Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.2120
Revised: December 9, 2013
Accepted: January 8, 2014
Published online: February 28, 2014
Processing time: 181 Days and 19.4 Hours
Leptomeningeal involvement is usually reported as a secondary event in advanced gastric carcinoma. Leptomeningeal carcinomatosis (LMC), as the initial manifestation of asymptomatic gastric cancer, is exceedingly rare with only a few cases reported in recent years. The presenting neurologic symptoms include headache, vomiting and seizures and are usually clinically atypical. The diagnosis of LMC is made via identification of malignant cells that originate from epithelial cells in the cerebrospinal fluid by cytological examination and provides cues to track the primary tumor. Endoscopic examinations are crucial to confirm the presence of gastric cancer, and imaging studies, especially gadolinium-enhanced magnetic resonance imaging of the brain, are sometimes helpful in diagnosis. Thus far, there is no standard therapy for LMC, and despite all measures, the prognosis of the condition is extremely poor. Here, we report on the clinical features and diagnostic procedures for a patient with occult gastric cancer with Bormann type I macroscopic appearance and poor differentiation in pathology, who presented with LMC-induced neurological symptoms as the initial clinical manifestation. Additionally, we review the similar cases reported over the past years, making comparison among cases in order to provide more information for the future diagnosis.
Core tip: Here we report on a patient, initially and alone, presented with neurological symptoms and signs without any clues indicating gastric cancer. Evidence shows that the tumor directly spread solely to the brain without involvement of any other organs or tissues. How do we make an accurate and rapid diagnosis? Cerebral spinal fluid cytological studies play a key role in diagnosis, finding malignant cells that originate from epithelial cells. A gastroscopic examination was performed, revealing a tumor in the gastric antrum which was classified as Bormann type I in macroscopic appearance, a rare type in Bormann classification, however, with poor differentiation in pathology. This patient survived for 4 mo without treatment. It is unclear whether this particular form of metastasis affects clinical outcomes.
- Citation: Guo JW, Zhang XT, Chen XS, Zhang XC, Zheng GJ, Zhang BP, Cai YF. Leptomeningeal carcinomatosis as the initial manifestation of gastric adenocarcinoma: A case report. World J Gastroenterol 2014; 20(8): 2120-2126
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/2120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.2120
Gastric cancer-induced leptomeningeal carcinomatosis (LMC) is less common; its incidence is only 0.14%-0.24% among all gastric cancers[1-3]. In very rare cases, LMC presents as the initial manifestation of an asymptomatic gastric adenocarcinoma where metastasis to the peritoneum and liver generally occur first. Herein, we report a case of LMC as the initial manifestation of a Bormann type I gastric adenocarcinoma with poor differentiation in pathology. The clinical features of this case and appropriate diagnostic procedures are discussed.
A 40-year-old woman, who had been diagnosed with adenomyosis and had undergone hysterectomy thereafter in December 2008, presented with complaints of a 2-mo headache and cervical pain. She was suspected to have an upper respiratory tract infection or to have caught a cold on July 21, 2010. At this time, she took a non-steroidal anti-inflammatory drug, and her symptoms were not alleviated. Her headache became increasingly severe 12 d after the onset of disease. On August 3, 2010, she visited a doctor due to a serious sustained sharp headache combined with projectile vomiting. A cranial computed tomography (CT) scan was performed, which indicated cerebral edema with mild ventricular dilatation; these findings were followed by administration of 20% mannitol to reduce intracranial pressure. Two days later, on August 5, 2010, she suffered a sudden loss of consciousness with limb twitching and an antiepileptic drug was administered. Afterwards, she was referred to another hospital due to the progression of the disease. Magnetic resonance imaging (MRI) revealed increased hydrocephalus and slight parenchymal swelling. Because meningeal irritation was noted, a lumbar puncture was performed and revealed that her cerebral spinal fluid (CSF) had an opening pressure of over 300 mmH2O, glucose concentration of 4.13 mmol/L compared to a serum level of 5.5 mmol/L, protein content of 480 mg/L, chloride concentration of 110 mmol/L, and increased leukocyte levels (red blood cell count, 0 cells; white blood cell count, 8-10 × 106 cells/L; lymphocyte percentage, 74%). A repeat lumbar puncture revealed decreased glucose concentrations (CSF level of 2.79 mmol/L compared with serum level of 6.1 mmol/L), increased protein (790 mg/L), high intracranial pressure (over 300 mm H2O), increased white blood cell count (25 × 106 cells/L) with a lymphocyte percentage of 75% and 0 counts of red blood cells, and a normal concentration of chloride (101.20 mmol/L, normal range 120-130 mmol/L).
Cerebrospinal fluid culture was negative for acid-fast bacillus, Cryptococcus neoformans and bacterium. During her hospital stay, the onset of 3 epileptic seizures occurred, which were noticed by her husband or nurse. Although screening for tuberculosis infection was negative, she was suspected of having tuberculous meningitis and was started on tuberculostatic drugs, including rifampicin, isoniazid and pyrazinamide, as well as dehydration and diuretic drugs since August 7, 2010. Nevertheless, no improvement was observed after 7 d of treatment. Afterwards, on August 14, 2010, she was referred to the Brain Center of the Guangdong Province Hospital of Traditional Chinese Medicine.
On physical examination, the patient’s body temperature was 36.3 °C; her pulse was 60 beats/min, her respiratory rate 18 breaths/min and blood pressure 120/70 mmHg. Fundoscopic examination revealed bilateral papilledema (1PD each) and retinal edema was observed in the posterior pole.
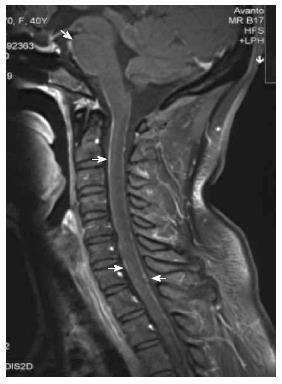
The patient’s neurological examination was notable for positive Kernig sign and Brudzinski’s sign. The initial blood chemistry tests and metabolic screening (e.g., electrolytes, kidney and liver function tests, blood glucose) were normal as were her routine stool and urination exams. The serum level of hepatitis B surface antigen was 287.1S/CO, and those of carcinoembryonic antigen (CEA), CA125, CA199, CA153 and alpha-fetoprotein in the serum were within normal limits. A repeat MRI scan was performed 1 week after admission, which indicated progressive linear and nodosity enhancement along the ventral surface of the brainstem, cerebellum and C1-T4 spinal cord (Figure 1). Expansion of the lateral ventricles, third ventricle and fourth ventricle was noted and communicating hydrocephalus was considered.
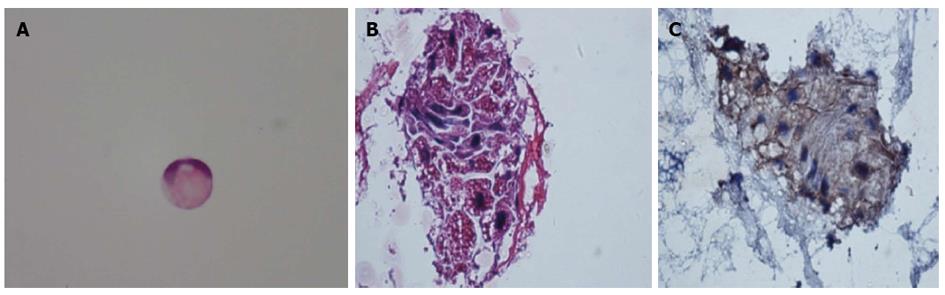
On August 12, 14, 17 and 18, 2010, additional lumber punctures were performed for a total of 4 taps during this period. The results of the CSF examination revealed an increase in CSF opening pressure (> 330 mmH2O for each tap), protein content (1470, 1140, 280 and 800 mg/L), WBC count (48, 7, 8, 7 × 106 cells/L) and CEA content (not determined on 12 August, 284.3, 351.9 and 347.0 ng/mL; serum level within normal limits), and a slight decrease in chloride concentration (108.4, 107.8, 109.5 and 108.9 mmol/L). Glucose levels were within or slightly lower than the lower limit of the normal range (3.06, 3.23, 2.14 and 3.13 mmol/L). CSF cytological analysis revealed malignant neoplastic cells (in particular, signet-ring cells, Figure 2) in the CSF specimen on the 14th and 17th of August, 2010. The diagnosis of LMC was made and the search for the primary tumor began. Immunohistochemical staining revealed that the CSF cells were positive for creatine kinase (CK) (Figure 2), which supports that the malignant neoplastic cells originated from the epithelium; this may be suggestive of the original tumor occurring in the esophagus and gastrointestinal tract, lungs or uterus. No expression of human epidermal receptor protein-2 (c-erbB-2; HER2) was detected via immunohistochemistry on de-stained CSF cytology slides.
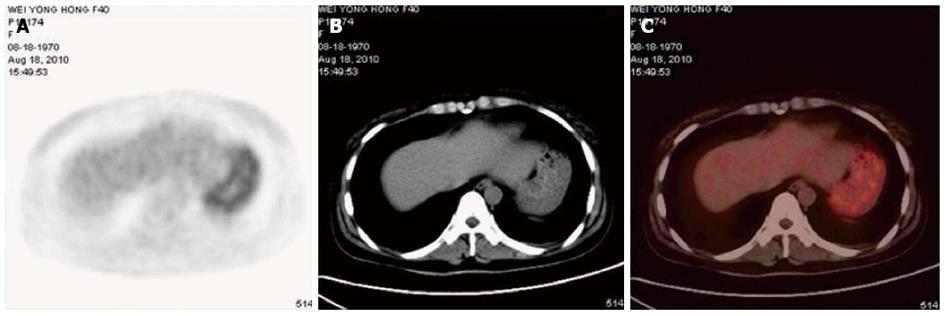
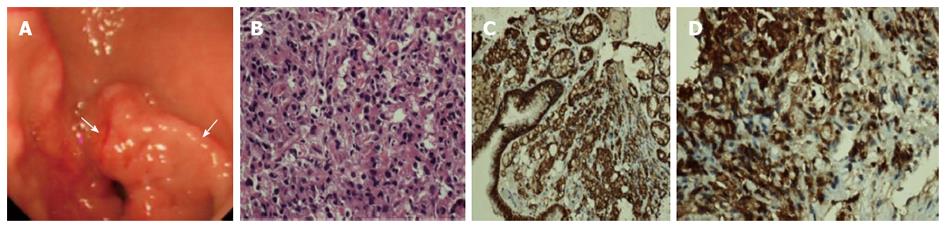
On August 18, 2010, the patient’s chest X-ray image was normal, and a whole-body position emission tomography (PET)/CT scan was conducted for further search for the original tumor. The PET/CT demonstrated that the stomach, which was not satisfactorily filled, had increased 18F-FDG uptake diffusely, with a maximum standardized uptake value (SUVmax) of 4.3 (mean 3.7) (Figure 3). This was subsequently explained by physiologic uptake. In addition to enlargement of the third and bilateral lateral ventricles and hydrocephalus, no abnormality was detected via PET/CT. Meanwhile, a gastroscopic examination was performed, revealing mass effect in the gastric antrum (Bormann class I) (Figure 4). Because the endoscopic ultrasound and enhanced CT did not reveal infiltration of the tumor, and there were no positive findings of metastasis to the lymph nodes on PET/CT, the patient was roughly considered TxN0M1 in clinical stage.
A subsequent histopathological examination of biopsies sampled during gastroscopy revealed poorly differentiated adenocarcinoma (Figure 4) with positive expression for CK and CEA (Figure 4), and partial positivity for CD68. An immunohistochemistry test was negative for HER2.
The patient was started on anti-tuberculostatic drugs, including isoniazid at a dose of 0.6 g per day, pyrazinamide at 1.5 g per day, and streptomycin at 0.5 g once, 2 times per day. The therapy was administered from August 7, 2010 until the day on which malignant neoplasm cells were found in the CSF. In deference to the patient’s will, no further anti-tumor treatment was administered. During the hospital stay, the patient’s condition worsened, and a neurological examination revealed nuchal rigidity and bilateral palsies of cranial nerves III, V, VI, VII, and XII. The patient was transferred to the hospital in her hometown, and after an unfortunate course of the disease, died on October 16, 2010, 4 mo after the initial presentation of her symptoms.
LMC is defined as diffuse spreading of malignant cells throughout the arachnoid membrane and the pia mater by propagation in the CSF. The majority of patients diagnosed with LMC have a prior cancer diagnosis. Meningeal involvement as a presenting symptom of malignancy is rare[4]. LMC is reportedly diagnosed clinically in 2%-4% of all cancer patients[5]; the prevalence of LMC in gastric cancer patients is as low as 0.14-0.24%[1-3], while the rate is 12%-14% in breast cancer, 10%-26% in lung cancer, and 17%-25% in melanoma[3].
To the best of our knowledge, the distinction and rarity of the present case are as follows: (1) this patient, initially and alone, presented with neurological symptoms and signs without any clues indicating gastric cancer, such as stomach ache, acid regurgitation, dyspepsia, anemia or occult fecal bleeding. This reminded us that the primary tumor may be occult without obvious symptoms or signs; this requires physicians to be prepared to consider alternative diagnostic decisions; (2) the gastroscopic examination identified the primary tumor as Bormann type I, oddly, with poor differentiation in pathology. Traditionally, Bormann type I gastric cancer has almost always been accompanied by high histopathological differentiation, while Bormann type III or IV gastric cancer is poorly differentiated in pathology[6,7]. Moreover, the macroscopic appearance of Bormann type I gastric cancer is less common than the other Bormann types; according to one large series of gastric cancer patients, the percentage of each Bormann classification was type I in 7% of patients, type II in 36% of patients, type III in 25% of patients and type IV in 26% of patients[8]; and (3) the biological behavior of this patient’s occult gastric adenocarcinoma is interesting. The tumor directly spread solely to the brain without involvement of any other organs or tissues such as the lungs, liver, bones or lymph nodes. It is unclear whether this particular form of metastasis affected the patient’s clinical outcome; she survived for 4 mo without any anti-tumor therapy. The striking clinical picture that this LMC patient presented raises interesting questions regarding the biology and metastatic behavior of certain subclasses of gastric cancer.
Previously, only 7 similar cases have been documented in 6 reports[9-14], the detailed information of whom, as well as our present case, is summarized in Table 1. By reviewing the literature on these 7 cases and the disease progression of our case, we identified multiple relevant diagnostic issues that may be of clinical use to make prompt and accurate diagnoses when similar cases are encountered.
| Ref. | Case (age/sex) | Gastroscopic classification | Histopathological stage | Involvement of other organs/tissues | Treatment | Survival from onset of initial symptoms |
| Present case | 40/F | Bormann I | AdenocarcinomaPoor differentiationSignet cell type | No | No | 4 mo |
| Deeb et al[12], 1997 | 53/M | Not classified2 | No | Intraventricular MTX | > 6 mo | |
| Braeuninger et al[9], 2005 | 68/M | Not classified2 | Lymph nodes | Intrathecal chemotherapy | 2 mo | |
| Lee et al[10], 2007 | 49/F | Bormann IV | Lymphadenopathy | No | ND | |
| Yamada et al[13], 2008 | 53/M | Bormann II | ND | Radiotherapy | 4.23 mo (127 d) | |
| Gdovinova et al[11], 2009; Case 1 | 40/F | ND | Lymphatic node, ovary, peritoneum, leptomeninges | No | 2 mo | |
| Gdovinova et al[11], 2009; Case 2 | 49/F | Not classified2 | ND | Yes1 | 2 mo | |
| Ohno et al[14], 2010 | 62/M | Not classified2 | ND | Radiotherapy | 3 mo (12 wk) |
The clinical symptoms and signs of LMC are usually organized into 3 categories: cerebral, cranial nerve and spinal. Out of the 8 cases considered, 7, including our case, initially presented with headache, nausea and vomiting (cerebral symptoms and signs), 1 with back pain and weakness of the lower extremities (spinal symptoms and signs), 3 with diplopia, 1 with visual loss and 1 with hearing loss (cranial nerve symptoms and signs). Multifocal involvement of the meninges in LMC accounts for a great amount of variability in clinical presentations, thus making early diagnosis extremely challenging. Therefore, complicated and non-specified clinical features are those of which clinicians remain acutely aware to further track primary diseases.
As to the diagnosis of LMC, the presence of neoplastic cells in the CSF is diagnostic. A diagnosis of LMC may be supported by neuroimaging evidence, especially the results of gadolinium-enhanced MRI. Because cytological examination of the CSF is the sine qua non of diagnosis, CSF sampling procedures and analysis warrant close attention. Repeated CSF cytological examination is sometimes necessary. The presence of malignant cells was confirmed after the 2nd CSF examination in 2 cases reported in the literature[11,12], while our case required 4 taps to confirm malignant cells. As previously reported, CSF cytological results are positive in 50% of all cases of meningeal metastasis from non-central nervous system neoplasms after a single lumbar tap and in 85% to 90% of cases after multiple taps[15]. Three simple measures can increase the likelihood of finding malignant cells in a CSF sample: (1) obtaining at least 10.5 mL of CSF for analysis[16]; (2) immediately processing the sample (within 1 h after collection[17]); and (3) obtaining CSF from a site adjacent to the affected central nervous system region[16].
Apart from cytological examination of the CSF, other CSF changes were observed in the 8 LMC cases described, such as increased opening pressure, increased protein content and white blood cell count and a glucose concentration within normal limits or slightly decreased (except that in 1 case with hypoglycorrhachia, glucose concentration was 0.6 mmol/L[11]; and our case with a glucose concentration of 2.79 mmol/L on the 1st CSF examination), which are supported throughout the literature[16]. Other notable CSF changes were described in these LMC cases, including increased lactate or CEA concentrations. Concerning the CSF markers of interest, their concentrations in CSF should be tested simultaneously with those in the serum to eliminate the possibility of passive diffusion[17]. As shown in our case, CSF CEA was within the range between 284.3 and 347.0 ng/mL, while that in the serum was within normal limits. Thus, when the CSF marker level is inappropriately high and the blood brain barrier is intact, these markers may be produced in the subarachnoid space by the tumor metastases.
With respect to clinical imaging techniques, as demonstrated in nearly all 8 LMC patients considered, brain CT and MRI without contrast yielded false negative results. However, 3 of the 8 patients obtained positive findings on gadolinium-enhanced MRI, such as nerve thickening and linear and punctiform enhancement of the leptomeninges. Leptomeningeal enhancement is suggestive of LMC, but not diagnostic. Therefore, one must consider other conditions that may produce similar imaging features, such as intracranial hypotension after craniotomy or lumber puncture, as well as infectious or inflammatory diseases. Although MRI is superior to CT with 1.5-2 times higher specificity and sensitivity[18,19], the rate of false negative MRI findings is approximately 30%; this could be further diminished by the use of greater amounts of gadolinium[20,21]. Additionally, it is important to perform MRI before CSF examination because spinal tap alone may induce long-lasting (weeks to months) diffuse meningeal enhancement[18]. When the primary cancer is unknown and CSF cytology is negative, MRI alone is not sufficient to establish an LMC diagnosis; histological confirmation is required before such a diagnosis can be made.
In the present case, whole-body PET/CT was performed to track the primary disease, which revealed increased glucose uptake in the stomach, with a SUVmax of 4.3. However, this abnormality was initially explained by physiological uptake, and close follow-up was suggested by the patient’s radiologist. As indicated by Dassen AE[22], FDG-PET appears to have little or no value in the primary detection of gastric cancer. They suggested that there is a clear difference in the sensitivity of FDG-PET between different histological carcinoma subtypes, particularly in the non-intestinal (i.e., diffuse) subtype. Carcinomas containing signet ring cells exhibit consistently low detectability by FDG-PET. Prior evidence indicates[23-28] that SUV counts between 7.7-13.2 were found in tubular gastric carcinoma and moderately differentiated carcinoma, which are significantly higher than those for mucinous adenocarcinoma and signet ring cell carcinoma (4.1-7.7).
Regarding the histopathological examination of primary gastric cancer, poorly differentiated adenocarcinoma was finally confirmed in all of the 8 LMC cases considered. This is consistent with previous reports indicating that poorly differentiated adenocarcinoma with signet ring cell features is the most frequently occurring type of LMC associated with GC in Japan[29]; this is likely to be true in Korea[7].
By consensus, the prognosis of LMC is poor. The median survival in untreated patients is 4-6 wk, which may increase to 4-6 mo with aggressive treatment in some cases[30,31] Out of the 8 LMC cases described, 3[9-11] had metastasis to distant organs other than the meninges was found, such as in the lymph nodes, ovaries, and peritoneum. Five of these patients received anti-tumor therapy and their survival ranged from 2-6 mo[9,11-14]. This indicates that treatment was not associated with a clinically significant different prognosis than non-treatment (2 mo in 1 case in literature[11], and 4 mo in our case). Various relevant and concerning questions arise including those surrounding the clinical biological behavior of rare and odd adenocarcinomas, as in the present case with gastroscopic Bormann type I and poor differentiation, and whether this factor partially influences metastasis and patient survival.
In summary, to the best of our knowledge, this case of LMC originating from an occult gastric adenocarcinoma is the first ever reported with Bormann type I macroscopic appearance, but with poor differentiation in pathology. Its direct invasion to the meninges resulting in LMC as the initial presentation is very interesting, especially considering that there was no evidence of metastasis to other organs or tissues. It is unclear whether this particular form of metastasis affects clinical outcomes; our patient survived for 4 mo without treatment. Early diagnosis requires highly aware and vigilant physicians. CSF cytological studies play a key role in diagnosis and neuroimaging examinations are helpful; however, they present certain limitations.
The patient presented with a sustained headache, cervical pain, projectile vomiting, and a sudden loss of consciousness with limb twitching and epileptic seizures.
The main clinical findings include positive Kernig’s sign and Brudzinski’s sign in neurological examination.
In this case, the differential diagnosis is tuberculous meningitis.
The cerebral spinal fluid (CSF) examination revealed an increase in CSF opening pressure, protein content, WBC count and carcinoembryonic antigen (CEA) content, and CSF cytological analysis revealed malignant neoplastic cells.
Magnetic resonance imaging indicated progressive linear and nodosity enhancement along the ventral surface of the brainstem, cerebellum and C1-T4 spinal cord and position emission tomography/computed tomography demonstrated that the stomach had increased 18F-FDG uptake diffusely, with a maximum standardized uptake value of 4.3 (mean 3.7).
A histopathological examination of biopsies sampled during gastroscopy revealed poorly differentiated adenocarcinoma with positive expression for creatine kinase (CK), CEA and partial positivity for CD68, while the CSF cells was positive for CK.
Leptomeningeal carcinomatosis, also known as neoplastic meningitis, occurs when malignant cells enter the leptomeningeal space via hematogenous dissemination or direct extension. The malignant cells are spread throughout the neuraxis by the flow of the CSF, leading to disease throughout the central nervous system. Leptomeningeal carcinomatosis leads to substantial morbidity and mortality, and there are few, if any, effective treatments.
The gastric cancer can metastasize to meninges alone without involvement of any other organs or tissues, and thus the patients may present with neurological abnormalities alone without any clue to the stomach. Early diagnosis requires highly aware and vigilant physicians. For diagnosis, CSF cytological studies play a key role and neuroimaging examinations are helpful; however, they present certain limitations.
The strength of this report is that this case of leptomeningeal carcinomatosis (LMC) originating from an occult gastric adenocarcinoma is the first ever reported with Bormann type I in macroscopic appearance, but with poor differentiation in pathology. Its direct invasion to the meninges resulting in LMC as the initial presentation is very interesting, especially considering that there was no evidence of metastasis to other organs or tissues. It is unclear whether this particular form of metastasis affects clinical outcomes.
P- Reviewers: Giovanni LC, Rajeshwari K, Zarogiannis S S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Giglio P, Weinberg JS, Forman AD, Wolff R, Groves MD. Neoplastic meningitis in patients with adenocarcinoma of the gastrointestinal tract. Cancer. 2005;103:2355-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Lee JL, Kang YK, Kim TW, Chang HM, Lee GW, Ryu MH, Kim E, Oh SJ, Lee JH, Kim SB. Leptomeningeal carcinomatosis in gastric cancer. J Neurooncol. 2004;66:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Oh SY, Lee SJ, Lee J, Lee S, Kim SH, Kwon HC, Lee GW, Kang JH, Hwang IG, Jang JS. Gastric leptomeningeal carcinomatosis: multi-center retrospective analysis of 54 cases. World J Gastroenterol. 2009;15:5086-5090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Olson ME, Chernik NL, Posner JB. Infiltration of the leptomeninges by systemic cancer. A clinical and pathologic study. Arch Neurol. 1974;30:122-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 377] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Pentheroudakis G, Pavlidis N. Management of leptomeningeal malignancy. Expert Opin Pharmacother. 2005;6:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Takahashi Y, Ellis LM, Ohta T, Mai M. Angiogenesis in poorly differentiated medullary carcinoma of the stomach. Surg Today. 1998;28:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kim M. Intracranial involvement by metastatic advanced gastric carcinoma. J Neurooncol. 1999;43:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ming SC. Classification of gastric carcinoma. Gastric Carcinoma. 2nd ed. Edinburgh: Churchill Livingstone 1986; 197. |
| 9. | Braeuninger S, Mawrin C, Malfertheiner P, Schildhaus HU, Seiler C, Dietzmann K, Lins H. Gastric adenocarcinoma with leptomeningeal carcinomatosis as the presenting manifestation: an autopsy case report. Eur J Gastroenterol Hepatol. 2005;17:577-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Lee HG, Lee B, Kim SM, Suh BJ, Yu HJ. A case of gastric adenocarcinoma presenting as meningeal carcinomatosis. Korean J Intern Med. 2007;22:304-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Gdovinova Z, Feketeova E, Szilasiova J, Havlikova E, Banik P. Meningeal carcinomatosis as the first manifestation of malignant carcinomatosis. Bratisl Lek Listy. 2009;110:490-495. [PubMed] |
| 12. | Deeb LS, Yamout BI, Shamseddine AI, Shabb NS, Uthman SM. Meningeal carcinomatosis as the presenting manifestation of gastric adenocarcinoma. Am J Gastroenterol. 1997;92:329-331. [PubMed] |
| 13. | Yamada T, Furukawa K, Yokoi K, Ohaki Y, Okada S, Tajiri T. Case of meningeal carcinomatosis with gastric cancer which manifested meningeal signs as the initial symptom; the palliative benefit of radiotherapy. J Nippon Med Sch. 2008;75:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Ohno T, Yokoyama Y, Aihara R, Mochiki E, Asao T, Kuwano H. Sudden bilateral sensorineural hearing loss as the presenting symptom of meningeal carcinomatosis of gastric cancer: report of a case. Surg Today. 2010;40:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Groves MD. Leptomeningeal disease. Neurosurg Clin N Am. 2011;22:67-78, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Martins SJ, Azevedo CR, Chinen LT, Cruz MR, Peterlevitz MA, Gimenes DL. Meningeal carcinomatosis in solid tumors. Arq Neuropsiquiatr. 2011;69:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Mason WP, Yeh SD, DeAngelis LM. 111Indium-diethylenetriamine pentaacetic acid cerebrospinal fluid flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology. 1998;50:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Chamberlain MC. Comparative spine imaging in leptomeningeal metastases. J Neurooncol. 1995;23:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Yousem DM, Patrone PM, Grossman RI. Leptomeningeal metastases: MR evaluation. J Comput Assist Tomogr. 1990;14:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Chamberlain MC, Sandy AD, Press GA. Leptomeningeal metastasis: a comparison of gadolinium-enhanced MR and contrast-enhanced CT of the brain. Neurology. 1990;40:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 165] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JF, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol. 2009;35:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, Lee JH, Ryu KW, Kim YW, Bae JM. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, Cho A, Lee JD. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582-1588. [PubMed] |
| 25. | Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Yeung HW, Macapinlac H, Karpeh M, Finn RD, Larson SM. Accuracy of FDG-PET in Gastric Cancer. Preliminary Experience. Clin Positron Imaging. 1998;1:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, Ido T, Yamaura G, Takahashi H, Fukuda H, Kanamaru R. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med. 2003;44:690-699. [PubMed] |
| 29. | Lisenko Y, Kumar AJ, Yao J, Ajani J, Ho L. Leptomeningeal carcinomatosis originating from gastric cancer: report of eight cases and review of the literature. Am J Clin Oncol. 2003;26:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Kim L, Glantz MJ. Neoplastic meningitis. Curr Treat Options Oncol. 2001;2:517-527. [PubMed] |
| 31. | Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 182] [Article Influence: 6.3] [Reference Citation Analysis (0)] |