Published online Mar 7, 2014. doi: 10.3748/wjg.v20.i9.2247
Revised: December 15, 2013
Accepted: January 14, 2014
Published online: March 7, 2014
Processing time: 129 Days and 11.4 Hours
Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic tumor, is a highly aggressive human cancer with the lowest five-year survival rate of any human maligancy primarily due to its early- metastasis and lack of response to chemotherapy and radiation. Recent research suggests that PDAC cells comprise a hierarchy of tumor cells that develop around a population of cancer stem cells (CSCs), a small and distinct population of cancer cells that mediates tumoregenesis, metastasis and resistance to standard treatments. Thus, CSCs could be a target for more effective treatment options. Interestingly, pancreatic CSCs are subject to regulation by some of key embryonic stem cell (ESC) transctiption factors abberently expressed in PDAC, such as SOX2, OCT4 and NANOG. ESC transcription factors are important DNA-binding proteins present in both embryonic and adult somatic cells. The critical role of these factors in reprogramming processes makes them essential not only for embryonic development but also tumorigenesis. Here we provide an overview of stem cell transcription factors, particularly SOX2, OCT4, and NANOG, on their expression and function in pancreatic cancer. In contrast to embryonic stem cells, in which OCT4 and SOX2 are tightly regulated and physically interact to regulate a wide spectrum of target genes, de novo SOX2 expression alone in pancreatic cancer cells is sufficient to promote self-renewal, de-differentiation and imparting stemness characteristics via impacting specific cell cycle regulatory genes and epithelial-mesnechymal transtion driver genes. Thus, targeting ESC factors, particularly SOX2, could be a worthy strategy for pancreatic cancer therapy.
Core tip: Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal human cancer due to its early metastasis and lack of response to chemoradiotherapy. Pancreatic cancer stem cells (CSCs) are implicated in tumorigenesis and metastasis as well as therapy resistance, therefore represent a potential target for effective therapeutic options. Recent publications including our own research demonstrate that key embryonic stem cell (ESC) factors, such as OCT4, NANOG and SOX2, are abbrently expressed in PDAC and contribute to pancreatic CSC-like characteristics, such as self-renewal and de-differentiation. This review aims to summarize our current knowledge on the role of ESC factors particulary SOX2 in regulating pancreatic CSC-like feature and implication for therapy.
- Citation: Herreros-Villanueva M, Bujanda L, Billadeau DD, Zhang JS. Embryonic stem cell factors and pancreatic cancer. World J Gastroenterol 2014; 20(9): 2247-2254
- URL: https://www.wjgnet.com/1007-9327/full/v20/i9/2247.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i9.2247
Pancreatic cancer is the fourth leading cause of cancer death in men and women in the United States. In 2012 alone, an estimated 43920 adults in the United States were diagnosed with pancreatic cancer and 37390 deaths from this disease ocurred[1]. About 280000 new cases of pancreatic cancer were recorded in 2008 worldwide. Pancreatic ductal adenocarcinoma (PDAC), the most common type of pancreatic cancer, is also the most lethal among the human solid tumors with a 5-year survival rate of less than 5 percent[2]. The main reasons for this outcome include lack of early detection, invasive behavior and intrinsic resistance to most chemo-/radio- and immuno-therapy strategies[3,4]. Recently, several studies have identified PDAC cancer stem cells (CSCs), which are highly tumorigenic and have the capacity to not only self-renew, but also generate differentiated progeny[3,5-7]. Pancreatic CSCs are also resistant to chemotherapies commonly used to treat patients with PDAC[8-10]. Thus, studies identifying key determinants in pancreatic cancer and pancreatic CSCs can provide both biomarkers of PDAC aggressiveness and potentially optimal targets to overcome chemoresistance (Table 1). Here we review how embryonic stem factors contributes to the agressiveness of this disease and the potential for targeted therapy.
