Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.1993
Revised: December 1, 2013
Accepted: January 14, 2014
Published online: February 28, 2014
Processing time: 154 Days and 5.6 Hours
Colorectal cancer is a lethal disease if not discovered early. Even though appropriate screening and preventive strategies are in place in many countries, a significant number of patients are still diagnosed at late stages of the disease. The management of metastatic colorectal cancer remains a significant clinical challenge to oncologists worldwide. While cytotoxic regimens constitute the main treatment of choice in this patient population, addition of the five biologics (bevacizumab, cetuximab, aflibercept, panitumumab and regorafenib) to these regimens has improved clinical outcomes. The most commonly used cytotoxic regimens include doublet combinations (FOLFOX/XELOX or FOLFIRI). Many clinical trials have been published and others are underway to compare the biologic agents with one another in order to prove the superiority of one regimen over another. Metastatic colorectal cancer patients have many treatment options; however, the optimal use and sequence of targeted agents remain to be determined. This review entails concise and updated clinical data on the management of metastatic colorectal cancer. The aim of the review is to determine where to fit the five biologic targets into the treatment algorithm of metastatic colorectal cancer patients and to derive treatment sequences that would achieve best clinical outcome based on the current available data.
Core tip: Metastatic colorectal cancer patients have many treatment options; however, the issue of best treatment sequence remains a challenge in this population. This review involves an in depth analysis of previous and most recent clinical advances in this field and aims to come out with treatment sequences that identify patient groups who are most likely to benefit from such sequences based on the current available data.
- Citation: Temraz S, Mukherji D, Shamseddine A. Sequencing of treatment in metastatic colorectal cancer: Where to fit the target? World J Gastroenterol 2014; 20(8): 1993-2004
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/1993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.1993
Colorectal cancer (CRC) is a lethal disease if not discovered early. Even though appropriate screening and preventive strategies are in place in many countries, a significant number of patients are still diagnosed at late stages of the disease. It is reported that approximately 20%-25% of patients present with distant metastatis at diagnosis[1,2]. Treatment goals for these patients are usually palliative rather than curative with the exception of a small number of patients with stage IV disease, liver-confined disease who may be surgically cured.
Recent advances in chemotherapy-based regimens have increased median overall survival (OS) for patients with metastatic CRC (mCRC) from 11-12 mo in the 5-fluorouracil (5-FU) era[3] to more than 24 mo in the era of biologic compounds and doublet/triplet chemotherapy regimens[4-6].
The continuum of care approach to the management of patients with metastatic rectal cancer is the same as that for patients with metastatic colon cancer. The three active conventional chemotherapy agents for mCRC are fluoropyrimidines, irinotecan and oxaliplatin. The most widely used cytotoxic backbone involves double-agent chemotherapy with either FOLFOX/XELOX or FOLFIRI with no significant differences between either regimen[7,8], while triple-agent chemotherapy (FOLFOXIRI), although achieving better progression free survival (PFS), response rate (RR) and OS than FOLFIRI in some trials[9,10], is only reserved to patients who can tolerate such an aggressive regimen. 5-FU/LV or capecitabine, which have been shown to be inferior to FOLFOX[11-13] and FOLFIRI[14,15] in terms of OS (with FOLFIRI regimen), PFS and RR, are still a treatment of choice in patients who cannot tolerate treatment with oxaliplatin and irinotecan. The addition of biological targets to these four cytotoxic regimens has shown better treatment outcomes in the majority of patients; however, debate still exists with regards to the best sequence of treatment, and which agents to be used in first line and then following progression. In the discussion that follows, we review the literature of clinical trials to come out with treatment sequences that achieve the best outcome in mCRC patients.
Data for this review were compiled using MEDLINE/PubMed, American Society of Clinical Oncology and European Society of Medical Oncology abstract databases published before July 2013. The search terms included colorectal cancer, bevacizumab, panitumumab, cetuximab, aflibercept and regorafenib. Information regarding ongoing clinical trials was obtained using the United Stated National Institute of Health’s online resource clinicaltrials.gov. Only articles published in English were considered.
Addition of bevacizumab to “weaker” cytotoxic regimens such as 5-FU/LV or to capecitabine yielded better PFS compared to the cytotoxic regimen alone in 3 clinical trials. The first phase II trial assessing the efficacy of adding bevacizumab to 5-FU/LV revealed that bevacizumab at 5 mg/kg every 2 wk resulted in increases of 3.8 mo in PFS (from 5.2 to 9.0 mo; P = 0.005) compared with 5-FU/LV alone. A statistically significant increase in RR was demonstrated for the bevacizumab arm compared with the control arm (40% vs 17%, P = 0.029). Median OS was improved in the bevacizumab arm but did not reach statistical significance[16]. In another phase II trial by Kabbinavar et al, patients were randomly assigned to 5-FU/LV/placebo (n = 105) or 5-FU/LV/bevacizumab (n = 104). RR and OS were better in the bevacizumab arm but they did not reach statistical significance. PFS was significantly better in the bevacizumab arm with 9.9 mo vs 5.5 mo in the placebo arm (P = 0.0002)[17]. Patients in this trial were non-eligible to receive irinotecan based-therapy and were ≥ 65 years. In the recent phase III trial by Cunningham et al, addition of bevacizumab to capecitabine in elderly patients ≥ 70 years was associated with significantly prolonged PFS, the primary end point, compared with capecitabine alone (9.1 mo vs 5.1 mo, P < 0.001)[18]. RR was also significantly improved in the bevacizumab plus capecitabine arm (19.3% vs 10.0%, P = 0.042). OS, a secondary endpoint, was longer in patients in the bevacizumab arm (20.7 mo vs 16.8 mo, P = 0.182) but did not reach statistical significance and the study was not powered to show a difference in OS between treatment arms. Therefore, patients receiving fluoropyrimidine regimens as part of their first-line treatment have prolonged PFS of about 9 mo from the addition of bevacizumab. The toxicity profile from adding bevacizumab was generally well tolerated in all 3 trials.
Bevacizumab: In a phase 3 trial by Hurwitz et al[19], patients were assigned to either receive irinotecan, bolus 5-FU and leucovorin (IFL) plus bevacizumab or the same cytotoxic regimen with placebo. Median OS (20.3 mo vs 15.6 mo, P < 0.001), PFS (10.6 mo vs 6.2 mo, P < 0.001) and RR (44.8% vs 34.8%, P = 0.004) were all superior in the bevacizumab group. Results from a phase III study that was initially meant to compare the safety and efficacy of 3 different irinotecan containing regimens in the first-line treatment of mCRC was later amended to compare FOLFIRI plus bevacizumab with mIFL plus bevacizumab. At the time when the results were first published, the median OS was not reached in the FOLFIRI arm[20]. A year later, the authors report a median OS of 28 mo in the FOLFIRI plus bevacizumab arm compared to 19.2 mo in the mIFL plus bevacizumab arm (P = 0.037). Differences in PFS and RR were not statistically significant between the 2 arms[21]. Based on the results from this trial, FOLFIRI plus bevacizumab was found to be superior to mIFL plus bevacizumab in the first-line treatment of mCRC. Two other clinical trials, the PACCE and AVIRI trials, of FOLFIRI plus bevacizumab thereafter reported consistent data with PFS reported to be 11.7 and 11.1 mo, OS 20.5 mo and 22.2 mo and RR 40% and 53.1%, respectively[22,23]. The median OS of 28 mo reported by Fuchs et al[20] was the highest survival reported when bevacizumab was added to FOLFIRI. The cytotoxic regimen FOLFIRI was shown to be superior to IFL, and addition of bevacizumab to both regimens yielded better results with FOLFIRI as is expected. Nevertheless, bevacizumab and FOLFIRI in the first-line treatment of mCRC is a superior regimen and is hence recommended in patients who can tolerate such a combination.
Panitumumab: In a single arm phase II trial, FOLFIRI plus panitumumab in the first line setting resulted in an overall RR of 49%, PFS of 7.6 mo and an R0 resection rate of hepatic metastasis of 7%. When stratified according to KRAS status, those with wild-type KRAS had better PFS (8.9 mo vs 7.2 mo), RR (56% vs 38%) and R0 resection rate (8% vs 5%) than those with mutated KRAS tumors[24].
Cetuximab: Cetuximab with FOLFIRI in the first line treatment of mCRC demonstrated significant clinical activity. In the CRYSTAL (Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer) trial, addition of cetuximab to FOLFIRI in patients with KRAS wild-type resulted in significantly better OS (23.5 mo vs 20 mo, P = 0.0093), PFS (9.9 mo vs 8.4 mo, P = 0.0012), RR (57.3% vs 39.7%, P < 0.001) and R0 resection rate (5.1% vs 2%, P = 0.0265) compared with FOLFIRI alone[25]. However, patients with mutated KRAS status failed to achieve improvement in survival and RRs.
Cetuximab vs bevacizumab: The German AIO (Arbeitsgemeinschaft Internistische Onkologie) KRK-0306 (FIRE-3) phase III randomized multicenter trial compared the efficacy of FOLFIRI-cetuximab to FOLFIRI-bevacizumab in 592 patients with wild-type KRAS mCRC who were not previously treated for metastatic disease[4]. The primary endpoint was the overall RR. Among the intent to treat (ITT) population, overall RR (62% vs 58%, P = 0.183) and PFS (10.0 mo vs 10.3 mo, P = 0.547) were similar between the cetuximab and bevacizumab arms, respectively. In those 526 patients assessable for efficacy, the overall RR was significantly higher in the FOLFIRI-cetuximab arm (72.2% vs 63.1%, P = 0.017). OS was significantly longer in patients treated with FOLFIRI-cetuximab (28.7 mo) compared with patients who received FOLFIRI-bevacizumab (25 mo, P = 0.017). The lack of correlation between PFS and OS in this trial is unclear and may be related to the subsequent therapies used after first-line treatment and also highlights the importance of choice of primary endpoint. In a subgroup analysis of the same trial for patients with mutated KRAS tumors, neither strategy demonstrated a clearly superior outcome[26]. Results from the US intergroup phase III C80405 trial which randomized patients to either cetuximab or bevacizumab with FOLFOX or FOLFIRI will help address this issue as well. But for now, and until data from other trials become available, the optimum biologic to be used with FOLFIRI based on the current available data seems to be cetuximab. In patients with mutated KRAS tumors, and even though bevacizumab did not seem to incur additional benefits over cetuximab in the subgroup analysis, it is still not recommended to use cetuximab/panitumumab-based regimens. And hence, FOLFIRI plus bevacizumab is a treatment option in patients with mutated KRAS tumors.
Bevacizumab: Addition of bevacizumab to FOLFOX or XELOX in the NO16966 trial reported only an increase in PFS when bevacizumab was added to FOLFOX or XELOX compared to the cytotoxic regimen alone (9.4 mo vs 8.0 mo, P = 0.0023). Median OS was 21.3 mo in the bevacizumab group and 19.9 mo in the placebo group (P = 0.07) and RR was similar between the two arms (47% vs 49%, P = 0.31)[27]. Other trials suggest that the addition of bevacizumab to an oxaliplatin-based regimen yields a similar magnitude of efficacy to that seen when bevacizumab is added to a FOLFIRI regimen. In four clinical trials, addition of bevacizumab to XELOX or FOLFOX resulted in PFS ranging between 10.3-11.4 mo, OS ranging between 20.3-24.5 mo and a RR ranging between 46%-50%[22,28-30]. However, in all these trials, addition of bevacizumab to oxaliplatin-based regimens was not compared to the cytotoxic regimen alone. The NO16966 trial was the only trial that involved this comparison and has shown that addition of bevacizumab improved PFS as reported in other phase III trials, but the observed trend in an improvement in OS did not reach statistical significance, which may be attributed to a shorter treatment duration in the bevacizumab arm (about 6 mo) as compared to other trials and that treatment until disease progression may be necessary to maximize the clinical benefit derived from bevacizumab therapy.
Results of the large observational BEAT trial of bevacizumab concluded that median PFS, TTP (time to treatment progression) and OS were consistent across the doublet regimens (FOLFOX, XELOX and FOLFIRI), suggesting thatthe efficacy of bevacizumab is not related to thechemotherapy regimen used[31]. Results of this have been confirmed in doublet combinations but not in triplet regimens. In a recent phase 2 trial of a head-to-head comparison between XELOX plus bevacizumab and XELIRI plus bevacizumab, the addition of bevacizumab to these two cytotoxic regimens yielded similar PFS (10.4 mo vs 12.1 mo, P = 0.3) and OS (24.4 mo vs 25.5 mo, P = 0.45) with no superiority of one regimen over the other[32]. Another clinical trial, MAVERICC, is underway comparing FOLFIRI plus bevacizumab vs FOLFOX plus bevacizumab. In this phase 2 prospective study, tumoral excision repair cross-complementation group 1 and plasma vascular endothelial growth factor A are employed as potential biomarkers for oxaliplatin- and bevacizumab-containing regimens, respectively (ClinicalTrials.gov Identifier: NCT01374425). While the magnitude of effect seems to be equivalent between FOLFIRI and FOLFOX, only further clinical trials addressing biomarkers of response to these cytotoxic regimens could stratify patients to either cytotoxic regimen.
Aflibercept: In a phase II trial assessing the efficacy of aflibercept when added to FOLFOX in the first-line treatment of mCRC, no significant improvement in RR and PFS was achieved. OS in that trial was not reported[5]. Hence, for now, aflibercept is not recommended in the first line treatment when added to a FOLFOX regimen. Its efficacy in the second-line setting was achieved when added to FOLFIRI which may also be of benefit if used in the first-line. However, no clinical trial has yet addressed this issue and so aflibercept’s use is limited to second-line treatment regimens that involve irinotecan naïve patients.
Panitumumab: In the phase III Panitumumab Randomized Trial in Combination with Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy (PRIME) study, addition of panitumumab to FOLFOX in the first-line treatment of patients with KRAS wild-type significantly improved PFS (9.6 mo vs 8.0 mo, P = 0.02). The overall increase in survival was not significant but was higher in the panitumumab group (23.9 mo vs 17.9 mo, P = 0.072) as well as the overall RR (55% vs 48%; P = 0.068) and R0 resection rate (8.3% vs 7.0%)[5].
Wild-type RAS (wild-type KRAS exons 2, 3, 4 and wild-type NRAS exons 2, 3, 4) was associated with significantly better OS (26 mo vs 20.2 mo, P = 0.04) and PFS (10.1 mo vs 7.9 mo, P < 0.01) in the panitumumab plus FOLFOX arm than the FOLFOX arm alone. In patients with wild-type KRAS exon 2 but mutated other RAS (KRAS exons 3, 4 or NRAS exons 2, 3, 4), the PFS and OS were not different between the two arms. Hence, patients with wild-type RAS have a statistically significant OS benefit when treated with panitumumab plus FOLFOX vs FOLFOX alone. Panitumumab is unlikely to benefit patients with any RAS mutations and BRAF mutation had no predictive value[33].
Cetuximab: Unlike the synergy seen between cetuximab and irinotecan, data on the efficacy of cetuximab with oxaliplatin-based regimens report conflicting results ranging from additive to detrimental effects of these two drugs. The phase 2 oxaliplatin and cetuximab in first-line treatment of metastatic colorectal cancer (OPUS) trial demonstrated that addition of cetuximab to FOLFOX4 regimen resulted in significant improvement in PFS (8.3 mo vs 7.2 mo, P = 0.0064), RR (57% vs 34%, P = 0.0027), R0 resection rate (12% vs 3%, P = 0.0242) but only a trend toward improvement in OS (22.8 mo vs 18.5 mo, P = 0.39)[34]. However, two recent phase 3 trials, the Medical Research Council Continuous Chemotherapy plus Cetuximab or Intermittent Chemotherapy with Standard Continuous Palliative Combination Chemotherapy with Oxaliplatin and Fluoropyrimidine in First-Line Treatment of Metastatic Cancer (MRC COIN) and Nordic Colorectal Cancer Biomodulation Group Study 7 (NORDIC VII) trials have raised more questions with regards to the efficacy of cetuximab with oxaliplatin-based regimens. The MRC COIN study involved 357 patients with KRAS wild-type in the cetuximab arm plus FOLFOX or XELOX and 358 patients with KRAS wild-type in the control arm (FOLFOX or XELOX without cetuximab). The investigators reported no differences in OS (17 mo vs 17.9 mo, P = 0.67) and PFS (8.6 mo vs 8.6 mo, P = 0.6) between cetuximab arm and control group, respectively. RR, on the other hand, was increased from 57% with chemotherapy alone to 64% with addition of cetuximab (P = 0.049)[35]. A post-hoc analysis; however, demonstrated improvement in PFS in the infusional FOLFOX plus cetuximab (P = 0.037) but not in the XELOX plus cetuximab group (P = 0.88). A PFS benefit was restricted to those patients with wild-type KRAS and those with no or only one metastatic site treated with 5-FU infusion therapy (P = 0.011).The number of patients receiving XELOX (n = 240) far exceeded those receiving FOLFOX (n = 117) which may have contributed to the negative outcomes seen in the cetuximab arms. Moreover, the COIN trial reported significant dose reductions in infusional 5-FU in the FOLFOX plus cetuximab arm compared to the control group (P = 0.016) and the XELOX plus cetuximab group received significant dose reductions of both oxaliplatin (P = 0.0018) and capecitabine (P = 0.004) compared to the control arm which may explain in part the lack of efficacy in the cetuximab arms. The Nordic VII trial investigated the efficacy of cetuximab when added to bolus 5-FU/LV/oxaliplatin (FLOX)[36]. The trial included 194 patients with wild-type KRAS; 97 patients received FLOX plus cetuximab and 97 received FLOX alone. An additional 130 patients with mutant KRAS tumors were randomized between the two arms. In patients with wild-type KRAS, a trend towards worse outcome was seen in terms of OS (20.1 mo vs 22 mo, P = 0.48) and PFS (7.9 mo vs 8.7 mo, P = 0.66) between the cetuximab arm and the control arm, respectively. Additionally, the RR did not differ between the two groups (46% vs 47%, P = 0.89). On the other hand, patients with mutated KRAS tumors exhibited a trend toward better prognosis when they were treated with cetuximab; PFS (9.2 mo vs 7.8 mo, P = 0.07), OS (21.1 mo vs 20.4 mo, P = 0.89) and RR (35% vs 23%, P = 0.31). Hence, both the COIN and NORDIC VII trials did not demonstrate an efficacy from the addition of cetuximab to oxaliplatin-based regimens. However, this was not the case in the OPUS trial which demonstrated a significant improvement in PFS when cetuximab was added to FOLFOX regimen. It seems that cetuximab is efficient when added to infusional 5-FU as seen in the OPUS trial, while capecitabine or bolus 5-FU are not associated with significant improvement in PFS. The PRIME trial also demonstrated a significant improvement in PFS when panitumumab was added to the FOLFOX regimen. The AIO KRK-0104 study randomly assigned 198 patients to either cetuximab plus XELIRI (n = 93) or cetuximab plus XELOX (n = 92)[37]. The trial was not powered to compare the two treatment regimens; however, the RR was similar for the two arms (46.1% in XELIRI vs 47.7% in XELOX arm). The PFS reported in this trial is lower than that reported in both the OPUS and CRYSTAL trials probably further indicating that cetuximab is more efficient with infusional 5-FU regimens than either bolus 5-FU or capecitabine regimens. It is of note that the OS reported in the trial was comparable to that observed in OPUS and Crystal trials. A recent meta-analysis that pooled results ofthe PRIME, OPUS, COIN, and NORDIC VII revealed that addition of cetuximab and panitumumab to oxaliplatin-based regimens in the first line setting significantly improved PFS (P = 0.03) and RR (P = 0.009) compared to chemotherapy alone but the difference in OS was not significant. OS and PFS were not significant when cetuximab and panitumumab were added to bolus 5-FU or capecitabine-based regimens compared with chemotherapy alone[38].
The recent results of the new EPOC study revealed detrimental results with the addition of cetuximab to chemotherapy (fluoropyrimidine and oxaliplatin) in patients with liver resectable metastases and KRAS wild-type tumors thus questioning the role of cetuximab in upfront therapy with oxaliplatin based regimens in this setting[39]. The study randomized 272 patients to chemotherapy alone or chemotherapy with cetuximab. The trial was stopped when the study met a protocol pre-defined futility analysis. PFS was significantly worse in the cetuximab arm (14.8 mo vs 24.2 mo, P < 0.048). The phase 2 OPUS trial was the only trial that supported the addition of cetuximab to FOLFOX and so until a phase 3 trial of cetuximab plus FOLFOX demonstrates superior clinical activity over FOLFOX alone, this cytotoxic regimen is still not recommended in the first-line treatment of mCRC patients and particularly in patients with resectable liver metastases.
In a pooled, retrospective analysis by Roock et al[40] of 579 mCRC patients who received cetuximab, patients with mutation in codon 13 (G13D) had significantly longer OS (7.6 vs 5.7 mo; P = 0.005) and PFS (4.0 mo vs 1.9 mo, P = 0.004) than patients with other KRAS mutations. In addition, OS was similar between patients with the G13D mutation and patients with wild-type KRAS. Moreover, pooled data from 1378 evaluable patients from the CRYSTAL and OPUS studies revealed significant variations in treatment effects for RR (P = 0.005) and PFS (P = 0.046) in patients with G13D-mutant tumors vs all other mutations[41]. Cetuximab plus chemotherapy vs chemotherapy alone significantly improved PFS (7.4 mo vs 6.0 mo, P = 0.039) and RR (40.5% vs 22.0%, P = 0.042) but not OS (15.4 mo vs 14.7 mo, P = 0.68) in patients with G13D-mutant tumors. However, the efficacy of cetuximab in patients with G13D mutations was inferior to those with wild-type KRAS. A study by Gajate et al[42] reported different results, patients with mutation in G13D did not differ significantly in PFS (4.96 mo vs 3.1 mo, P = 0.72) and OS (8.2 mo vs 14.6 mo, P = 0.084) from other KRAS mutations. Also, as seen in pooled data from the CRYSTAL and OPUS studies, patients with KRAS wild-type tumors have a longer PFS (7.3 mo, P = 0.025) and OS (19.0 mo, P = 0.004) than patients with G13D-mutated tumors[42]. Moreover, the finding of cetuximab benefit in patients with G13D mutations was not reproducible with panitumumab in other pooled retrospective analysis of 3 trials with the use of FOLFOX with or without panitumumab in the first-line setting (PRIME trial), FOLFIRI with and without panitumumab in the second-line setting and best supportive care with and without panitumumab in the salvage setting[43]. No mutant KRAS allele was consistently identified as a predictive factor for PFS or OS in either the control arm or the panitumumab arm[43]. Prospective randomized trials in patients with G13D mutations are needed before any conclusions could be made about the potential benefit from cetuximab (or panitumumab). One such trial is currently open to accrual[44].
Panitumumab vs bevacizumab: The PEAK study was the first prospective trial to compare bevacizumab to an anti-EGFR monoclonal antibody in combination with an oxaliplatin-based regimen[45]. Median PFS was 10.9 mo with panitumumab and 10.1 mo with bevacizumab (P = 0.35). Median OS has not been reached with panitumumab and was 25.4 mo with bevacizumab (P = 0.14). The overall RRs were 58% and 54% and the resection rates were 13% and 11% for the panitumumab and bevacizumab arms, respectively.
In a prospective-retrospective analysis of the PEAK, patients with wild-type RAS receiving panitumumab had a PFS of 13.1 mo while those receiving bevacizumab had a PFS of 9.5 mo (P = 0.02)[46]. OS in the panitumumab arm was not reached while in the bevacizumab arm OS was 29 mo (P = 0.06). In patients with wild-type KRAS exon 2 but mutated KRAS (exons 3 or 4) or mutated NRAS (exons 2, 3 or 4), both the PFS (7.8 mo vs 8.9 mo, P = 0.44) and OS (not reached vs 21.6 mo, P = 0.5) were comparable between the panitumumab and bevacizumab arms. In this first-line estimation study in patients with wild-type RAS mCRC, PFS and OS favored panitumumab plus FOLFOX relative to bevacizumab plus FOLFOX.
Bevacizumab: Bevacizumab with triple cytotoxic regimens seems to be superior to doublet regimens. Recently, Falcone et al[6] reported the results of the Tribe trial where they sought to confirm the superiority of FOLFOXIRI over FOLFIRI when bevacizumab is added to both regimens. FOLFOXIRI plus bevacizumab significantly increased PFS (median 9.5 mo vs 11.9 mo, P = 0.001) and RR (53% vs 64%, P = 0.015) when compared to FOLFIRI plus bevacizumab. Median OS for FOLFOXIRI/bevacizumab was 31.0 mo compared with 25.8 mo in the FOLFIRI/bevacizumab group (P = 0.054). Grade 3-4 neurotoxicity, diarrhea, stomatitis, and neutropenia were significantly higher (P < 0.05) in patients receiving FOLFOXIRI/bevacizumab; while the incidence of febrile neutropenia, serious adverse events, and treatment-related deaths were similar among the two groups. Preliminary results of the OPAL trial assessing the safety of FOLFOXIRI with bevacizumab in the first-line setting in 96 patients revealed that the incidence of adverse events was as previously reported by Falcone et al[6] and that the regimen was well tolerated among the patient population included in the study. An interesting activity of FOLFOXIRI/bevacizumab was seen in BRAF mutated cancers; however, the numbers were low to derive any definite conclusions[6]. FOLFOXIRI regimen has been shown to be superior to FOLFIRI alone in the first line treatment of mCRC[9,10] and whether an additional benefit is employed from the addition of bevacizumab is unclear. The superiority of FOLFOXIRI plus bevacizumab over FOLFOX plus bevacizumab has also been reported in the phase 2 OLIVIA trial[48]. The R0 resection rate was significantly higher (48.8% vs 23.1%, P = 0.017), RR was higher but did not reach statistical significance and PFS data are still immature but favor the FOLFOXIRI arm. The results suggest that FOLFOXIRI-bevacizumab improves resection rates, RR, and long-term outcomes vs FOLFOX-bevacizumab in patients with initially unresectable colorectal liver metastases. Grade ≥ 3 adverse events occurred in 84% of patients in the FOLFOX arm compared to 95% in the FOLFOXIRI arm and included neutropenia (35% vs 48%), febrile (8% vs 13%) and diarrhea (14% vs 28%).
A clinical trial comparing FOLFOXIRI plus bevacizumab to FOLFOXIRI alone could define the magnitude of effect from the addition of bevacizumab. Moreover, BRAF-mutated microsatellite stable tumors have a poor prognosis[49] and could hence be good candidates to an aggressive regimen such as FOLFOXIRI plus bevacizumab. Also, receiving FOLFOXIRI-bevacizumab as first-line treatment limits choices in subsequent treatment arms, an issue that questions the importance of second and third line treatments. Among elderly Medicare metastatic CRC patients who survived at least 1 year after diagnosis, first-line therapy improved both short and long-term survival[50]. Second and subsequent chemotherapy lines reduced short-term mortality (2 years); however, they didn’t add any additional long term survival benefit (5 years) as compared to first-line therapy. So, should we worry about the sequential treatment strategy or should we provide the best upfront treatment? Only clinical trials addressing the benefit of first and subsequent lines of therapy between several treatment sequences can answer this question.
Cetuximab: Data on cetuximab with FOLFOXIRI is still premature. Two small trials reported high RRs of 79 and 81%, OS of 35 and 24.7 mo, and one trial reported a PFS of 9.5 mo[51,52]. Toxicity will likely be a problem with such a combination. But till now, the only biologic target whose efficacy with FOLFOXIRI has been proven in phase III trials is bevacizumab. A trial comparing the FOLFOXIRI regimen alone to FOLFOXIRI plus biologics is needed to assess the efficacy of biologics with this cytotoxic regimen.
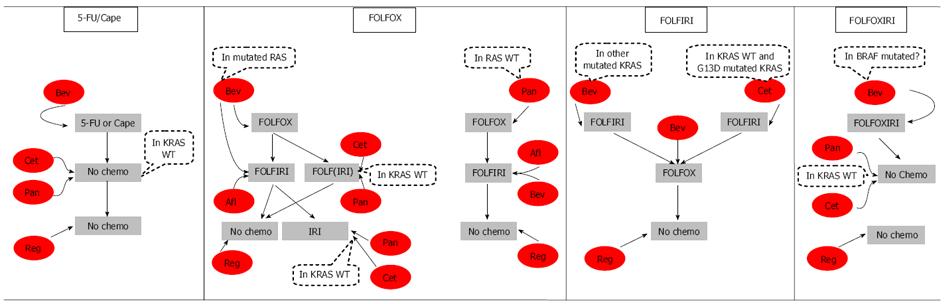
Subsequent treatment options following progression on the 4 aforementioned cytotoxic backbones and their associated targets are summarized in Figure 1.
Patients progressing on 5-FU or capecitabine with bevacizumab in the first-line are unlikely to receive any regimen containing irinotecan or oxaliplatin in subsequent lines of therapy. Therefore, patients progressing on first line 5-FU or capecitabine-bevacizumab have only the option of EFGR monoclonal antibodies in the second line setting if they have KRAS wild type tumors then regorafenib as their last treatment line[53-56]. Patients with mutated KRAS can only receive regorafenib as their second treatment line since anti-EGFR therapy in this patient population is not recommended.
Patients receiving the FOLFOX regimen with bevacizumab in the first-line setting receive the alternative cytotoxic regimen FOLFIRI following progression[57-59]. The TML trial enrolled 820 patients with unresectable mCRC who progressed within 3 mo after discontinuing first-line treatment with a bevacizumab-containing chemotherapy regimen. Patients were randomized to receive either oxaliplatin-based or irinotecan-based chemotherapy (depending on what they received first line) plus bevacizumab (n = 409) or chemotherapy alone (n = 411). Results of the primary analysis showed a significant improvement in OS (11.2 mo vs 9.8 mo, P = 0.006) and PFS (5.7 mo vs 4.1 mo, P < 0.0001) in favor of the bevacizumab plus chemotherapy arm[60]. RR were comparable between the two treatment arms (5.4% vs 3.9%, P = 0.3113). In a post hoc subgroup analysis of the trial, patients progressing on oxaliplatin-based chemotherapy with bevacizumab and crossing over to irinotecan-based chemotherapy with bevacizumab had a prolonged OS (12 mo vs 10 mo, P = 0.052) and PFS (6.2 mo vs 4.2 mo, P = 0.0005) compared to the chemotherapy alone arm. The BEBYP trial, conducted by the Gruppo Oncologico Nord Ovest, also supported the results of the TML trial[61]. A significant clinical benefit was associated with continuing bevacizumab after first-line bevacizumab-containing chemotherapy. At a median follow-up of 18 mo, median PFS was 6.77 mo in the bevacizumab arm compared to 4.97 mo in the chemotherapy-alone arm (P = 0.006). In the phase 3 VELOUR trial, addition of aflibercept to FOLFIRI in patients who progressed on an oxaliplatin-based regimen resulted in significant improvement in OS (13.5 mo vs 12.06 mo, P = 0.0032) and PFS (6.90 mo vs 4.67 mo, P < 0.0001) compared to FOLFIRI plus placebo[62]. The OS and PFS were comparable to those achieved with bevacizumab and FOLFIRI and prove the superiority of aflibercept with FOLFIRI over FOLFIRI alone. Hence, aflibercept with FOLFIRI constitutes another treatment of choice in patients progressing on first-line FOLFOX plus bevacizumab. Following progression on this regimen and having received all standard therapies, patients with mutated KRAS can be administered regorafenib monotherapy as their final treatment line.
A subset of patients with mutated RAS includes patients with wild-type KRAS. In this subset of patients, panitumumab or cetuximab plus FOLFIRI or irinotecan could be a treatment option. FOLFIRI plus panitumumab resulted in significantly better PFS (5.9 mo vs 3.9 mo, P = 0.004) and a trend toward improved OS compared to FOLFIRI alone that did not reach statistical significance and which may have been attributed to the large number of patients receiving anti-EGFR therapy following progression[63]. The EPIC trial, which evaluated irinotecan monotherapy with irinotecan plus cetuximab in patients pre-treated with FOLFOX, revealed that cetuximab added to irinotecan significantly improved PFS (4.0 mo vs 2.6 mo, P≤ 0.0001) and RR (16.4% vs 4.2%, P < 0.0001) but not OS[64]. In these trials, cetuximab and panitumumab resulted in significantly better PFS and RR but not OS while bevacizumab and aflibercept were associated with significantly better OS compared to chemotherapy alone. In a head-to-to head comparison between panitumumab and bevacizumab with FOLFIRI following progression on oxaliplatin-based chemo and bevacizumab, the SPIRITT trial revealed no significant difference in OS and PFS between the two arms. However, RR was higher in the panitumumab arm (28% vs 16%)[65]. The worst of grade 3/4 adverse events were recorded for 78% of the panitumumab arm vs 65% of the bevacizumab arm but this did not appear to impact discontinuation rates (29% vs 25% rates of discontinuation due to adverse events, respectively). Another phase 2 trial is currently recruiting participants to compare the efficacy of cetuximab vs bevacizumab with chemotherapy following progression on bevacizumab and chemotherapy in the first-line setting (ClinicalTrials.gov Identifier: NCT01442649). At this point, the choice of whether to use anti-EGFR therapy or bevacizumab with FOLFIRI partly depends on the patient’s clinical situation. If the patient is suffering from large tumor burden and is progressing rapidly, then panitumumab may be a better choice since it is associated with a higher response rate. But if skin toxicity is a concern, bevacizumab should be used.
Patients with wild-type KRAS have two options; either bevacizumab/aflibercept with FOLFIRI or panitumumab/cetuximab with FOLFIRI. Patients receiving bevacizumab/afliberceptplus FOLFIRI have the chance to be given irinotecan plus cetuximab as a third treatment line. In this setting, 55 heavily pretreated patients whose disease had progressed during or within an oxaliplatin-based first-line chemotherapy and an irinotecan-based second-line regimen were given irinotecan and cetuximab. This regimen in the third-line treatment resulted in a median PFS of 4.7 mo and median OS of 9.8 mo[66]. Finally, their last treatment line will involve regorafenib. On the other hand, patients with wild-type KRAS receiving panitumumab or cetuximab in the second line setting with FOLFIRI can only be administered regorafenib following progression.
Patients with wild-type RAS who receive first-line therapy with panitumumab and FOLFOX, are administered either aflibercept or bevacizumab with the FOLFIRI regimen which both have shown a survival benefit over chemotherapy alone[60,62]. Following progression on either of these lines, the last treatment of choice remaining for these patients is regorafenib since they have progressed on all standard therapies.
Patients with KRAS wild-type tumors progressing on FOLFIRI plus cetuximab should receive the FOLFOX regimen with bevacizumab. Aflibercept with FOLFOX did not show any significant improvement in the first-line setting and so it is not recommended in the second-line setting. Moreover, the GOIM (Gruppo Oncologico Dell’ Italia Meridionale) trial is underway to assess the efficacy of FOLFOX with or without cetuximab following progression on cetuximab plus FOLFIRI[67]. Until the results of this trial become available, bevacizumab is used in this setting with the FOLFOX regimen. The ECOG (Eastern Cooperative Oncology Group) Study E3200 assessed the efficacy of bevacizumab plus FOLFOX in patients previously treated with fluoropyrimidine and irinotecan to FOLFOX alone and found that OS (12.9 mo vs 10.8 mo, P = 0.0011), PFS (7.3 mo vs 4.7 mo, P < 0.0001) and RR (22.7% vs 8.6%, P≤ 0.0001) were all significantly higher in the bevacizumab group compared to the FOLFOX regimen alone[68]. Patients progressing on bevacizumab and FOLFOX benefit from regorafenib monotherapy in the third-line setting. Regorafenib is approved for the treatment of mCRC patients who progressed on standard therapies and was shown to be superior to supportive care in the CORRECT trial[69].
Patients with mutated KRAS, who cannot receive anti-EGFR therapy as part of their treatment, receive FOLFIRI plus bevacizumab and then cross over to FOLFOX plus bevacizumab after progression. In the TML trial, the post hoc analysis revealed that patients receiving irinotecan-based regimens with bevacizumab and then receiving bevacizumab with oxaliplatin-based regimens after progression had prolonged PFS (5.4 mo vs 3.8 mo, P < 0.0001) and OS (10.9 mo vs 9.3 mo, P = 0.0454) than patients in the chemotherapy alone arm. The last line of therapy available for these patients involves regorafenib which yielded an OS of 6.4 mo compared to best supportive care alone which yielded an OS of 5.0 mo (P = 0.0052)[69].
Patients progressing on the FOLFOXIRI plus bevacizumab regimen and having wild-type KRAS status benefit from irinotecan and cetuximab in the second treatment line. In a phase 2 trial of 40 patients progressing on at least one line of chemotherapy, biweekly cetuximab biweekly and irinotecan resulted in a RR of 22.5%, PFS of 3.4 mo and OS of 8 mo[70]. As their last treatment line, patients could receive regorafenib. On the other hand, if patients had mutated KRAS tumors, then their second treatment option would be regorafenib.
First-line treatment involves four cytotoxic backbones to which biologic targeted agents have been added. The effect of these targeted agents ranges from synergistic to detrimental and hence it is crucial to know where to fit these compounds into the management of mCRC patients. 5-FU or capecitabine is a weak regimen limited to elderly patients and those who cannot tolerate aggressive regimens. The addition of bevacizumab to this cytotoxic regimen yielded better PFS of up to 9 mo[16-18].
FOLFOX (or XELOX) is arguably the doublet cytotoxic regimen most commonly used in the first-line treatment of mCRC. The combination of EGFR-targeted therapy with this regimen has shown conflicting results with cetuximab but not with panitumumab. Addition of panitumumab to this regimen yielded an OS and PFS benefit in patients with wild-type RAS compared to bevacizumab[46]. Hence, patients with wild-type RAS are good candidates for FOLFOX plus panitumumab regimens while patients exhibiting any RAS mutation are candidates for FOLFOX plus bevacizumab. The other doublet cytotoxic regimen used in the first-line treatment is FOLFIRI. In a head-to-head comparison between bevacizumab and cetuximab with this regimen, cetuximab seems to be superior to bevacizumab[4]. Hence, cetuximab with FOLFIRI is limited to patients with KRAS-wild type and possible mutated KRAS with G13D mutations while other mutated KRAS tumors are more likely to benefit from FOLFIRI with bevacizumab. The results of the Intergroup C80405 study are eagerly awaited and it is hoped that results of this study will reveal the optimal first-line regimen for chemotherapy doublet plus targeted therapy. As for the triplet cytotoxic regimen FOLFOXIRI, and even though it was associated with significantly more adverse events when added to bevacizumab than either FOLFIRI or FOLFOX regimen, it resulted in the longest reported PFS and OS[6,48]. Cetuximab with this regimen yielded very high RRs but the data are still immature in this setting[51,52].
As outlined in Figure 1, second and third-line treatment options will depend on the drugs used in the first line setting. Biomarkers such as RAS mutation status remain of key importance. For patients with RAS wild-type tumors who have received anti-angiogenic rather than EGFR-targeted therapy in the first-line setting there is a choice to be made whether to continue anti-angiogenic therapy and switch the chemotherapy backbone, reserving EGFR-targeted therapy to the third line, or switch both chemotherapy and targeted therapy. We have no definitive data to guide this decision however there appears to be an advantage to the use of cetuximab in combination with irinotecan over oxaliplatin. Regorafenib has shown a survival advantage over placebo in heavily pre-treated patients and we are awaiting further work to identify biomarker that might help us select which patients are more likely to benefit from this therapy.
Current options for the management of metastatic CRC involve the use of four cytotoxic chemotherapy regimens and five targeted therapeutic agents. The optimal use and sequencing of these agents has yet to be determined. A major concern regarding clinical trials designed to compare one regimen with another is the large number of patients crossing over to the alternative regimen which may hinder the exact interpretation of OS. To overcome such a drawback, treatment sequences should be compared from line one up to subsequent treatment lines. In such a way, the efficacy of the whole treatment sequence is compared to another treatment sequence with the OS, PFS, RR and R0 resection rates compared across all treatment lines. Such trials are beginning to emerge and are currently underway (ClinicalTrials.gov Identifier: NCT01910610 and NCT01878422). As we learn more about the biology of this disease and biomarkers for treatment selection, we hope to improve outcomes for all patients.
P- Reviewers: Classen CF, Langner C S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Van Cutsem E, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Yoo PS, Lopez-Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer. 2006;6:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, O’Connell M, Sargent P, Piedbois P. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766-3775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Heinemann V, von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, Heintges T, Lerchenmueller J, Kahl C, Seipelt G. Randomized comparison of FOLFIRI plus cetuximab vs FOLFIRI plus bevacizumab as first-line treatment of KRAS wild-type metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3). J Clin Oncol. 2013;31 suppl:Abstr LBA3506. |
| 5. | Pericay C, Folprecht G, Saunders M, Thomas A, Roh JK, Lopez R, Höhler T, Kim JS, Zilocchi C, Boëlle E. Phase 2 Randomized, Noncomparative, Open-Label Study Of Aflibercept And Modified Folfox6 In The First-Line Treatment Of Metastatic Colorectal Cancer (Affirm). Ann Oncol. 2012;23 suppl 4:iv5-iv18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Falcone A, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Trenta P, Tomasello G, Ronzoni M, Ciuffreda L. FOLFOXIRI/bevacizumab (bev) vs FOLFIRI/bev as first-line treatment in unresectable metastatic colorectal cancer (mCRC) patients (pts): Results of the phase III TRIBE trial by GONO group. J Clin Oncol. 2013;31 suppl:Abstr 3505. |
| 7. | Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866-4875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 8. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts S. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 887] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 10. | Masi G, Vasile E, Loupakis F, Cupini S, Fornaro L, Baldi G, Salvatore L, Cremolini C, Stasi I, Brunetti I. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [PubMed] |
| 12. | Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441-9442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136-147. [PubMed] |
| 14. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [PubMed] |
| 15. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2221] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 16. | Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60-65. [PubMed] |
| 17. | Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697-3705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 656] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 18. | Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, Jonker D, Osborne S, Andre N, Waterkamp D. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 474] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 19. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7723] [Article Influence: 367.8] [Reference Citation Analysis (1)] |
| 20. | Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779-4786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 598] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 21. | Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26:689-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 23. | Sobrero A, Ackland S, Clarke S, Perez-Carrión R, Chiara S, Gapski J, Mainwaring P, Langer B, Young S. Phase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancer. Oncology. 2009;77:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Köhne CH, Hofheinz R, Mineur L, Letocha H, Greil R, Thaler J, Fernebro E, Gamelin E, Decosta L, Karthaus M. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol. 2012;138:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3120] [Article Influence: 195.0] [Reference Citation Analysis (1)] |
| 26. | Stintzing S, Fischer von Weikersthal L, Decker T, Vehling-Kaiser U, Jäger E, Heintges T, Stoll C, Giessen C, Modest DP, Neumann J. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23:1693-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2267] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 28. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 999] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 29. | Díaz-Rubio E, Gómez-España A, Massutí B, Sastre J, Abad A, Valladares M, Rivera F, Safont MJ, Martínez de Prado P, Gallén M. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist. 2012;17:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky G, Mainwaring P. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol. 2012;30:3588-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 411] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 32. | Schmiegel W, Reinacher-Schick A, Arnold D, Kubicka S, Freier W, Dietrich G, Geißler M, Hegewisch-Becker S, Tannapfel A, Pohl M. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: a randomized phase II study of the AIO colorectal study group. Ann Oncol. 2013;24:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Oliner K, Douillard J, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Analysis of KRAS/NRAS and BRAF mutations in the phase III PRIME study of panitumumab (pmab) plus FOLFOX vs FOLFOX as first-line treatment (tx) for metastatic colorectal cancer (mCRC). J Clin Oncol. 2013;31 suppl:Abstr 3511. |
| 34. | Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 35. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (2)] |
| 36. | Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 420] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 37. | Moosmann N, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, Dietzfelbinger H, Oruzio D, Klein S, Zellmann K, Decker T. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104--a randomized trial of the German AIO CRC study group. J Clin Oncol. 2011;29:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Wen F, Tang R, Sang Y, Li M, Hu Q, Du Z, Zhou Y, Zhang P, He X, Li Q. Which is false: oxaliplatin or fluoropyrimidine? An analysis of patients with KRAS wild-type metastatic colorectal cancer treated with first-line epidermal growth factor receptor monoclonal antibody. Cancer Sci. 2013;104:1330-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Primrose J, Falk S, Finch-Jones M, Valle J, Sherlock D, Hornbuckle J, Gardner-Thorpe J, Smith D, Imber C, Hickish T. A randomized clinical trial of chemotherapy compared to chemotherapy in combination with cetuximab in k-RAS wild-type patients with operable metastases from colorectal cancer: The new EPOC study. J Clin Oncol. 2013;31 suppl:Abstr 3504. |
| 40. | De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 584] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 41. | Tejpar S, Celik I, Schlichting M, Sartorius U, Bokemeyer C, Van Cutsem E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012;30:3570-3577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 42. | Gajate P, Sastre J, Bando I, Alonso T, Cillero L, Sanz J, Caldés T, Díaz-Rubio E. Influence of KRAS p.G13D mutation in patients with metastatic colorectal cancer treated with cetuximab. Clin Colorectal Cancer. 2012;11:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, Wiezorek J. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 44. | Segelov E, Wilson K, Gebski V, Waring P, Tabernero J, Wasan H, Ciardiello F, Desai J, Underhill C, Karapetis C. ICE CREAM: Irinotecan cetuximab evaluation and the cetuximab response evaluation among patients with G13D mutation. J Clin Oncol. 2013;31 suppl:Abstr TPS3649. |
| 45. | Schwartzberg L, Rivera F, Karthaus M, Fasola G, Canon J, Yu J, Go W. PEAK (study 20070509): A randomized phase II study of mFOLFOX6 with either panitumumab (pmab) or bevacizumab (bev) as first-line treatment (tx) in patients (pts) with unresectable wild-type (WT) KRAS metastatic colorectal cancer (mCRC). J Clin Oncol. 2012;30 suppl 34:Abstr 446. |
| 46. | Schwartzberg L, Rivera F, Karthaus M, Fasola G, Canon J, Yu J, Oliner K, Go W. Analysis of KRAS/NRAS mutations in PEAK: A randomized phase II study of FOLFOX6 plus panitumumab (pmab) or bevacizumab (bev) as first-line treatment (tx) for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC). J Clin Oncol. 2013;31 suppl:Abstr 3631. |
| 47. | Stein A, Atanackovic D, Hildebrandt B, Stuebs P, Steffens C, Brugger W, Hapke G, Illerhaus G, Bluemner E, Bokemeyer C. FOLFOXIRI plus bevacizumab (BEV) in patients (pts) with previously untreated metastatic colorectal cancer (mCRC): Preliminary safety results from the OPAL study. J Clin Oncol. 2012;30 suppl 34:Abstr 515. |
| 48. | Gruenberger T, Bridgewater JA, Chau I, Alfonso PG, Rivoire M, Lasserre S, Waterkamp D, Adam R. Randomized, phase II study of bevacizumab with mFOLFOX6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: Resectability and safety in OLIVIA. J Clin Oncol. 2013;31:(abstr 3619). |
| 49. | Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 50. | Hanna N, Onukwugha E, Bikov K, Zheng Z, Seal B, Mullins C. Comparative analysis of second and subsequent chemotherapy lines on short- and long-term survival of elderly Medicare metastatic colon cancer patients. J Clin Oncol. 2012;30 suppl 34:Abstr 455. |
| 51. | Garufi C, Torsello A, Tumolo S, Ettorre GM, Zeuli M, Campanella C, Vennarecci G, Mottolese M, Sperduti I, Cognetti F. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer. 2010;103:1542-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 52. | Assenat E, Desseigne F, Thezenas S, Viret F, Mineur L, Kramar A, Samalin E, Portales F, Bibeau F, Crapez-Lopez E. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist. 2011;16:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 1283] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 54. | Hecht JR, Patnaik A, Berlin J, Venook A, Malik I, Tchekmedyian S, Navale L, Amado RG, Meropol NJ. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007;110:980-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 55. | Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1481] [Article Influence: 82.3] [Reference Citation Analysis (1)] |
| 56. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1469] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 57. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2282] [Cited by in RCA: 2199] [Article Influence: 104.7] [Reference Citation Analysis (1)] |
| 58. | Recchia F, Saggio G, Nuzzo A, Lalli A, Lullo LD, Cesta A, Rea S. Multicentre phase II study of bifractionated CPT-11 with bimonthly leucovorin and 5-fluorouracil in patients with metastatic colorectal cancer pretreated with FOLFOX. Br J Cancer. 2004;91:1442-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Bidard FC, Tournigand C, André T, Mabro M, Figer A, Cervantes A, Lledo G, Bengrine-Lefevre L, Maindrault-Goebel F, Louvet C. Efficacy of FOLFIRI-3 (irinotecan D1,D3 combined with LV5-FU) or other irinotecan-based regimens in oxaliplatin-pretreated metastatic colorectal cancer in the GERCOR OPTIMOX1 study. Ann Oncol. 2009;20:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 894] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 61. | Masi G, Loupakis F, Salvatore L, Cremolini C, Fornaro L, Schirripa M, Granetto C, Miraglio E, Di Costanzo F, Antonuzzo L. Second-line chemotherapy (CT) with or without bevacizumab (BV) in metastatic colorectal cancer (mCRC) patients (pts) who progressed to a first-line treatment containing BV: Updated results of the phase III “BEBYP” trial by the Gruppo Oncologico Nord Ovest (GONO). J Clin Oncol. 2013;31 suppl:Abstr 3615. |
| 62. | Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1023] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 63. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 757] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 64. | Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 710] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 65. | Cohn A, Hecht J, Dakhil S, Saleh M, Piperdi B, Cline-Burkhardt V, Tian U, Go W. SPIRITT (study 20060141): A randomized phase II study of FOLFIRI with either panitumumab (pmab) or bevacizumab (bev) as second-line treatment (tx) in patients (pts) with wild-type (WT) KRAS metastatic colorectal cancer (mCRC). J Clin Oncol. 2013;31 suppl:Abstr 3616. |
| 66. | Vincenzi B, Santini D, Rabitti C, Coppola R, Beomonte Zobel B, Trodella L, Tonini G. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: a single centre phase II trial. Br J Cancer. 2006;94:792-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Ciardiello F, Maiello E, Pisconti S, Giuliani F, Barone C, Rizzo M, Bordonaro R, Montesarchio V, Cinieri S, Martinelli E. Optimal treatment strategy in KRAS wild type (wt) metastatic colorectal cancer (mCRC): Cetuximab plus FOLFIRI followed by FOLFOX4 with or without cetuximab-The Capri trial from the Gruppo Oncologico Dell’Italia Meridionale (GOIM). J Clin Oncol. 2013;31 suppl:Abstr e14565. |
| 68. | Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1724] [Article Influence: 95.8] [Reference Citation Analysis (1)] |
| 69. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2108] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 70. | Martín-Martorell P, Roselló S, Rodríguez-Braun E, Chirivella I, Bosch A, Cervantes A. Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: results of a phase II single institution trial. Br J Cancer. 2008;99:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |