Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.2051
Revised: November 6, 2013
Accepted: November 12, 2013
Published online: February 28, 2014
Processing time: 225 Days and 14.6 Hours
AIM: To characterize longitudinally the inflammation and the gut microbiota dynamics in a mouse model of dextran sulfate sodium (DSS)-induced colitis.
METHODS: In animal models, the most common method used to trigger colitis is based on the oral administration of the sulfated polysaccharides DSS. The murine DSS colitis model has been widely adopted to induce severe acute, chronic or semi-chronic colitis, and has been validated as an important model for the translation of mice data to human inflammatory bowel disease (IBD). However, it is now clear that models characterized by mild intestinal damage are more accurate for studying the effects of therapeutic agents. For this reason, we have developed a murine model of mild colitis to study longitudinally the inflammation and microbiota dynamics during the intestinal repair processes, and to obtain data suitable to support the recovery of gut microbiota-host homeostasis.
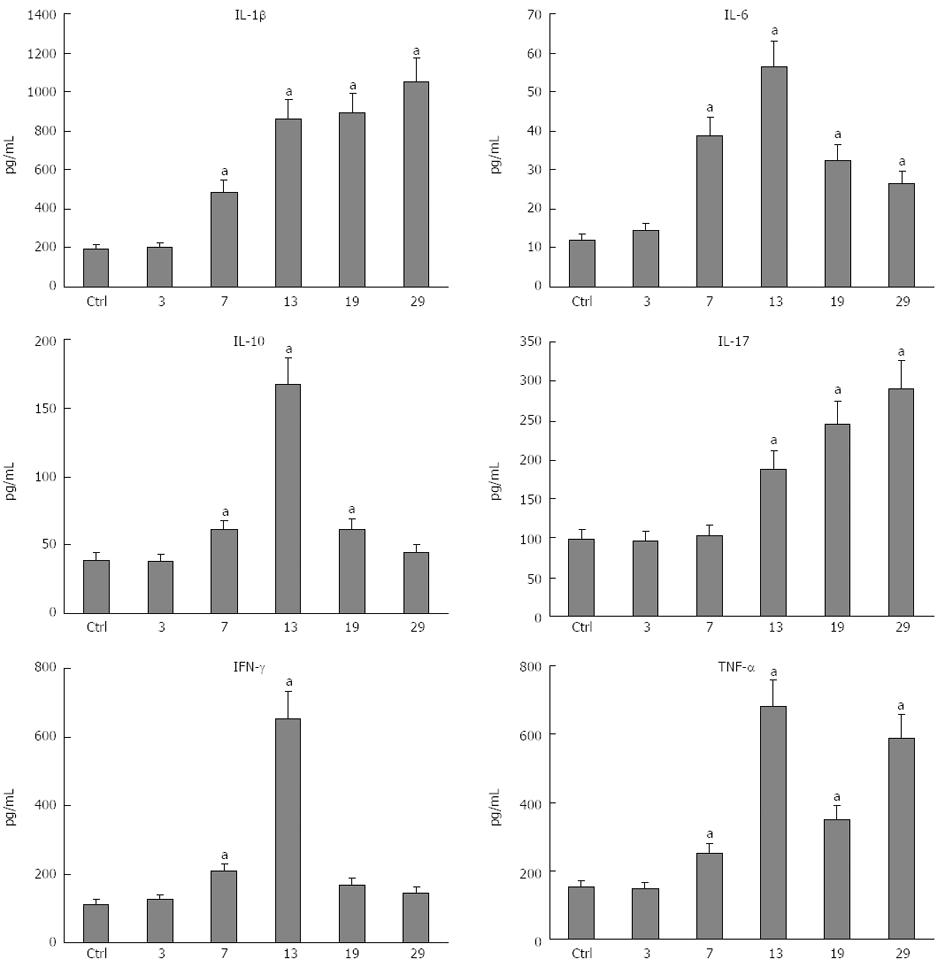
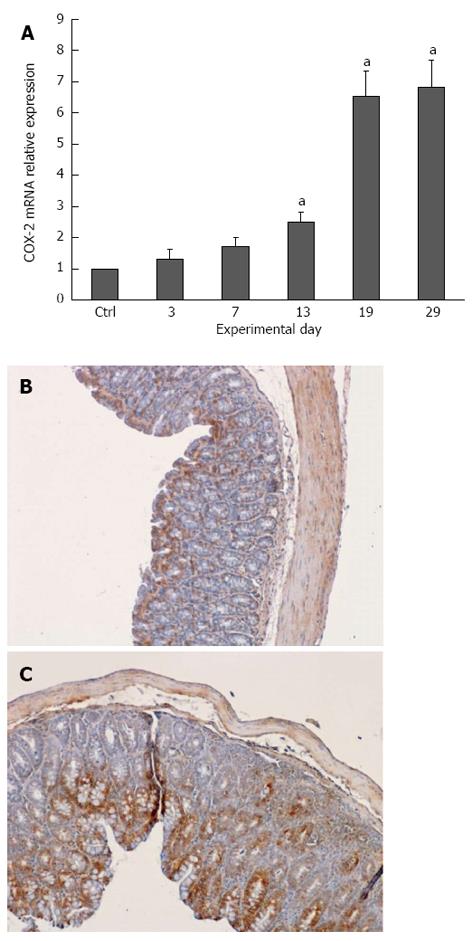
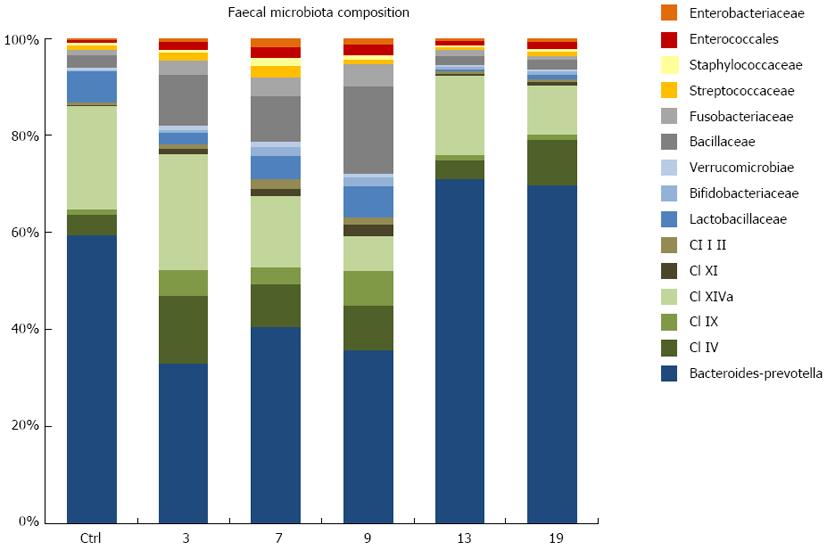
RESULTS: All plasma cytokines evaluated, except IL-17, began to increase (P < 0.05), after 7 d of DSS administration. IL-17 only began to increase 4 d after DSS withdrawal. IL-1β and IL-17 continue to increase during the recovery phase, even when clinical signs of colitis had disappeared. IL-6, IL-10 and IFN-γ reached their maxima 4 d after DSS withdrawal and decreased during the late recovery phase. TNFα reached a peak (a three- fold increase, P < 0.05), after which it slightly decreased, only to increase again close to the end of the recovery phase. DSS administration induced profound and rapid changes in the mice gut microbiota. After 3 d of DSS administration, we observed a major reduction in Bacteroidetes/Prevotella and a corresponding increase in Bacillaceae, with respect to control mice. In particular, Bacteroidetes/Prevotella decreased from a relative abundance of 59.42%-33.05%, while Bacillaceae showed a concomitant increase from 2.77% to 10.52%. Gut microbiota rapidly shifted toward a healthy profile during the recovery phase and returned normal 4 d after DSS withdrawal. Cyclooxygenase 2 expression started to increase 4 d after DSS withdrawal (P < 0.05), when dysbiosis had recovered, and continued to increase during the recovery phase. Taken together, these data indicated that a chronic phase of intestinal inflammation, characterized by the absence of dysbiosis, could be obtained in mice using a single DSS cycle.
CONCLUSION: Dysbiosis contributes to the local and systemic inflammation that occurs in the DSS model of colitis; however, chronic bowel inflammation is maintained even after recovery from dysbiosis.
Core tip: Experimental animal models of colitis are important for investigating the physiopathological mechanisms underlying inflammatory bowel disease (IBD) in humans. Murine dextran sulfate sodium colitis models have been widely adopted and validated as relevant models for the translation of mice data to human IBD. Nevertheless, it is clear that models characterized by mild intestinal damages are more accurate for studying the effects of therapeutic agents. In this study, we developed a reproducible mild chronic colitis model, which allows the evaluation of the intestinal repair processes, the modulation of systemic inflammation and the recovery of the gut microbiotic homeostasis.
- Citation: Fazio LD, Cavazza E, Spisni E, Strillacci A, Centanni M, Candela M, Praticò C, Campieri M, Ricci C, Valerii MC. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J Gastroenterol 2014; 20(8): 2051-2061
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/2051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.2051
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), is more common in the populations of developed countries. Assessment of the efficacy of novel and adjunct IBD therapies requires experimental animal models resembling human IBD. There is no ideal animal model for IBD, and myriad methods have been designed to induce colitis in mice, rats and other animals. Dextran sulfate sodium (DSS)-induced colitis is one of the most commonly used models. DSS-colitis reflects many of the clinical features of UC[1-3]. For example, changing the DSS concentration or administration cycles can easily induce acute, chronic or relapsing colitis. Moreover, the dysplasia that frequently occurs after the chronic phase of DSS colitis resembles the clinical course of human UC[4]. Recent reports have focused on the multifunctional role of DSS for in vivo colitis modeling[5]. In the most widely used DSS murine model, animals are treated with 3% DSS in their drinking water for seven days. This provides a model of acute intestinal injuries that permits clinical monitoring of colitis using parameters such as weight loss, stool consistency and blood in the stool. Together these parameters yield an average clinical score that is a powerful comparable number for identifying potential differences among groups over the total duration of the experiment. However, the devastating intestinal injuries caused by 3% DSS remain for up to ten days after DSS administration. Therefore, they do not provide a sensitive system to evaluate the role of therapeutic agents with different efficacies[5], or the role of the various parameters involved in intestinal repair. By contrast, the milder seven-day 1% DSS treatment model seems to be a powerful means of evaluating the effect of most therapeutic agents and the repair phase of colitis. However, the 1% DSS model would not be characterized as a disease according to traditional disease activity indices and hence prevents clinical monitoring[5]. Histopathology, with quantification of morphological and immunological changes in the colon during and after 1% DSS treatment, is necessary to identify differences among groups.
The molecular events taking place after DSS ingestion and those leading to established colitis are not completely understood, but are of primary importance to understand the strengths and weaknesses of this model. DSS is a sulfated polysaccharide with a variable molecular weight (MW) ranging from 5 to 1400 kDa. DSS is rapidly depolymerized in the stomach, reaching the cecum with a MW between 750 and 5000 Da, and it is reasonable to assume that these smaller sulfated polysaccharides are responsible for the observed colon damage[6]. Hence, the MW of DSS is considered a major factor in the induction of colitis. The most severe colitis was obtained in BALB/c mice using DSS with a MW of 40 kDa; higher or lower MWs resulted in milder forms of colitis[7]. For this reason, some companies have developed DSS specifically designed for the induction of colitis, and these specific products are strongly recommended to obtain much more repeatable results. DSS metabolism in the gut also involves the formation of complexes between DSS fragments and medium-chain fatty acids (MCFA), which are enriched in the large bowel[8]. The high toxicity of DSS-MCFA complexes explains why only the large bowel, and especially the terminal colon, is inflamed by the DSS moieties. Once it enters into colonocytes, DSS impairs major cellular functions by inhibiting the activity of cellular enzymes, such as ribonuclease[8] and iNOS[9], and ultimately causes cell cycle arrest and apoptosis in colonocytes[6], and probably in other colonic wall cells. By interfering with the intestinal barrier function, DSS is also able to stimulate local and systemic inflammation by locally increasing the expression of cyclooxygenase-2 (COX-2) and by inducing the secretion of a variety of cytokines and other inflammatory mediators that spread from the colon to the blood[10].
The importance of the microbiota and microbe-mucosa crosstalk in the pathogenesis of IBD is supported by several animal model studies. Colitis severity is dependent on the commensal bacterial strains maintained in gnotobiotic animals[11], and DSS treatment has been associated with a major shift in the composition of the intestinal microbiota, whose dynamics rapidly shift toward an unhealthy state[12-14]. Moreover, antibiotic administration has been shown to improve both IBD and DSS-induced colitis[15], indicating that the microbiota play a critical role in this disease, as well as in the DSS model system. This view is supported by evidence showing that the simple ingestion of a lysate of microbial cells belonging to the Firmicutes, considered a healthy-type phylum, reduced DSS-induced experimental colitis in mice[14].
The present study aimed to characterize longitudinally the inflammation and the gut microbiota dynamics in a highly sensitive DSS-induced murine model of colitis.
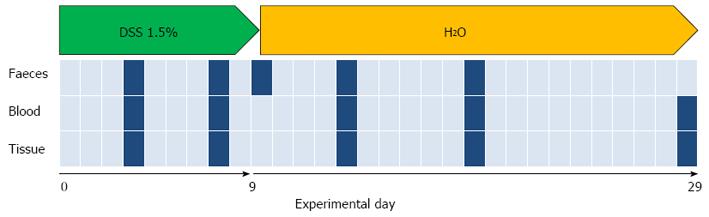
Twenty-four male 8-week-old C57BL/6 mice were purchased from Charles River Laboratories (Lecco, Italy). The animals were housed in a controlled environment in collective cages at 22 ± 2 °C and 50% humidity, under a 12-h light/dark cycle. Mice were allowed to acclimate to these conditions for at least 14 d before inclusion in the experiments and had free access to food and water throughout the study. Colitis was induced in 12 mice by oral administration of dextran sulfate sodium (DSS for colitis, TdB Consultancy, Sweden, MW 40000). DSS was added at a concentration of 1.5% in tap water. DSS-tap water was freshly prepared every day and administered to the mice for 9 d (day 0-9), followed by 21 d of tap water (day 10-29). The average amount of DSS taken was recorded daily. The control group (n = 12) received only tap water. A schema of the experimental design is shown in Figure 1. The experiment, which was approved by the institutional review board of the University of Bologna and performed according to Italian and European guidelines, was repeated three times.
The disease activity index (DAI) was calculated by the combined score of weight loss, stool consistency and bleeding, as detailed in Table 1. All parameters were scored from day 1 to day 29.
| Stool consistency | Bleeding | Weight loss |
| 0 = Formed | 0 = Normal color stool | 0 = No weight loss |
| 1 = Mild-soft | 1 = Brown color | 1 = 5%-10% weight loss |
| 2 = Very soft | 2 = Reddish color | 2 = 11%-15% weight loss |
| 3 = Watery stool | 3 = Bloody stool | 3 = 16%-20% weight loss |
| 4 ≥ 20% weight loss |
Mice (n = 2) were anesthetized and sacrificed by cervical dislocation on day 3 (after 3 d of DSS intake), 7 (after 7 d of DSS intake), 13 and 19. The colon was excised, rinsed with saline solution, fixed in 4% formalin and embedded in paraffin. Of 4 µm sections were obtained, stained with hematoxylin-eosin and observed for a histological assessment of epithelial damage by a pathologist who was blinded to the samples’ origins.
Blood samples (200 μL) were taken from the tail vein on days 3, 7, 13, 19, and 29. Blood, collected in Eppendorf tubes containing sodium citrate, was centrifuged at 1000 RPM for 10 minutes, and the plasma was collected and stored at -80 °C until BioPlex analysis. Cytokine levels were determined using a multiplexed mouse bead immunoassay kit (Bio-Rad, CA, United States). The six-plexed assays (IL-1α, IL-6, IL-10, IL-17A, IFN-γ and TNFα) were performed in 96-well filter plates, as previously described[16], following the manufacturer’s instructions. Microsphere magnetic beads coated with monoclonal antibodies against the different target analytes were added to the wells. After incubation for 30 min, the wells were washed and biotinylated secondary antibodies were added. After incubation for 30 min, the beads were washed and incubated for 10 min with streptavidin-PE conjugated to the fluorescent protein, phycoerythrin (streptavidin/phycoerythrin). After washing, the beads (a minimum of 100/analyte) were analyzed in the BioPlex 200 instrument (BioRad). The concentrations of the samples were estimated from a standard curve using a fifth-order polynomial equation and expressed as pg/ml after adjusting for the dilution factor (Bio-Plex Manager software 5.0). Samples below the detection limit of the assay were recorded as zero. The intra-assay coefficient of variance averaged 15%.
Total RNA from colon samples was extracted using the Trizol® reagent (Life Technologies, CA, United States), according to the manufacturer’s instructions. Extracted RNA samples were treated with DNase I to remove any genomic DNA contamination using DNA-free kit (Ambion, United States) and reverse-transcribed using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Canada). COX-2 and β-actin mRNAs were reverse-transcribed using random hexamer primers (Fermentas, Canada). Real-time polymerase chain reaction (PCR) was used to analyze COX-2 and β-actin mRNA levels using the SYBR® Select Master Mix (Life Technologies, CA, United States) and StepOnePlusTM system (Applied Biosystems, CA, United States), according to the manufacturer’s instructions. The melting curve data were collected to check PCR specificity. Each cDNA sample was analyzed as triplicate. COX-2 mRNA levels were normalized against β-actin mRNA and relative expressions were calculated using the 2-2ΔCt formula. The COX-2 primer pair was: 5’- TTC TCT ACA ACA ACT CCA TCC TC -3’ and 5’- GCA GCC ATT TCC TTC TCT CC -3’ (247 bp product); the β-actin primer pair was: 5’- ACC AAC TGG GAC GAC ATG GAG -3’ and 5’- GTG GTGGTG AAG CTG TAG CC -3’ (380 bp product).
Tissue sections (4 µm) were mounted on slides, sections were deparaffinized with xylene and rehydrated through a series of graded alcohols, and then incubated overnight at 4 °C with anti-COX-2 antibody (Cayman Chemicals, Ann Arbor, MC, United States) at a 1:200 dilution in PBS/BSA-1.5%. The primary antibody was omitted from control slides. Sections were then incubated with secondary anti-rabbit antibody for 15 min at room temperature and then reacted with 3,3-diaminobenzidine tetrahydrochloride for 1 min. Sections were then counterstained with hematoxylin.
The intestinal mice microbiota were characterized using the fully validated diphylogenetic DNA microarray platform, HTF-Microbi.Array[17]. Targeting 33 phylogenetically related groups, this ligation detection reaction (LDR)-based Universal Array covers up to 95% of the mammalian gut microbiota[18]. Gut microbiota analysis was performed at day 3, 7, 9, 13 and 19. The QIAamp DNA Stool Mini Kit (Qiagen) was used to extract total DNA from fecal material, according to the modified protocol reported previously[17]. The final DNA concentration was determined using a NanoDrop ND-1000 instrument (NanoDrop Technologies). A nearly full-length portion of the 16S rDNA gene was amplified using universal forward primer 27F and reverse primer 1492R, according to a protocol described previously[17]. PCR amplifications were performed in a Biometra Thermal Cycler T Gradient (Biometra, Göttingen, Germany). The PCR products were purified using the High Pure PCR CleanupMicrokit (Roche, Mannheim, Germany), eluted in 30 µL of sterile water and quantified using a NanoDrop ND-1000. Slide chemical treatment, array production, the LDR protocol and hybridization conditions were performed as reported previously[19,20]. Briefly, LDR reactions were carried out in a final volume of 20 µL containing 500 fmol of each LDR-UA HTF-Microbi.Array probe[18], 50 fmol of PCR product and 25 fmol of the synthetic template (5’-AGCCGCGAACACCACGATCGACCGGCGCGCGCAGCTGCAGCTTGCTCATG-3’). The LDR products were hybridized on Universal Arrays, setting the probe annealing temperature at 60 °C. All arrays were scanned and processed according to the protocol and parameters described previously[17]. Fluorescence intensities were normalized on the basis of the synthetic ligation control signal[18]. The relative abundance of each bacterial group was obtained by calculating the relative fluorescence contribution of the corresponding HTF-Microbi.Array probe as a percentage of the total fluorescence.
Al data were expressed as the mean ± SEM of at least three independent determinations. Statistical differences between groups were determined by one-way ANOVA by using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, United States). Differences were considered statistically significant at P < 0.05.
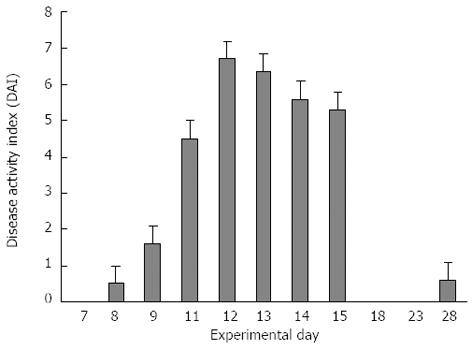
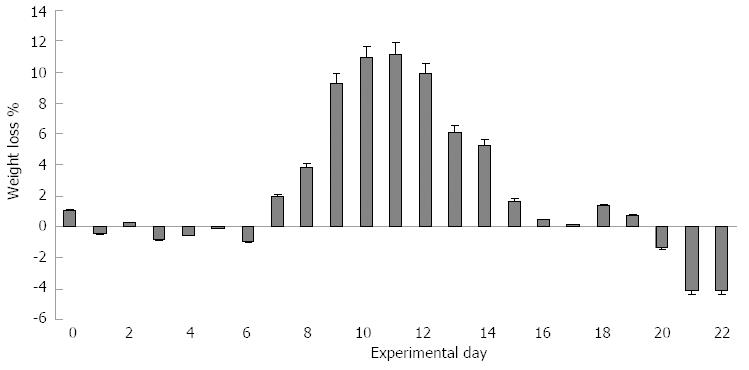
Mice started to show mild clinical signs of disease after the end of the 1.5% DSS treatment (day 9), as indicated by the simultaneous increase in stool consistency and bleeding index (maximum DAI score = 2). The most evident clinical signs were recorded between days 11 and 15 (Figure 2), with a significant weight loss that peaked between days 9 and 12 (Figure 3) with a maximum DAI score = 7.
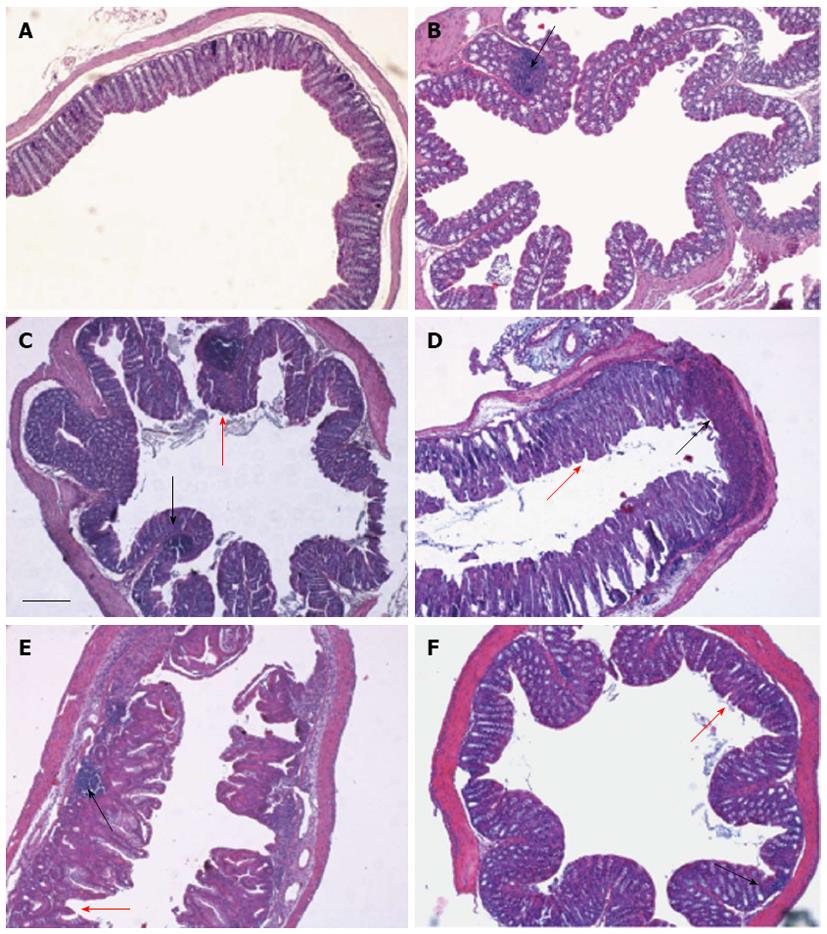
Histological evaluation of the colon was made from the colocecal junction to the anus. Overall, the tissue damage tended to be limited to the terminal colon and rectum regions, and could be classified as mild to moderate colitis (Figure 4). After 3 d of DDS treatment (Figure 4B), the colonic mucosa appeared normal, except for focal inflamed areas in which we observed leukocyte infiltration, with a prevalence of granulocytes, indicating active inflammatory colitis, and solid lymphatic follicles. After 7 d of DDS treatment (Figure 4C), the terminal colonic mucosa showed the same features of spotted focal leukocyte infiltrations, with a prevalence of lymphocytes, showing the colitis had shifted toward a chronic status, with mucus discharge, architectural abnormalities and depletion of goblet cells. At day 13 (Figure 4D), the lymphocyte infiltration was widespread, and epithelial damage was evident, with complete crypt disappearance, mucosal erosion in some areas and a mild thickening of the muscularis mucosa. During weight recovery, at day 19 (Figure 4E), leukocyte infiltration tended to return to being focalized. Mucosal erosions were still evident, with flattened villi. At day 29 (Figure 4F), significant focal infiltration, also involving the glands, was still present, with villi that still appeared flattened.
All cytokines evaluated, except IL-17, started to increase after 7 d of DSS administration (Figure 5). The IL-17 plasma level started to increase at day 13. IL-1β (Figure 5A) had a sudden four-fold increase at day 13, when colitis reached its maximum DAI score, and slightly increased until day 29, when clinical signs of colitis had disappeared, reachingβ its peak plasma level. IL-6 (Figure 5B) started to increase at day 7 and reached a peak at day 13 (a 4.5 fold increase), after which it decreased to reach a value twice the normal level by the end of the experiment (day 29). IL-10 (Figure 5C) reached a peak at day 13 (a 4.2 fold increase), after which it decreased to resume a physiological level at the end of the experiment (day 29). IL-17 (Figure 5D) started to increase at day 13 and continuously increased to a peak at day 29 (a three-fold increase), which was the end of the experiment. IFN-γ (Figure 5E) increased at day 7, reaching a peak at day 13 (a six-fold increase) and then rapidly decreased to resume a physiological level at the end of the experiment (day 29). Finally, TNFα (Figure 5F) increased at day 7, reaching a peak at day 13 (a three-fold increase), after which it slightly decreased at day 19 to increase again to the peak level at the end of the experiment (day 29). At the end of the recovery (day 29), IL-1β, IL-17 and TNFα were at their maximum levels, indicating that colitis had become chronic.
COX-2 plays a crucial role in the production of many lipid mediators involved in intestinal inflammation and is one of the targets of IBD pharmacological therapy; therefore, we analyzed COX-2 mRNA expression in colon tissue during DSS-induced colitis. Interestingly, COX-2 mRNA started to increase (by two-fold) only 5 d after DSS removal and continue to increase (up to seven-fold) until the end of the recovery phase, a trend that mirrored the chronicization of the inflammatory process (Figure 6).
To characterize the reaction of the gut microbiota in our DSS murine model of mild colitis, the temporal dynamics of the fecal microbiota of DSS-treated mice was compared with that of healthy controls. In particular, for DSS-treated mice, the gut microbiota was characterized in control mice and at day 3, 7, 9, 13, 19 (Figure 7). The fecal microbiota of healthy control mice was sampled at the same time points. According to our data, DSS administration prompted profound and rapid changes in the mice microbiota (Figure 7). After 3 d of DSS treatment, we observed a major reduction of Bacteroidetes/Prevotella and a corresponding increase in Bacillaceae, with respect to control mice. In particular, Bacteroidetes/Prevotella decreased from a relative abundance (rel.ab.) of 59.42% to 33.05%, while Bacillaceae showed a concomitant increase from 2.77% to 10.52%. During the course of colitis, from day 7 to day 9, there was a progressive increase in the rel.ab. of Bacillaceae (from 10.52% to 17.90%), Lactobacillaceae (from 2.07% to 6.55%), Verrucomicrobiae (from 0.82% to 1.07%), Enterococcales (from 1.52% to 2.07%) and Enterobacteriaceae (from 0.66% to 1.18%), and a parallel progressive reduction of members of the Clostridium cluster XIVa (from 23.79% to 7.19%). On the other hand, the progression of colitis did not affect Bacteroidetes/Prevotella, which remained constant at the low rel.ab value detected 3 d after DSS administration. Unlike DSS-treated mice, healthy control mice showed a constant gut microbiota profile throughout the study. Interestingly, during recovery from DSS-induced colitis, at day 13 and 19, we observed a rapid shift of the gut microbiota toward a healthy profile, comparable to that shown by healthy control mice. In particular, 5 d after the interruption of DSS administration, the mice microbiota recovered a rel.ab. value of Bacteroidetes/Prevotella and Bacillaceae similar to that observed in healthy controls (71.08% and 1.79%, respectively).
The DSS murine model described here induces a milder colitis than the classical 3% DSS model, with a mortality rate very close to zero. Moreover, the signs and symptoms of colitis induced by this model are much more homogeneous in all the treated mice. We are aware that the responses to DSS observed in laboratory animals not only depend on DSS type and treatment protocol[21]; however, in our hands, this model has proven to be highly reproducible.
Histological examination of the colon of 1.5% DSS-treated mice showed that the mucosal damage starts to be evident 6 d before the DAI started to increase. This early damage was limited to the terminal colon mucosa and ascended toward the proximal colon when colitis severity increased. Colitis showed peak histological damage at day 13, in association with the maximum DAI. Histological damage remained evident even when the DAI score had returned to zero and the weight loss had been completely recovered. The persistence of histological damage, lymphocyte infiltration and COX-2 overexpression after complete clinical recovery (day 29) mimics the features of chronic UC in humans. The histological changes in this model are much easier to follow compared with those observed in the 3% DSS model, in which epithelial architecture is constantly lost for many days[5].
Circulating cytokine levels are indicative of the whole inflammatory profile. While studies in IBD patients have focused on serum cytokines, most investigations in mice models tended to evaluate tissue-derived cytokines, losing information on systemic inflammation. Circulating IL-1β, IL-6, IL-17 and TNFα play a key role in the pathogenesis of IBD[22]. IL-1β and IL-6 levels correlate with IBD activity. IL-17 is a delayed-type immune reaction cytokine produced by Th17 and by CD8+ T cells during chronic inflammation. Even if its role in IBD remains controversial[23], it seems to have a prominent pro-inflammatory role in the DSS model[24,25]. IL-10 is the most important antinflammatory cytokine in humans. Its role has been extensively studied in IL-10 knockout mice, and IL-10 mRNA expression in the inflamed mucosa is increased in UC patients, but decreased in CD patients[26,27]. IFNγ secretion has been linked to IL-17 secretion[28] in experimental colitis and its relative mRNA expression transiently increases during DSS-induced acute colitis, with a peak close to the maximum DAI score[5]. TNFα is a master cytokine in IBD pathogenesis, and its orchestrating role in colonic inflammation was verified by the efficacy of anti- TNFα therapy in IBD[29]. The serum TNFα level correlates with clinical activity both in UC and CD[29]. Alex and collaborators[30] reported that acute DSS colitis in mice significantly increases circulating IL-1β, TNFα, IL-6 and IL-17, while chronic colitis increases IL-4, IL-10, IL-6 and IFNγ; however, they only analyzed a single time point for each condition. In our model, while IL-6, IFNγ and IL-10 peaked at day 13 (maximum DAI score) and decreased during the recovery of colitis, IL-1β, IL-17 and TNFα levels remained high, even when the symptoms and signs of colitis had disappeared. Thus, while circulating IL-6, IFNγ and IL-10 levels seem to correlate with the major clinical signs, IL-1β, IL-17 and TNFα mainly correlate with histological damage that persists and becomes chronic.
DSS (2%-5%) administration for 5-7 d has been used to develop an acute form of colitis, while an inflammatory condition reminiscent of human chronic IBD can be induced by repeated DSS cycles[31]. Our model permits the development of chronic colitis using a single DSS cycle.
To the best of our knowledge, this is the first study to characterize the gut microbiota trajectory in a mouse model of DSS-induced colitis. In particular, the longitudinal approach allowed the assessment of microbiotic changes immediately after the induction of colitis, during the course of disease progression and during the recovery phase. According to our data, the induction of colitis rapidly compromises the homeostasis of the gut microbial ecosystem, leading to a dramatic reduction of Bacteroidetes/Prevotella, a major mutualistic group of the mice gut microbiota, and a corresponding increase in Bacillaceae. Confirming these findings, a rapid decrease in Bacteroidetes was previously observed in mice models of DSS-induced colitis[32]. Moreover, disease progression in our model was associated with a gradual, but weak, increase in the pro-inflammatory gut microbiotic components Enterococcales and Enterobacteriaceae, the minor symbiotic member Lactobacillaceae, and the mucus-degrading Verrucomicrobiae Akkermansia muciniphila. On the other hand, during the progression of colitis, we also noted a gradual decrease in members of the Clostridium cluster XIVa. As a major component of a healthy gut microbiota, this cluster is involved in the production of short-chain fatty acids, which are microbial metabolites essential for several aspects of the host physiology: nutrition, immune modulation and protection from pathogen colonization[33]. Taken together, these data demonstrate a progressive impairment of the gut microbiota with advancing colitis, resulting in a dysbiotic profile that can violate mutualism and support the disease. Interestingly, at the end of DSS administration, during weight recovery, we observed a rapid shift of the gut microbial community toward a healthy profile. Within two days of the end of DSS administration, the mice gut microbiota showed rel.ab. values of Bacteroidetes/Prevotella, Bacillaceae, Enterococcales, Enterobacteriaceae, Lactobacillaceae, Verrucomicrobiae and Clostridium cluster XIVa similar to those observed in healthy controls. These data demonstrated the high degree of resilience of the gut microbiota, which showed a potential for rapid recovery of its healthy mutualistic profile after DSS-induced dysbiosis.
One of the unanswered questions regarding IBD and DSS-induced colitis is to establish to what extent the dysbiosis is a contributory cause of the local and systemic inflammation, especially during the recovery phase. Dysbiosis can cause increased mucus secretion[7] and exacerbate intestinal inflammation, which further contribute to the microbiota shift. In DSS-colitis, microbiota homeostasis is rapidly compromised. After 3 d of DSS treatment, the microbiota is profoundly changed, and this alteration is maintained during DSS administration. When the maximum DAI was reached, the microbiota were observed to be returning to a healthy composition. Thus, dysbiosis precedes the systemic inflammation that starts to increase after 7 d of DSS treatment, reaching its maximum when the maximum DAI is reached and remaining high until the late recovery phase.
COX-2 is a very good marker of colonocytes and colon mucosa inflammation. Its expression in the colon of DSS-treated mice starts to increase only when the maximum DAI is reached and remains very high until the late recovery phase. The 10 d delay between the dysbiosis and the increased COX-2 expression in colonocytes suggests that dysbiosis alone is not able to trigger COX-2 expression. On the other hand, recovery from dysbiosis is not sufficient to ameliorate the inflammatory profile of DSS colitic mice, nor the inflammation of their colonocytes.
These results emphasize that the microbiota certainly contribute to intestinal inflammation, but also that the pro-inflammatory response elicited by DSS in the colon wall continues even when the animals recover from dysbiosis. It is therefore reasonable to assume that the observed deviations in the gut microbiota structure can foster changes in cytokine expression[34]. However, the interactions between the microbiota and the immune system are very complicated, and remain to be elucidated in detail[35]. More studies are required to be able to draw conclusions regarding this point.
The overexpression of COX-2 in colonocytes associated with leukocyte mucosal infiltration creates a pro-inflammatory loop from which it is difficult to escape. Moreover, circulating IL-10, one of the major anti-inflammatory cytokines, decreases during the recovery phase until it returns to basal levels at the end of the recovery, when both COX-2 expression and circulating IL-1β, IL-17 and TNFα are at their maximum levels. It is very likely that this kind of pro-inflammatory loop, which is responsible for the chronicity of DSS-induced colitis, is also activated in UC patients.
Decreasing the DSS concentration to 1.5% and increasing the treatment’s duration to 9 d induces chronic colitis with a short milder acute phase, followed by a mild chronic active disease. This mild disease is a much more accurate condition for studying the dynamics of colitis during clinical remission. This model also represents a step forward in reducing the suffering of animals and, given the very low mortality rate, it allows a reduction in the number of animals required to obtain statistically significant results.
Authors thank Dr. A. Sardo for technical and moral support.
Progress in understanding the molecular basis of inflammatory bowel disease (IBD) in humans has accelerated, thanks to the generation of animal models of colitis. Experimental colitis permits the study of complex physiopathological mechanisms, which cannot be simulated in vitro or in silico. The most commonly used method to trigger colitis in animal models is based on oral administration of a sulfated polysaccharide called dextran sulfate sodium (DSS). This model has been validated as a relevant model for the translation of mice data to human inflammatory bowel diseases.
The etiology of IBDs remains largely unknown, and their prevalence is increasing in developed countries, with the total number of IBD patients estimated as between 1 and 1.5 million in the United States. A genetic basis for IBD has long been recognized, because of the increased familial risk. However, significant discordance for Crohn’s disease (CD) in twins, and a much less robust phenotypic concordance for ulcerative colitis (UC), suggest that environmental factors play a major role in IBDs pathogenesis. Among these, the gut microbiota seems to have a crucial role in CD and UC, because an altered immune response to normal microbiota has been identified as a common feature in IBD patients.
This study represents a step forward in the use of the DSS model in preclinical studies. It describes new experimental procedures for dissecting the role of microbiome-immune system interactions in the pathogenesis of colitis and the evaluation of new possible IBDs treatments.
IBDs, including CD and UC, are chronic inflammatory disorders of the intestine. DSS is a synthetic sulfated polysaccharide composed of dextran with sulfated glucose. It is capable of triggering colitis in mice by binding to medium-chain-length fatty acids present in the colon, thereby inducing inflammation.
The study is well designed and very interesting for evaluating treatments for ulcerative colitis with mild to moderate activity. It describes a new murine model of colitis, based on the administration of 1.5% DSS. The results are very interesting and have a strong potential for being used as a benchmark for further studies that evaluate the possible treatments of colitis.
P- Reviewers: Rajendran VM, Sipos F, Taxonera C, Vetvicka V S- Editor: Wen LL L- Editor: Stewart GJ E- Editor: Zhang DN
| 1. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1254] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 2. | Melgar S, Karlsson L, Rehnström E, Karlsson A, Utkovic H, Jansson L, Michaëlsson E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim. 2000;49:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol. 2011;2011:342637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Rose WA, Sakamoto K, Leifer CA. Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases. BMC Immunol. 2012;13:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Araki Y, Bamba T, Mukaisho K, Kanauchi O, Ban H, Bamba S, Andoh A, Fujiyama Y, Hattori T, Sugihara H. Dextran sulfate sodium administered orally is depolymerized in the stomach and induces cell cycle arrest plus apoptosis in the colon in early mouse colitis. Oncol Rep. 2012;28:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2012;7:e32084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Coburn LA, Gong X, Singh K, Asim M, Scull BP, Allaman MM, Williams CS, Rosen MJ, Washington MK, Barry DP. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One. 2012;7:e33546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Kim DJ, Kim KS, Song MY, Seo SH, Kim SJ, Yang BG, Jang MH, Sung YC. Delivery of IL-12p40 ameliorates DSS-induced colitis by suppressing IL-17A expression and inflammation in the intestinal mucosa. Clin Immunol. 2012;144:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 284] [Article Influence: 18.9] [Reference Citation Analysis (1)] |
| 11. | Samanta AK, Torok VA, Percy NJ, Abimosleh SM, Howarth GS. Microbial fingerprinting detects unique bacterial communities in the faecal microbiota of rats with experimentally-induced colitis. J Microbiol. 2012;50:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Berry D, Schwab C, Milinovich G, Reichert J, Ben Mahfoudh K, Decker T, Engel M, Hai B, Hainzl E, Heider S. Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012;6:2091-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 13. | Lombardi VR, Etcheverría I, Carrera I, Cacabelos R, Chacón AR. Prevention of chronic experimental colitis induced by dextran sulphate sodium (DSS) in mice treated with FR91. J Biomed Biotechnol. 2012;2012:826178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Rath HC, Schultz M, Freitag R, Dieleman LA, Li F, Linde HJ, Schölmerich J, Sartor RB. Different subsets of enteric bacteria induce and perpetuate experimental colitis in rats and mice. Infect Immun. 2001;69:2277-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 662] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 16. | Candela M, Consolandi C, Severgnini M, Biagi E, Castiglioni B, Vitali B, De Bellis G, Brigidi P. High taxonomic level fingerprint of the human intestinal microbiota by ligase detection reaction--universal array approach. BMC Microbiol. 2010;10:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Candela M, Rampelli S, Turroni S, Severgnini M, Consolandi C, De Bellis G, Masetti R, Ricci G, Pession A, Brigidi P. Unbalance of intestinal microbiota in atopic children. BMC Microbiol. 2012;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Castiglioni B, Rizzi E, Frosini A, Sivonen K, Rajaniemi P, Rantala A, Mugnai MA, Ventura S, Wilmotte A, Boutte C. Development of a universal microarray based on the ligation detection reaction and 16S rrna gene polymorphism to target diversity of cyanobacteria. Appl Environ Microbiol. 2004;70:7161-7172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Consolandi C, Severgnini M, Castiglioni B, Bordoni R, Frosini A, Battaglia C, Bernardi LR, Bellis GD. A structured chitosan-based platform for biomolecule attachment to solid surfaces: application to DNA microarray preparation. Bioconjug Chem. 2006;17:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 646] [Article Influence: 49.7] [Reference Citation Analysis (1)] |
| 21. | Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5848-5861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 194] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Shen W, Durum SK. Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease. Neurochem Res. 2010;35:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 25. | Melgar S, Yeung MM, Bas A, Forsberg G, Suhr O, Oberg A, Hammarstrom S, Danielsson A, Hammarstrom ML. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol. 2003;134:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434-1444. [PubMed] |
| 27. | He Y, Lin LJ, Zheng CQ, Jin Y, Lin Y. Cytokine expression and the role of Thl7 cells in mice colitis. Hepatogastroenterology. 2012;59:1809-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Reimund JM, Wittersheim C, Dumont S, Muller CD, Baumann R, Poindron P, Duclos B. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn’s disease. J Clin Immunol. 1996;16:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 202] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 30. | Bento AF, Leite DF, Marcon R, Claudino RF, Dutra RC, Cola M, Martini AC, Calixto JB. Evaluation of chemical mediators and cellular response during acute and chronic gut inflammatory response induced by dextran sodium sulfate in mice. Biochem Pharmacol. 2012;84:1459-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2011;17:917-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3076] [Article Influence: 236.6] [Reference Citation Analysis (0)] |
| 33. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3922] [Cited by in RCA: 3459] [Article Influence: 216.2] [Reference Citation Analysis (0)] |
| 34. | Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1619] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 35. | Chen VL, Kasper DL. Interactions between the intestinal microbiota and innate lymphoid cells. Gut Microbes. 2013;5:Epub ahead of print. [PubMed] |