| Gene | Biological role/behaviour | PDAC implications | Ref. |
| OCT4 | Overexpressed in 69% of PDAC. Pro-oncogenic role | Correlation with N1/M1 status and indicative of worse prognosis | Polvani et al[47], 2013 |
| Overexpressed in 48.8% of PDAC. Induces cell proliferation, migration and invasion | Contribution to metastasis and drug resistance | Lu et al[50], 2013 | |
| Overexpressed in human cell lines | Multidrug resistance and metastasis | Wang et al[52], 2013 | |
| Induction of tumorigenic capacity | Chemo-resistance | Wang et al[53], 2013 | |
| Overexpressed in 79.2% metaplastic ducts | Early carcinogenesis and worse prognosis | Wen et al[49], 2010 | |
| SOX2 | Overexpressed in poorly differentiated human tumors | Correlation to aggressiveness | Sanada et al[54], 2006 |
| Ectopic expression in 19.3% of PDAC. Promotes cancer cell proliferation/dedifferentiation | Rapid tumor progression and poor differentiation | Herreros-Villanueva et al[38], 2013 | |
| Induction of tumorigenic capacity | Chemo-resistance | Wang et al[53], 2013 | |
| NANOG | Overexpressed in 53.5% of PDAC. Induces proliferation, migration and invasion | Associated with eraly stage carcinogenesis and worse overall survival | Lu et al[50], 2013 |
| Overexpressed in cells capable of initiating spheres | Resistance to 5-FU treatment | Lonardo et al[68], 2013 | |
| Overexpressed in pancreatic tumors | Contribution to carcinogenesis and correlates to worse prognosis | Wen et al[49], 2010 |
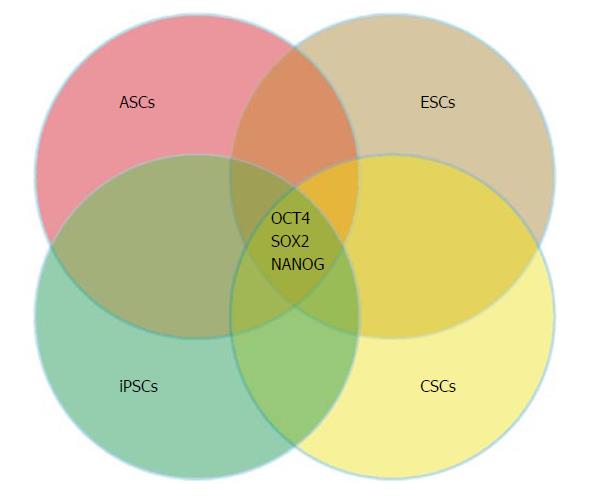
Stem cells (SCs) are traditionally defined as cells that can both self-renew and generate a progeny that are capable of following more than a single differentiation pathway[11]. Currently, four types of SCs have been described[12]. The first two are physiologically present at different stages of life, namely, the embryonic stem cells (ESCs) and the somatic or adult stem cells (ASCs). The ESCs are the best studied SCs and knowledge derived from ESCs research has guided the investigations of other types of SCs. ASCs are postnatal derivatives of ESCs located throughout the body. ASCs have been shown to retain co-expression of at least three of the core transcription factors characteristic of ESCs (OCT4, KLF4, and SOX2). Similar to ESCs, the presence of a balanced network of core stem markers, rather than the overt expression of a single factor, contributes to maintainenance of ASC characteristics. The third SC type is induced pluripotent stem cells (iPSCs), which are artificially engineered from a non-pluripotent cell, such as via somatic cell nuclear transfer or reprograming with gene transfer. The generation of iPSCs represents a milestone achievement in SC research, which not only breaks the dogma that somatic cell differentiation is an irreversible process, but also makes possible a new approach for regenerative medicine without controversial use of embryos. The fourth SC type is CSCs, also referred to as cancer initiating cells (CICs), which are defined as those cells within a tumor that can self-renew, produce differentiated progeny, and drive tumorigenesis. The ability of cancer cells to form nonadherent spheroids in vitro culture is frequently used as a surrogate of stemness. Unlike ESCs, CSCs are highly heterogenous with great variation among the markers for each tumor type.
ESCs are derived from the inner cell mass (ICM) of the preimplantation mammalian embryo and can be maintained indefinitely in culture[13]. By definition, ESCs are pluripotent. They are able to give rise to all somatic and the three germ cell lineages of the developing embryo. Pluripotency is maintained through self-renewal, which allows ESCs to duplicate themselves without losing the ability to differentiate. This can be achieved via both symmetric and asymmetric cell divisions[14].
Over the last decade, there has been accumulating evidence indicating that the maintenance of pluripotency in ESCs is governed by core genetic and epigenetic regulators, which allow self-renewal and prevents specific differentiation pathways. Recent progress on the molecular mechanism(s) governing stem cells pluripotency has provided critical insights into the role of nine core transcription factors OCT4 (POU5F1), NANOG, SOX2, Dppa4, Dppa5, Sall4, Utf1, Rex2, and Rif1 in maintaining mouse cells in the undifferentiated stage[15-18]. Among these genes, OCT4, NANOG, and SOX2, referred to as pluripotency genes, are highly expressed in the ICM. The perfect balance of these proteins maintains pluripotency and self-renew in ESC during the first days of embryonic development[18]. Broadly, the pluripotency genes have been shown to be common to all SC types (Figure 1). In contrast to OCT4, NANOG and SOX2, c-MYC, an important oncogene as well as a reprogramming factor for pluripotency[17], is highly heterogeneous in cells from the ICM. However, it is not always considered a pluripotency gene in ESCs. The activity of these three core pluripotency genes regulates and coordinates the expression of a second set of core genes, which include transcription factos, cell surface markers, ABC transporters, and enzymes. Together, these proteins orchestrate the specific stem cells properties[19].
SOX2 and OCT4 form a protein complex in the nucleus of ESCs. This complex is auto-regulated in a loop that, transcriptionally, also induces the expresion of pluripotency genes (most importantly NANOG), cell cycle, apoptosis, DNA repair, chromatin structure genes, and genes regulating endoderm, mesoderm, and ectoderm differentiation. Thus, tight control of all these genes may allow ICM cells to exit from their inherent developmental program, as they acquire the ability to self-renew, while retaining pluripotency as ESCs[20]. Finally, when the expression of these pluripotency genes decreases in a properly regulated way, an induction in the expression of early differentiation markers occurs. These markers include ectoderm markers (Pax6, Otx1, Neurod1, Nes, Lhx5, and Hoxb1), mesoderm markers (Tbx2, T, Nkx2-5, Myod1, Myf5, Mesdc1, Mesdc2, Kdr, Isl1, Hand1 and Eomes), endoderm markers (Onecut1, Gata4, Gata5, and Gata6), and extraembryonic markers (Cdx2 and Tpbpa).
iPSCs were first derived by the transduction of mouse and human fibroblasts through integrating viruses carrying four transcription factors: OCT4, SOX2, MYC and Krupple-like factor 4 (KLF4)[21], also referred as the Yamanaka factors. Takhashi and Yamanaka[21] broke a dogma in developmental biology by showing that mammalian somatic cell differentiation is a reversible process[17,21]. By transfecting human somatic cells with the four Yamanaka factors, they were able to revert the differentiated cells to an embryonic-like state. Because these newly generated cells showed the morphology, pluripotency, and capacity to form teratomas similar to ESCs, they named these cells iPSCs. Later, Yu et al[22] further demonstrated that the combination of OCT4, NANOG, SOX2 and Lin28, also called Thomson Factors, was able to produce iPSCs. Both, Yamanaka and Thomson Factors are Reprogramming Factors as Reprogramming is the process that converts differentiated cells back to pluripotent cells, namely the reversal of differentiation.
Recently, new methods have been developed to reprogram human somatic cells with or without MYC[22,23] and to combine only some of the reprogramming transcription factors with chemical inhibitors[24-26]. However, the fact remains that OCT4, SOX2, MYC, and KLF4 reside at the heart of the reprogramming process. Given that the transcription factors in this network not only associate with one another, but also associate with many of the same proteins in the network, there is a high degree of interdependence between these transcription factors. Thus, it is not surprising that the levels of SOX2 and OCT4 need to be controlled carefully for optimal production of iPSC, or that small changes in the levels of these master regulators can lead to dramatically altered cell fates. However, it remains to be determined how their levels affect the molecular efficiency of reprogramming. Given the strict requirement for SOX2 and OCT4 during development, their key roles in ESC differentiation, and the pronounced differences in reprogramming when their levels are not optimized, additional efforts should be made to determine why small changes in the levels of these two master regulators alters the behavior of pluripotent stem cells.
Although initially discovered in hematopoietic malignancies, such as acute myelogenous leukemia and chronic myelogenous leukemia[27,28], CSCs were later described in various solid tumors, including glioblastoma[29], melanom[30], prostate[31], colon[32] head and neck squamous cell carcinoma[33], breast[34], ovarian[35], bladder[36], lung[37] and pancreatic cancer[6,7,38,39]. In these malignancies, a small population of CSCs can self-renew and differentiate into all of the other cell types forming the bulk tumoral population. However, the bulk of tumor cells lack the ability to differentiate into other subpopulations of cancer cells and thus possess limited self-renewal capacity. In addition, it has been shown that CSCs have tumor initiation capacity, forming xenograph tumors in mice and are radio- and chemo-resistant, contributing to lack of therapeutic response in patients[39].
Although several proteins have been proposed as CSC markers, there is great variation between tumor types[40,41]. This variation might be the result of the lack of standardized techniques to obtain and analyze CSCs, as well as the intrinsic plasticity of these cells[40]. Since CSCs express many genes in common with early ESCs, primarily OCT4, NANOG, and SOX2, the picture that emerged was that these transcription factors could also work together as part of a highly integrated network to regulate pluripotency and self-renewal in tumors. Nevertheless, the heterogeneity of tumors and the plasticity that characterize CSCs render the expression pattern of these transcription factors highly heterogeneous in different tumors and even within the same tumor.
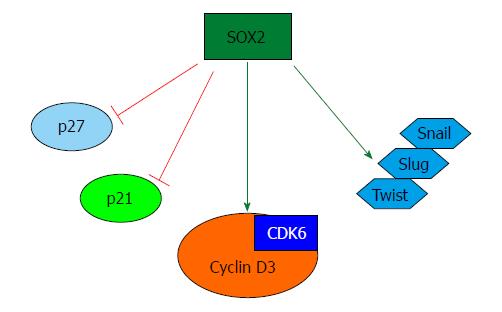
Several publications show that overexpression of OCT4, SOX2 and NANOG, together or separately, led to tumor transformation, tumorigenicity, tumor metastasis, and even distant recurrence after chemoradiotherapy[42]. It is well known that these transcription factors are more frequently overexpressed in poorly differentiated tumors (compared to well differentiated tumors) and, in theory, that the expression level of the pluripotent transcription factors should decrease with the differentiation of cells[43]. In this regard, how these genes contribute to specific CSC properties has not been fully elucidated. Based on data obtained from iPSCs, several mechanisms have been proposed to explain the properties that these transcription factors could be imparting on CSCs. For example, once these transcription factors are overexpressed, they might activate several genes whose promoters are accessible to them. These “first responders” must then engage the epigenetic machinery to remodel the chromatin through histone modification and DNA methylation. In this process, genes critical for pluripotency must be switched on, while genes responsible for differentiation must be turned off and kept off[44]. From this data, it is clear that OCT4, NANOG and SOX2 are master regulators, which together drive the transition from a somatic cell to either a CSC or iPSC (Figure 2).
As mentioned above, CSCs have also been described in PDAC (Table 1). Originally Li et al[6] identified human pancreatic CSCs as CD44+/CD24+/ESA+. A few months later, Hermann et al[7] showed that CD133 and CXCR4 are also expressed in cells with CSC properties. In addition, some other markers such as c-Met[5] and aldehyde dehydrogenase 1 activity (ALDH1)[45] have been demonstrated in pancreatic CSCs. Recently, some reports describe the presence of a side population (SP) of cells in pancreatic cancer, a chemoresistant population of cells that could be enriched in CSCs. Additionally, this data indicates that SP cells express pancreatic CSC markers (CXCR4, CD133) and multidrug resistance genes (ABCB1), associating these cells with candidate therapeutic targets and potential prognostic value[46].
The regulation and characterization of CSCs in various types of human cancer, in which SOX2, OCT4 and NANOG are important players, is currently a hot topic. However, the number of specific publications analyzing their role in pancreatic cancer is very limited. In particular, a literature search on PUBMED database using the terms ‘‘OCT4’’, ‘‘NANOG’’ and ‘‘SOX2’’ together with ‘‘pancreatic cancer’’, showed 24, 27, and 20 published articles, respectively. Furthermore, only a few of these articles discuss these factors in the context of CSCs (Table 1). Polvani et al[47] found that OCT4 is expressed in 69% of PDAC and that this expression correlates with N1/M1 status and clinical stage, being an independent prognostic factor for worst outcomes. In agreement with several breast cancer publications[48], patients with OCT4+ PDAC have a shorter survival, suggesting this ESC factor as a marker of poor prognosis. Importantly, high levels of OCT4 and NANOG in human pancreatic cancer tissues were found to be associated with early stages of carcinogenesis[49] and correlate with worse prognosis[50]. Additionally OCT4 seems to contribute to multidrug-resistance and metastasis[51,52]. Wang et al[53] recently demonstrated that SP cells positive for NANOG, OCT4 and SOX2 possessed aggressive growth, invasion, migration and drug-resistance properties.
To date, very little is known regarding how OCT4 and NANOG contribute to pancreatic CSC properties at the molecular level. Interestingly, recent studies suggest that SOX2 is aberrantly expressed in a significant fraction of pancreatic tumors. Initially, Sanada et al[54] analyzed 14 cases of human PDAC immunohistochemically, and observed weak expression of SOX2 in pancreatic intraepithelial neoplasia (PanIN-3) lesions. They also observed relatively high and frequent expression in invasive and poorly differentiated PDAC. Later, it was shown that at the mRNA level, SOX2 expression driven by hedgehog-EGFR signaling is necessary for tumor-initiating pancreatic cancer cells[55]. Very recently, the molecular mechanism underlying SOX2 regulation of pancreatic cancer stemness has been elucidated. Using primary human cancer tissues and cell lines (L3.6, Bxpc3, CFPAC-1, Panc1 and Panc04.03), our group demonstrated a critical role for SOX2 in promoting cell proliferation, dedifferentiation and impartment of stem cell-like features to pancreatic cancer cells[38]. In particular, SOX2 gene suppression arrested cells at the G1 phase and its overexpression alone was sufficient to drive cell proliferation by facilitating G1/S transition. Mechanistically, G1 arrest in SOX2 knockdown cells is associated with a marked induction of p21Cip1 and p27Kip1, two key cyclin/CDK inhibitors, whereas SOX2 overexpression induces G1/S-specific cyclin D3 expression. All of three cell cycle regulators were identified as bona fide SOX2 regulatory targets. SOX2 also confers pancreatic cancer cell stemness and its overexpression alone is sufficient to drive sphere-formation and expression of CSC markers[7,38,45,56], as well as induce EMT drivers such as Snail, Slug and Twist (Figure 2). Consistently, loss of miR-145 elevates SOX2 and impairs differentiation in pancreatic tumors[57].
It is now evident that the core stem cell factors OCT4[16], SOX2[58], and NANOG[59] play essential roles in the maintenance of pluripotency and self-renewal of ESCs, ASCs, iPSCs and CSCs. These stem cell factors promote self-renewal by interacting with other transcription factors (Stat3, Hesx1, Zic3), critical cell signaling molecules (Hedgehog, TCF3, FGF2, LEFTY2)[60], and have been found aberrantly expressed in several types of human tumors including pancreatic cancer[61-63]. Although ESCs and CSCs share the property of self-renewal, they also reveal distinct features in that ESCs favor differentiation, whereas CSCs are more biased toward proliferation and inhibition of apoptosis. In particular, SOX2 has demonstrated OCT4 and/or NANOG independent activity in pancreatic cancer cells in promoting cell proliferation, survival, and/or de-differentiation[38]. Recent work by Polvani et al[47] further supports this statement demonstrating that OCT4 silencing reduces OCT4 and increases NANOG, but does not alter SOX2 expression.
SOX2 immunoreactivity has been demonstrated in PanIN lesions, as well as moderately and poorly differentiated tumors, which is consistent with previous reports showing an enrichment of SOX2 in pancreatic CSCs[64], as well as a decreased expression after anti-ESCs therapies[55,65]. Since SOX2 appears to be a key factor aberrantly expressed in PDAC and confers CSCs-like properties[38], targeting SOX2 or its upstream regulator(s) may be exploited for therapeutic purposes. Recent reports demonstrate that using poly (lactide-co-glucolide) to knockdown DCLK1 results in an increase in miR-145 associated with decreased puripotency factors including SOX2, and consequently, tumor growth arrest in xenografts[57,66]. Lastly, data from Sobrevals et al[67] elucidates the relevance of uPAR-controlled oncolytic adenoviruses in the elimination of pancreatic CSCs. Along these lines, C-Met inhibitors have been demonstrated to overcome gemcitabine resistance and stem cell signaling through downregulation of CSC markers including SOX2[65]. Strategies to target CSCs for cancer therapy have been proposed and are under investigation. For instance, metformin directed against pancreatic CSC has been shown to reduce tumor burden and prevent disease progression[68]. Disulfiram, an ALDH inhibitor, was tested in vitro and in vivo demonstrated a capacity to suppress pancreatic CSCs[69]. Promising results suggest that HAb18G/CD47 or Phospho-valproic acid (MDC-1112) could also be a promising target in pancreatic cancer surrogating anti-STAT3 therapies[70,71]. More recently, HAb18G/CD147 has been identified as another promising therapeutic target for highly aggressive pancreatic cancer and a surrogate marker in the STAT3-targeted molecular therapies, such as by phospho-valproic acid (MDC-1112), a novel valproic acid derivative. Since targeting CSCs has been demonstrated to be a viable therapeutic strategy against pancreatic cancer, a better undertanding of OCT4, NANOG and particularly SOX2 on their expression and regulatory circuitry in PDAC will facilitate the design of individualized therapies for PDAC patients.
P- Reviewers: Scaggiante B, Vickers MM S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 3. | Kumar-Sinha C, Wei I, Simeone DM. Emerging frontiers in pancreatic cancer research: elaboration of key genes, cells and the extracellular milieu. Curr Opin Gastroenterol. 2012;28:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Hamacher R, Schmid RM, Saur D, Schneider G. Apoptotic pathways in pancreatic ductal adenocarcinoma. Mol Cancer. 2008;7:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218-2227.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2427] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 7. | Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1987] [Cited by in RCA: 2137] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 8. | Ottinger S, Klöppel A, Rausch V, Liu L, Kallifatidis G, Gross W, Gebhard MM, Brümmer F, Herr I. Targeting of pancreatic and prostate cancer stem cell characteristics by Crambe crambe marine sponge extract. Int J Cancer. 2012;130:1671-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett. 2013;338:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Spivakov M, Fisher AG. Epigenetic signatures of stem-cell identity. Nat Rev Genet. 2007;8:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 12. | Alvarez CV, Garcia-Lavandeira M, Garcia-Rendueles ME, Diaz-Rodriguez E, Garcia-Rendueles AR, Perez-Romero S, Vila TV, Rodrigues JS, Lear PV, Bravo SB. Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J Mol Endocrinol. 2012;49:R89-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Surani MA, Hayashi K, Hajkova P. Genetic and epigenetic regulators of pluripotency. Cell. 2007;128:747-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 14. | Xi R, Xie T. Stem cell self-renewal controlled by chromatin remodeling factors. Science. 2005;310:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631-642. [PubMed] |
| 16. | Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2725] [Cited by in RCA: 2670] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 17. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18178] [Article Influence: 956.7] [Reference Citation Analysis (0)] |
| 18. | Tang F, Barbacioru C, Bao S, Lee C, Nordman E, Wang X, Lao K, Surani MA. Tracing the derivation of embryonic stem cells from the inner cell mass by single-cell RNA-Seq analysis. Cell Stem Cell. 2010;6:468-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 411] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 19. | Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 397] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14305] [Article Influence: 841.5] [Reference Citation Analysis (0)] |
| 22. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7589] [Cited by in RCA: 7242] [Article Influence: 402.3] [Reference Citation Analysis (0)] |
| 23. | Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2072] [Cited by in RCA: 1974] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 24. | Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 25. | Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Schöler HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 516] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 27. | Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730-737. [PubMed] |
| 28. | Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319-325. [PubMed] |
| 29. | Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821-5828. [PubMed] |
| 30. | Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320-4333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 413] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 31. | Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153-3161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 32. | O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2977] [Cited by in RCA: 3048] [Article Influence: 160.4] [Reference Citation Analysis (0)] |
| 33. | Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1548] [Cited by in RCA: 1614] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 34. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7830] [Cited by in RCA: 7722] [Article Influence: 351.0] [Reference Citation Analysis (0)] |
| 35. | Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 1007] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 36. | Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J, Chang HY, van de Rijn M. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:14016-14021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 494] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 37. | Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1596] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 38. | Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR, de Narvajas AA, Gomez TS, Simeone DM, Bujanda L. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 39. | Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883-190; discussion 1883-190;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 935] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 40. | Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013;144:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1455] [Cited by in RCA: 1345] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 42. | Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2057] [Cited by in RCA: 2019] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 43. | Bernhardt M, Galach M, Novak D, Utikal J. Mediators of induced pluripotency and their role in cancer cells - current scientific knowledge and future perspectives. Biotechnol J. 2012;7:810-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Pei D. Regulation of pluripotency and reprogramming by transcription factors. J Biol Chem. 2009;284:3365-3369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 46. | Van den Broeck A, Vankelecom H, Van Delm W, Gremeaux L, Wouters J, Allemeersch J, Govaere O, Roskams T, Topal B. Human pancreatic cancer contains a side population expressing cancer stem cell-associated and prognostic genes. PLoS One. 2013;8:e73968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Polvani S, Tarocchi M, Tempesti S, Mello T, Ceni E, Buccoliero F, D’Amico M, Boddi V, Farsi M, Nesi S. COUP-TFII in pancreatic adenocarcinoma: Clinical implication for patient survival and tumor progression. Int J Cancer. 2014;134:1648-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Nagasaki S, Suzuki T, Miki Y, Akahira J, Shibata H, Ishida T, Ohuchi N, Sasano H. Chicken ovalbumin upstream promoter transcription factor II in human breast carcinoma: possible regulator of lymphangiogenesis via vascular endothelial growth factor-C expression. Cancer Sci. 2009;100:639-645. [PubMed] |
| 49. | Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 50. | Lu Y, Zhu H, Shan H, Lu J, Chang X, Li X, Lu J, Fan X, Zhu S, Wang Y. Knockdown of Oct4 and Nanog expression inhibits the stemness of pancreatic cancer cells. Cancer Lett. 2013;340:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Quint K, Tonigold M, Di Fazio P, Montalbano R, Lingelbach S, Rückert F, Alinger B, Ocker M, Neureiter D. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int J Oncol. 2012;41:2093-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Wang D, Zhu H, Zhu Y, Liu Y, Shen H, Yin R, Zhang Z, Su Z. CD133(+)/CD44(+)/Oct4(+)/Nestin(+) stem-like cells isolated from Panc-1 cell line may contribute to multi-resistance and metastasis of pancreatic cancer. Acta Histochem. 2013;115:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Wang X, Liu Q, Hou B, Zhang W, Yan M, Jia H, Li H, Yan D, Zheng F, Ding W. Concomitant targeting of multiple key transcription factors effectively disrupts cancer stem cells enriched in side population of human pancreatic cancer cells. PLoS One. 2013;8:e73942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Sanada Y, Yoshida K, Ohara M, Oeda M, Konishi K, Tsutani Y. Histopathologic evaluation of stepwise progression of pancreatic carcinoma with immunohistochemical analysis of gastric epithelial transcription factor SOX2: comparison of expression patterns between invasive components and cancerous or nonneoplastic intraductal components. Pancreas. 2006;32:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Eberl M, Klingler S, Mangelberger D, Loipetzberger A, Damhofer H, Zoidl K, Schnidar H, Hache H, Bauer HC, Solca F. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med. 2012;4:218-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 56. | Penchev VR, Rasheed ZA, Maitra A, Matsui W. Heterogeneity and targeting of pancreatic cancer stem cells. Clin Cancer Res. 2012;18:4277-4284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One. 2013;8:e73940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 58. | Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1695] [Cited by in RCA: 1696] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 59. | Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643-655. [PubMed] |
| 60. | Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1833] [Cited by in RCA: 1847] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 61. | Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 772] [Cited by in RCA: 766] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 62. | Cox JL, Wilder PJ, Desler M, Rizzino A. Elevating SOX2 levels deleteriously affects the growth of medulloblastoma and glioblastoma cells. PLoS One. 2012;7:e44087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Leis O, Eguiara A, Lopez-Arribillaga E, Alberdi MJ, Hernandez-Garcia S, Elorriaga K, Pandiella A, Rezola R, Martin AG. Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene. 2012;31:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 64. | Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 65. | Hage C, Rausch V, Giese N, Giese T, Schönsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245-256. [PubMed] |
| 67. | Sobrevals L, Mato-Berciano A, Urtasun N, Mazo A, Fillat C. uPAR-controlled oncolytic adenoviruses eliminate cancer stem cells in human pancreatic tumors. Stem Cell Res. 2014;12:1-10. [PubMed] |
| 68. | Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One. 2013;8:e76518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 69. | Kim SK, Kim H, Lee DH, Kim TS, Kim T, Chung C, Koh GY, Kim H, Lim DS. Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy-resistant cancer cells. PLoS One. 2013;8:e78130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Mackenzie GG, Huang L, Alston N, Ouyang N, Vrankova K, Mattheolabakis G, Constantinides PP, Rigas B. Targeting mitochondrial STAT3 with the novel phospho-valproic acid (MDC-1112) inhibits pancreatic cancer growth in mice. PLoS One. 2013;8:e61532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Li L, Tang W, Wu X, Karnak D, Meng X, Thompson R, Hao X, Li Y, Qiao XT, Lin J. HAb18G/CD147 promotes pSTAT3-mediated pancreatic cancer development via CD44s. Clin Cancer Res. 2013;19:6703-6715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |