Yes | LM (-)/VM (-) | 17 | No recur |
| 8 | F | 42 | GCP | Epigastric pain | Upper | 8 | No | No | 2 | Yes | LM (-)/VM (-) | 17 | No recur |
| 9 | F | 46 | GCP | None | Upper | 10 | No | No | 9 | Yes | LM (-)/VM (-) | 12 | No recur |
| 10 | M | 58 | GCP | Epigastric pain | Middle | 15 | No | No | 4 | Yes | LM (-)/VM (-) | 5 | No recur |
| 11 | F | 48 | GCP | Globus | Lower | 9 | No | No | 12 | Yes | LM (-)/VM (-) | 43 | No recur |
| 12 | F | 44 | Leiomyoma | None | Middle | 5 | No | No | 6 | Yes | LM (-)/VM (-) | 43 | No recur |
| 13 | F | 70 | Leiomyoma | None | Middle | 5 | No | No | 7 | Yes | LM (-)/VM (-) | 45 | No recur |
| 14 | F | 46 | GCP | None | Lower | 12 | No | No | 1 | Yes | LM (-)/VM (-) | 12 | No recur |
| 15 | F | 65 | Leiomyoma | None | Upper | 6 | No | Hot burn | 6 | Yes | LM (-)/VM (-) | 45 | No recur |
| Characteristics | Values |
| Age, yr (mean ± SD) | 50.3 ± 9.67 |
| Sex, male | 6 (40) |
| Tumor location | |
| Upper esophagus | 5 (33.3) |
| Middle esophagus | 5 (33.3) |
| Lower esophagus | 5 (33.3) |
| Tumor size, mm (mean ± SD) | 6.93 ± 3.15 |
| Tumor size, mm | |
| > 10 | 2 (13.3) |
| 5-10 | 10 (66.6) |
| < 5 | 3 (20.0) |
In all patients, endoscopic en bloc resection was achieved. In one patient, the vertical margin was involved, but during the follow up period of 48 mo, no evidence of local recurrence was found in spite of no additional treatment being given. The mean procedural time was 8.86 ± 3.66 min. No evidence of severe complications such as perforation and bleeding (including delayed bleeding after hospital discharge) occurred. Minor complications such as chest pain (2/15, 13.3%) and heartburn (3/15, 20.0%) were reported but they symptoms were controlled by proton pump inhibitors, ulcermin and/or analgesics. Histologic examination revealed 10 granular cell tumors (66.6%), four leiomyomas (16.6%) and one lipoma (6.6%) (Table 3). No recurrence was observed during the mean follow up period of 45 ± 3.5 mo (range: 5-64 mo).
| Characteristics | Values |
| Endoscopic complete resection | 15 (100) |
| pathologic complete resection | 14 (93.3) |
| Lateral margin | 15 (100) |
| Vertical margin | 14 (93.3) |
| Procedure time, min (mean ± SD) | 8.86 ± 3.66 |
| Major complication | |
| bleeding | 0 (0) |
| perforation | 0 (0) |
| Minor complication | |
| Hot burn | 2 (13.3) |
| Chest pain | 3 (20.0) |
| Recurrence on follow up | 0 (0) |
| Pathologic outcomes | |
| Granular cell tumor | 10 (66.6) |
| Leiomyoma | 4 (16.6) |
| Lipoma | 1 (6.6) |
Recently, due to the increased use of high resolution endoscopy and routine health checkups, esophageal subepithelial tumors have been detected more frequently and referred to academic hospitals for EUS. Although EUS plays an integral part in evaluating such tumors, its accuracy in delineating the layer of origin and making a specific diagnosis is limited and subject to the operator’s experience. Usually, after excluding lipomas, vascular lesions or cysts by EUS, gastrointestinal subepithelial tumors apparently originating from the submucosal layer need pathologic confirmation[13]. To achieve this, simple endoscopic biopsy was the first approach used. However, the diagnostic yield with the use of this simple biopsy technique from the luminal side, even with the “bite on bite” technique, is limited (less than 38% diagnostic rate), although the use of jumbo forceps increases the yield to about 60% but at the expense of an increased incidence of bleeding that requires endoscopic hemostasis in a third of patients[14,15].
Although the conventional EMR technique for biopsy of gastrointestinal submucosal tumors is a simple procedure, this technique is sometimes associated with margin involvement and crush injury of the resected specimens, which leads to difficulty in pathologic evaluation and often necessitates additional surgical intervention. To overcome these shortcomings, EMR-B has been described as an effective assessment and treatment modality[16]. It is known that EMR-B is safe to remove the subepithelial tumor in the submucosal layer less than 10 mm on any sites of digestive tract.
With EMR-B, tumors can be frontally viewed with a hood attached to the endoscope and lifted sufficiently by endoscopic suction. In this way, undamaged circular resected specimens can be obtained and EMR-B provides a deeper resection margin compared with conventional EMR[16]. In the present study, the en bloc resection rate and complete pathologic resection rate were 100% and 93.3%, respectively. In addition, no serious complications such as perforation or delayed bleeding occurred.
According to our research results, most of the tumors were granular cell tumors[17-21]. Although the natural history of granular cell tumor is unclear, most such tumors are known to have a benign clinical course. However, approximately 1.5%-2.7% of cases have malignant potential[17,20,22,23]. On endoscopy, a granular cell tumor presents as a submucosal lesion that is gray-white to yellowish in color. On EUS, it appears as a sub-mucosal homogeneous hypoechogenic mass with well-defined margins[24,25]. No generally accepted management of his tumor has yet been established because the precise natural course of the lesion is unknown. Nevertheless, several authors recommend endoscopic resection as a safe and effective treatment option[26,27].
This study had several limitations. First, because this was a retrospective study, there may have been a potential bias when retrospectively reviewing the outcome of the endoscopic resection. Secondly, all of the endoscopic procedures were performed by two skilled endoscopists who had had more than 5 years of therapeutic endoscopic experience at academic hospitals. Thirdly, this study was based on a limited experience at a single center. In conclusion, in cases of esophageal tumors originating in the submucosal layer, we consider that endoscopic resection might be necessary if esophageal biopsy results are inconclusive. After exclusion of cystic and vascular lesions by EUS, EMR-B might be considered safe and effective for the diagnosis and treatment of lesions measuring less than 10 mm in diameter.
After exclusion of cystic and vascular lesions, the pathologic diagnosis of an esophageal tumor originating in the submucosa is necessary. However, endoscopic ultrasonography (EUS) is not conclusive and the diagnostic yield from esophageal simple biopsy is low. This study aimed to evaluate the safety and feasibility of endoscopic resection using band ligation (EMR-B) for the diagnostic and therapeutic removal of tumors located in the esophageal subepithelial region having originated from the submucosa.
This study aimed to evaluate the safety and feasibility of EMR-B for the diagnostic and therapeutic removal of tumors located in the esophageal subepithelial region having originated from the submucosa.
According to our research results, EMR-B is recommended for the solid tumor of less than 1 cm from the submucosal layer of origin, except for cystic or vascular lesion in our endoscopy center.
Although majority of the tumors originated from submucosal layer are benign, tumors with malignant potential such as neuroendocrine tumors or lymphoma may be found as subepithelial tumor originated from submucosal layer. Such as, if gastrointestinal subepithelial tumors originated from submucosal layer are suspected by EUS, endoscopic resection is recommanded.
For EMR-B, endoscope was inserted into the esophagus. After careful inspection, a solution was injected submucosal layer around the lesion to lift it off the muscle layer. A single-channel endoscope with a band ligation device attached to its tip was reinserted into the esophagus. The lesion was then aspirated into the ligator device, followed by deployment of the elastic band. Snare resection was performed below the band.
This study aimed to evaluate the safety and feasibility of EMR-B for diagnostic and therapeutic removal of tumors located in the esophageal subepithelial region having originated from the submucosa. The results are interesting and may represent a effective for the diagnosis and treatment of lesions measuring less than 10 mm in diameter.
P- Reviewer: Ali AEM, Fujita T, Gentili A, Gornals JB, Lo GH, Silva G S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Lim YJ, Son HJ, Lee JS, Byun YH, Suh HJ, Rhee PL, Kim JJ, Rhee JC. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010;16:439-444. [PubMed] |
| 2. | Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20-23. [PubMed] |
| 4. | Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Lim CS, Park SJ, Park MI, Moon W, Kim HH, Lee JS, Kim BJ, Ku DY. Successful endoscopic mucosal resection of a low esophageal carcinoid tumor. Clin Endosc. 2013;46:576-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202-208. [PubMed] |
| 7. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88-92. [PubMed] |
| 9. | Kim GH, Park do Y, Kim S, Kim DH, Kim DH, Choi CW, Heo J, Song GA. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009;15:3376-3381. [PubMed] |
| 10. | Ji JS, Lee BI, Choi KY, Kim BW, Choi H, Huh M, Chung WC, Chae HS, Chung IS. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009;24:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Choi CW, Kang DH, Kim HW, Park SB, Jo WS, Song GA, Cho M. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol. 2013;47:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Khashab MA, Pasricha PJ. Conquering the third space: challenges and opportunities for diagnostic and therapeutic endoscopy. Gastrointest Endosc. 2013;77:146-148. [PubMed] |
| 14. | Buscaglia JM, Nagula S, Jayaraman V, Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal DV. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012;75:1147-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006;64:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Ono A, Fujii T, Saito Y, Matsuda T, Lee DT, Gotoda T, Saito D. Endoscopic submucosal resection of rectal carcinoid tumors with a ligation device. Gastrointest Endosc. 2003;57:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Goldblum JR, Rice TW, Zuccaro G, Richter JE. Granular cell tumors of the esophagus: a clinical and pathologic study of 13 cases. Ann Thorac Surg. 1996;62:860-865. [PubMed] |
| 18. | Voskuil JH, van Dijk MM, Wagenaar SS, van Vliet AC, Timmer R, van Hees PA. Occurrence of esophageal granular cell tumors in The Netherlands between 1988 and 1994. Dig Dis Sci. 2001;46:1610-1614. [PubMed] |
| 19. | Yasuda I, Tomita E, Nagura K, Nishigaki Y, Yamada O, Kachi H. Endoscopic removal of granular cell tumors. Gastrointest Endosc. 1995;41:163-167. [PubMed] |
| 20. | Rubesin S, Herlinger H, Sigal H. Granular cell tumors of the esophagus. Gastrointest Radiol. 1985;10:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Shikuwa S, Matsunaga K, Osabe M, Ofukuji M, Omagari K, Mizuta Y, Takeshima F, Murase K, Otani H, Ito M. Esophageal granular cell tumor treated by endoscopic mucosal resection using a ligating device. Gastrointest Endosc. 1998;47:529-532. [PubMed] |
| 22. | Ohmori T, Arita N, Uraga N, Tabei R, Tani M, Okamura H. Malignant granular cell tumor of the esophagus. A case report with light and electron microscopic, histochemical, and immunohistochemical study. Acta Pathol Jpn. 1987;37:775-783. [PubMed] |
| 23. | Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22:779-794. [PubMed] |
| 24. | Moreira LS, Dani R. Treatment of granular cell tumor of the esophagus by endoscopic injection of dehydrated alcohol. Am J Gastroenterol. 1992;87:659-661. [PubMed] |
| 25. | Palazzo L, Landi B, Cellier C, Roseau G, Chaussade S, Couturier D, Barbier J. Endosonographic features of esophageal granular cell tumors. Endoscopy. 1997;29:850-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Battaglia G, Rampado S, Bocus P, Guido E, Portale G, Ancona E. Single-band mucosectomy for granular cell tumor of the esophagus: safe and easy technique. Surg Endosc. 2006;20:1296-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kahng DH, Kim GH, Park do Y, Jeon MS, Yi JW, Choi YY, Song GA. Endoscopic resection of granular cell tumors in the gastrointestinal tract: a single center experience. Surg Endosc. 2013;27:3228-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |