INTRODUCTION
Hepatic artery pseudoaneurysms (HAPs) are false aneurysms formed when a tear of a hepatic arterial wall leads to a peri-artery hematoma and HAPs can be caused by medical procedures, trauma, inflammatory or infectious conditions[1]. Though well described, HAPs are rare occurrences[2-4], even more so when caused by a liver biopsy and hence few liver biopsy related HAPs have been reported in the literature[5-8]. HAPs most commonly present with abdominal pain, hematemesis, anemia, hypovolemia and jaundice[9,10]. In spite of being rare, HAPs can be a deadly complication if not diagnosed and can lead to massive gastrointestinal (GI) bleeding from hemobilia and aortoenteric fistulas[4].
Diagnosis of HAPs require a high index of suspicion especially after iatrogenic procedures, and angiography should be performed when HAPs are suspected[4]. At present, the most effective treatment for HAPs are endovascular embolization, with rare instances of surgeries performed when embolization fails[1,7,11-14]. We report on a patient with a small HAP following liver biopsy that was initially undetectable by angiography and had been increasingly symptomatic for three months before being detected by angiography. This was treated successfully with endovascular embolization.
CASE REPORT
A 43-year-old woman presented with right upper quadrant (RUQ) pain and hematemesis. She had a past medical history of hypertension, hematemesis one year ago from an endoscopically proven Mallory-Weiss tear and sarcoidosis for several years involving her lungs and liver. A month prior, she had a percutaneous liver biopsy for persistent elevation of her alkaline phosphatase (ALP). The pathology findings were consistent with hepatic sarcoidosis. Subsequently, she was admitted to a local hospital for epigastric pain and hematemesis and was diagnosed by upper endoscopy with a Mallory-Weiss tear. She was treated conservatively and was discharged after a day of observation.
Upon admission, her hemoglobin (Hgb) was 99 g/L, aspartate aminotransferase (AST) 360 IU/L, alanine aminotransferase (ALT) 299 IU/L, ALP 532 IU/L, and total bilirubin (T-BILI) 2.7 mg/dL (direct bilirubin 1.9 mg/dL). Ultrasonography showed heterogenous echogenic material in her gallbladder suggestive of biliary sludge, stones or hemorrhage. Magnetic resonance cholangiopancreatography (MRCP) showed nonenhancing material in the gallbladder with no biliary ductal dilation. Endoscopic retrograde cholangiopancreatography (ERCP) revealed blood draining from the papilla indicative of hemobilia. Hepatic angiography showed no source for hemobilia. The patient’s symptoms resolved, her Hgb remained stable and liver enzymes improved. She was discharged to follow-up for a possible elective cholecystectomy.
Three weeks later she presented with RUQ pain and hematemesis. ERCP demonstrated non-bleeding erythematous gastropathy and a normal cholangiogram. MRCP showed heterogenous nonenhancing material within the gallbladder, likely representing blood. Laparoscopic cholecystectomy was performed, her symptoms and liver enzymes improved before discharge.
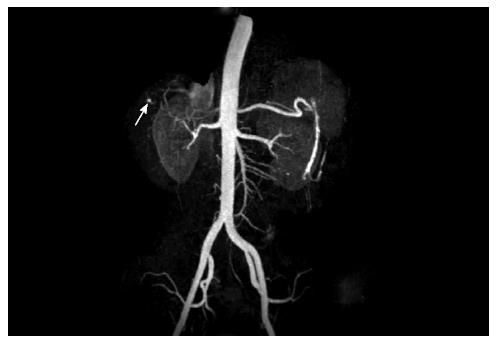
After one week, she re-presented with intermittent hematochezia. She reported taking nonsteroidal antiinflammatory drugs (NSAIDs) for intermittent RUQ discomfort and recently discontinuing pantoprazole. She was afebrile and hemodynamically stable. Her Hgb was 66 g/L, AST 93 IU/L, ALT 137 IU/L, ALP 716 IU/L, T-BILI 1.2 mg/dL. Colonoscopy showed clotted blood in the colon without any bleeding lesions and an upper endoscopy revealed an oozing gastric ulcer that was clipped with successful hemostasis. She was discharged and one week later she presented with severe RUQ pain with fever and chills, but without an overt GI bleed. Computed tomography demonstrated a wedge shaped region of low attenuation in the periphery of segments VI and VII of the liver, suggestive of segmental hepatic infarction. Magnetic resonance imaging (MRI) and angiography (MRA) demonstrated early abscesses within segments VI and VII, with patent hepatic arteries and portal vein branches. The aspirate showed Gram-positive cocci. She was started on antibiotics with an improvement of her symptoms and was subsequently discharged. Abdominal MRI after 1 week revealed improvement in the size of the abscesses, but showed multiple filling defects within the common bile duct (CBD) with mild enhancement of the CBD. She presented with RUQ pain and hematemesis, with elevated liver enzymes, but repeat ERCP was unremarkable. MRA revealed a small 5-mm intrahepatic pseudoaneurysm arising from the superior branch of the anterior division of the right hepatic artery (RHA) with a hypoattenuated lesion in segment VII, suggestive of infarction in the territory supplied by the RHA branch. This was confirmed by subsequent angiography (Figure 1). In retrospect, the review of the prior MRA revealed a less than a 1-mm pseudoaneurysm in the same location. Coil embolization of the pseudoaneurysm was performed with excellent angiographic result. One month later, MRI showed marked improvement in the size and appearance of the abscesses. The patient continued to do well with no further abdominal pain, GI bleed or any rise in her liver enzymes for one and a half years after the embolization.
Figure 1 Angiography findings.
A 5-mm intrahepatic pseudoaneurysm (arrow) was detected arising from the superior branch of the anterior division of the right hepatic artery.
DISCUSSION
HAPs are rare conditions[2-4] and are usually due to iatrogenic causes, including liver biopsies, cholecystectomy, transhepatic biliary drainage, and inadvertent surgical injuries[8,15-17]. Percutaneous liver biopsy (PLB) is a common and safe procedure with low mortality and morbidity rates[18,19]. There have been only a few case reports on HAPs caused by liver biopsy, but they describe larger pseudoaneurysms that are severely symptomatic shortly after the procedure[5-7]. Angiography is the most sensitive method that is available to detect HAP[4]. Our patient, however, remained increasingly symptomatic for three months before the HAP could be detected by imaging and treated successfully. A review of the literature did not reveal any case reports of HAPs as small as 1-mm causing persistent and recurrent symptoms for a prolonged course. This diminutive HAP was complicated by multiple episodes of hemobilia, hemorrhagic cholecystitis requiring cholecystectomy, and RHA thrombosis, which led to abdominal pain, elevation of liver enzymes, and hepatic infarction complicated by liver abscess formation.
This case demonstrates that a small HAP can avoid detection by angiography at an early stage. A high clinical suspicion and close clinical/radiological follow up is needed in symptomatic patients with history of liver biopsy despite an initial negative work up. In spite of the rarity of HAPs, the high prevalence of liver biopsies and the severity of the consequences of not detecting them make the recognition of this complication crucial in clinical practice.
Therapeutic modalities to treat HAPs include open surgery and endovascular approach. In light of the rarity of the disease, there have been no randomized comparisons between the two approaches. Open surgical repair is usually associated with complications such as intra-abdominal infection and hepatobiliary diseases, leading to higher morbidity and mortality associated with this approach[20]. Therefore, the endovascular approach has become the first line treatment for HAPs, leaving surgical repair as the salvage therapy only if the former fails[21]. The most commonly used endovascular technique is the endovascular ablation of the proximal feeding artery of the HAP either by coil or glue[14]. After ablation therapy, patients should be closely monitored for sac reperfusion and any potential end-organ ischemia that responds to a repeat endovascular treatment[22]. However, the risk of end-organ damage is clinically significant only if the embolization procedure involves major arterial supplies[23]. In these cases, stent placement of the major artery followed by coil embolization of collateral feeding arteries can be used to reduce the risk of ischemia[24].
P- Reviewers: Wang DR, Yu B S- Editor: Wen LL L- Editor: A E- Editor: Ma S