Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1371
Revised: November 3, 2013
Accepted: December 5, 2013
Published online: February 7, 2014
Processing time: 174 Days and 3.5 Hours
Microcystic/reticular schwannoma is a recently described variant of schwannoma with a predilection for the gastrointestinal tract. Due to overlapping features with other tumors, unawareness of this tumor type may lead to diagnostic and therapeutic pitfalls. We here report a case of microcystic/reticular schwannoma arising in the meso-appendix of a 43-year-old woman. The tumor was incidentally discovered by computed tomography scan for unrelated reasons. A laparoscopic operation was performed shortly after admission. Histological examination revealed a circumscribed tumor with a striking microcystic and cribriform architecture. Immunohistochemically, the tumor cells were diffusely positive for S100 protein, glial fibrillary acid protein and protein gene product 9.5, which were consistent with a peripheral nerve sheath tumor. The patient remains well with no signs of recurrence at a 10-mo follow-up. To our knowledge, this is the first case of microcystic/reticular schwannoma arising in the meso-appendix. Albeit very rare, microcystic/reticular schwannoma should be included in the differential diagnosis of appendiceal tumors.
Core tip: Microcystic/reticular schwannoma is a recently described variant of schwannoma with a predilection for the gastrointestinal (GI) tract. The striking reticular growth pattern and myxoid background may cause confusion with several other tumors commonly seen in the GI tract, in particular a gastrointestinal stromal tumor with prominent myxoid change and a signet ring cell carcinoma, especially on small biopsies. Herein we report for the first time a microcystic/reticular schwannoma arising primarily in the meso-appendix to highlight its existence and enhance pathologist’s and clinician’s awareness of this under-recognized variant of schwannoma so as to avoid misdiagnosis and mistreatment.
- Citation: Tang SX, Sun YH, Zhou XR, Wang J. Bowel mesentery (meso-appendix) microcystic/reticular schwannoma: Case report and literature review. World J Gastroenterol 2014; 20(5): 1371-1376
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1371.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1371
Schwannoma usually arises in the subcutaneous tissue of the distal extremities or the head and neck region of adult patients with no sex predilection. Occasionally, the tumor may also involve a wide variety of anatomic sites, including the mediastinum, gastrointestinal tract, retroperitoneum, spinal cord, cerebellopontine angle and bone. Apart from those arising in the setting of neurofibromatosis type 2 (NF2), most schwannomas are solitary and sporadic lesions, although a small percentage of cases are associated with von Recklinghausen’s disease. The typical schwannoma is characterized by a biphasic pattern composed of alternating Antoni A and Antoni B areas. In addition to the classic type, approximately 11 morphological variants have been recognized. These variants include ancient (degenerated) schwannoma[1], cellular schwannoma[2], plexiform schwannoma[3], melanotic schwannoma[4,5], epithelioid schwannoma[6], hybrid schwannoma/neurofibroma[7], hybrid schwannoma/perineurioma[8], gastrointestinal schwannoma[9], neuroblastoma-like schwannoma[10], lipoblastic schwannoma[11], and microcystic/reticular schwannoma[12]. Frankly speaking, these variants simply represent the morphological heterogeneity in schwannoma with no distinct relationship to the clinical behavior. Nevertheless, unawareness of these rare variants will lead to diagnostic pitfalls and risk of mistreatment of the patients. We present here a case of microcystic/reticular schwannoma arising in the vermiform appendix, a unique site that has not been described before. The clinical and pathological features of all 13 cases of microcystic/reticular schwannoma originated in the gastrointestinal tract were summarized.
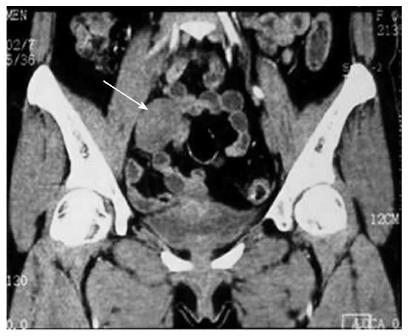
A 43-year-old woman presented with a pelvic mass which was incidentally discovered by computed tomography (CT) scan during a check-up for mild abdominal pain. On physical examination, the abdomen was soft and flat with no tenderness on deep palpation and no palpable mass could be identified. However, CT scan revealed an isodense mass in the right lower quadrant, measuring 4.5 cm × 3.4 cm × 4.6 cm in size (Figure 1). Slow and persistent enhanced signal was observed following administration of contrast. Her medical history was unremarkable. There was no clinical manifestation of either neurofibromatosis type 1 (NF1) or type 2 (NF2). With a suspicion of a gastrointestinal stromal tumor (GIST) or a leiomyoma, the patient was admitted for scheduled surgery. During laparoscopic operation, a solid well-circumscribed mass was found in the distal end of the meso-appendix, measuring approximately 4 cm in diameter. The patient recovered uneventfully after surgery and is now well with no evidence of recurrence at a 10-mo follow-up.
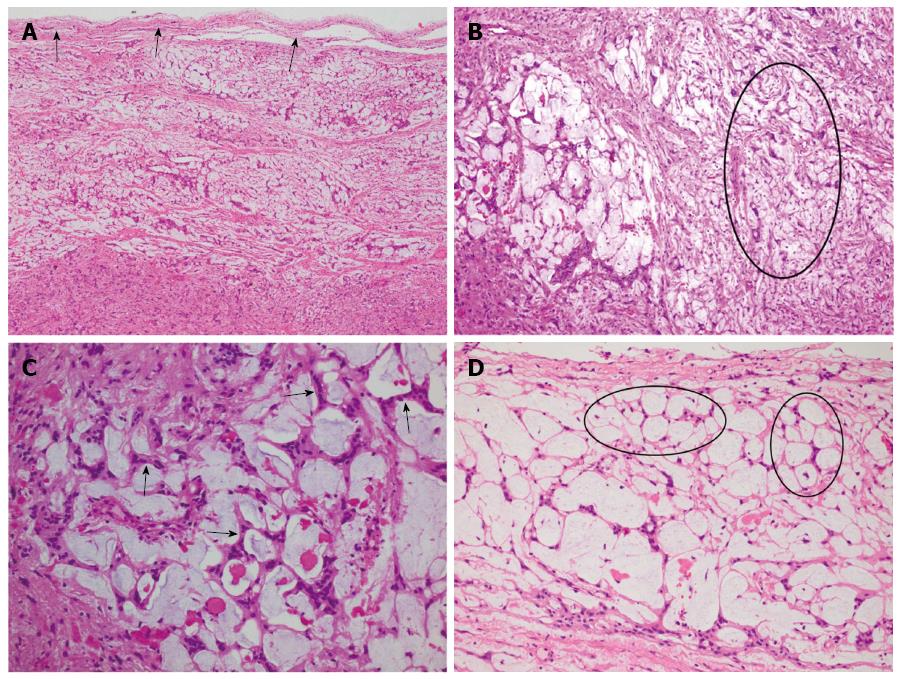
The submitted specimen consisted of white to gray fragmentary tissues measuring 4.0 cm × 4.0 cm × 1.9 cm in volume. It had a homogeneous gelatinous texture, showing no features of necrosis, hemorrhage or cystic degeneration. At scanning magnification, the tumor was well circumscribed and surrounded by a thin fibrous capsule (Figure 2A). It was composed of relatively alternating fibrillary and myxoid areas (Figure 2B). There was transition between these two areas. Tumor cells were spindle-shaped with eosinophilic cytoplasm and ovoid or tapered nuclei harboring small inconspicuous nucleoli. They had generally bland appearance without nuclear pleomorphism or degenerative atypia. Mitotic figure was scarce with less than 3/50 HPF. In fibrillary areas, the tumor cells were generally arranged in irregular fascicles with no palisading or Verocay body formation, whereas in myxoid areas, they were arranged in anastomosing or intersecting strands creating a striking lace-like or microcystic growth pattern (Figures 2C and D). In focal areas, remarkable perivascular lymphocytic aggregates were present.
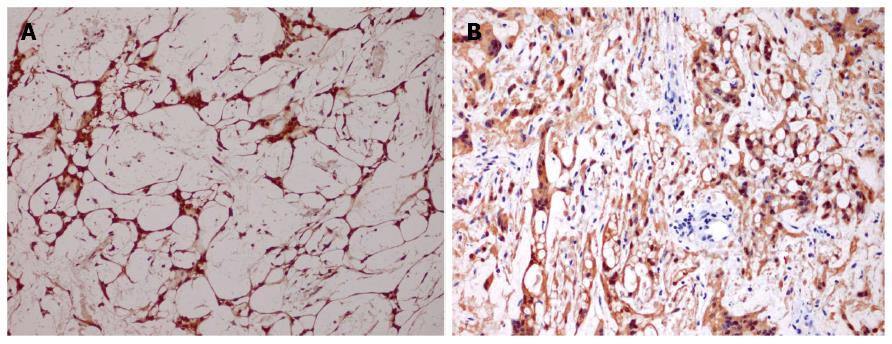
Immunohistochemistry was performed on paraffin-embedded sections using the standard EnVision method. The tumor cells showed diffuse and strong nuclear and cytoplasmic staining of S100 protein (Figure 3A). They were also positive for glial fibrillary acid protein (GFAP) and protein gene product 9.5 (PGP9.5) (Figure 3B). Focal staining was also found with CD117. MIB-1 showed low proliferative activity with index less than 1%. A few CD34 positive cells were observed among the stromal cells, indicating the presence of fibroblast or perineurial cells within the tumor. Tumor cells were all negative for CD57, DOG1, desmin, alpha smooth muscle actin, pancytokeratin (AE1/AE3), epithelial membrane antigen (EMA), synaptophysin, CD31 and P53.
We did sequencing analysis of exons 9, 11, 13, and 17 of c-KIT gene to exclude the possibility of GIST. Polymerase chain reaction assays were carried out using oligonucleotide primer pairs as previously described. Direct sequencing was performed on the ABI Prism 310 DNA sequencer. The result demonstrated that the tumor did not harbor c-KIT gene mutation.
Microcystic/reticular schwannoma is a rare variant of schwannoma. Including the current case, only 22 cases have been reported in the literature[12-21]. Due to its rarity, this tumor type has remained under-recognized. As there are overlapping features with a wide variety of neoplasms, this tumor type may be misdiagnosed and potentially lead to mistreatment of the patients. Therefore, enhanced awareness of its characteristic features is imperative for pathologists to avoid diagnostic pitfalls. To enhance the recognition, we present here an additional case of microcystic/reticular schwannoma and undertake a brief review of the literature.
Taking the current case into account, there are 13 cases of microcystic/reticular schwannoma arising primarily in the gastrointestinal tract, accounting more than a half of all cases. The overall median and average ages of patients with gastrointestinal microcystic/reticular schwannoma at diagnosis were 68 years and 66 years, respectively (range, 32-93 years). There was a predilection for female patients, with a male/female ratio of 1:3.3 (Table 1). Clinically, the majority of patients presented with an asymptomatic mass discovered incidentally by imaging examinations during routine check-up or at operation for other unrelated reasons. A few patients complained of indigestion, bowel habit change or epigastric pain[13,16]. Occasionally, the tumor appeared as an intestinal polypoid lesion at colonoscopy[12,14]. To date, none has clinical evidence of NF1 or NF2. The tumors ranged in size from 0.4 to 4.0 cm (median, 1.3 cm; mean, 1.5 cm). With regard to the site, 4 cases occurred in the colon, 3 cases in the small intestine, 2 cases in the cecum, 2 cases in the stomach, and 1 case each in the rectum and meso-appendix.
| Ref. | Sex/age (yr) | Location | Size (cm) | Symptoms | Gross appearance | Outcome (mo) |
| Liegl et al[12] | F/73 | Rectum | 0.85 | Asymptomatic | Circumscribed, not encapsulated | Died at 361 |
| Liegl et al[12] | F/72 | Stomach | 2.0 | Asymptomatic | Circumscribed, not encapsulated | ANED at 24 |
| Liegl et al[12] | M/68 | Cecum | 0.4 | Asymptomatic | Circumscribed with focal infiltration | ANED at 24 |
| Liegl et al[12] | F/93 | Jejunum | 1.6 | Asymptomatic | Circumscribed, not encapsulated | ANED at 7 |
| Liegl et al[12] | M/78 | Small intestine | 0.8 | Asymptomatic | Circumscribed with focal infiltration | “Recent case” |
| Lee et al[13] | F/32 | Ascending colon | 1.4 | Bowel habit change | Circumscribed with focal infiltration | UA |
| Agaimy et al[14] | F/67 | Cecum | 1.0 | A polyp at colonoscopy | Circumscribed with focal infiltration | ANED at 12 |
| Agaimy et al[14] | F/67 | Mid-jejunum | 2.2 | Incidental finding | UA | ANED at 2 |
| Kienemund et al[15] | F/70 | Sigmoid colon | 0.7 | UA | Circumscribed, not encapsulated | UA |
| Kienemund et al[15] | F/70 | Sigmoid colon | 1.3 | UA | UA | UA |
| Chetty et al[16] | F/63 | Stomach | 1.9 | Epigastric pain | Circumscribed, not encapsulated | ANED at 60 |
| Trivedi et al[18] | M/61 | Sigmoid colon | 0.7 | Incidental finding | Circumscribed, not encapsulated | ANED at 24 |
| Our case | F/43 | Meso-appendix | 4.0 | Incidental finding | Circumscribed and encapsulated | ANED at 10 |
Except for the 4 tumors showing focal infiltration between smooth muscle fibers or extending into the mucosa[12-14], the other gastrointestinal microcystic/reticular schwannomas were all well circumscribed. On histology, microcystic/reticular schwannoma differs from a classic schwannoma in many ways. The former lacks the distinctive features that are typically noted in a classic schwannoma, namely alternating areas of Antoni A and Antoni B, presence of palisading or Verocay bodies, aggregates of foamy histiocytes, and hyalinized blood vessels. The hallmark of microcystic/reticular schwannoma is the presence of a striking reticular and microcystic architecture, a feature not observed in any other variants of schwannoma.
Regardless of the preferential location in the gastrointestinal tract, microcystic/reticular schwannoma is different from another variant of so-called gastrointestinal schwannoma[9]. Gastrointestinal schwannoma is relatively more common than microcystic/reticular schwannoma. This type of schwannoma tends to occur in the stomach, although intestines can be occasionally involved. Histologically, it is composed of spindled Schwann cells displaying frequently a microtrabecular or microfascicular pattern and characterized by a peritumoral lymphocytic cuff. Although focal myxoid change can be observed in a few gastrointestinal schwannomas, prominent microcystic pattern is never seen.
Besides variants of schwannoma, there are other types of benign peripheral nerve sheath tumor occurring in the gastrointestinal tract which may cause confusion among them. These tumors include perineurioma and hybrid schwannoma/perineurioma. Perineurioma is composed of bland spindle cells with long bipolar cytoplasmic processes embedded in fine collagenous stroma which sometimes appears myxoid[22]. The typical feature of “pseudo-onion bulb” arrangement of tumor cells around a central axon will help arrive at a correct diagnosis. However, the reticular variant of perineurioma may present diagnostic dilemma. This unusual morphologic variant occurs preferentially in the soft tissue with no case of gastrointestinal origin documented[23]. Immunostaining with a panel of antibodies including EMA, claudin-1, S100 protein and GFAP will facilitate the differential diagnosis. Hybrid schwannoma/perineurioma is a benign tumor consisting of intimately mixed components of plump spindled Schwann cells and slender perineurial cells. This novel type of peripheral nerve sheath tumor can also occur in the gastrointestinal tract[8]. However, tumor cells are frequently arranged in a storiform or fascicular pattern and the stroma rarely undergoes myxoid change. Double immunostaining of S100 protein and EMA can clearly highlight the two components.
Occasionally, GIST may have myxoid change. Although the majority of GIST arise in stomach and intestines, involvement of appendix is not uncommon[24]. Indeed, it has been acclaimed that GIST represents the most common type of appendiceal mesenchymal tumors[25]. As the treatment varies greatly, a distinction between GIST and microcystic/reticular schwannoma is warranted. Appendiceal GISTs are usually incidental findings. Some cases may masquerade as appendicitis. Microscopically, they are indolent tumors composed of spindle cells with prominent extracellular collagen globules, known as skeinoid fibers. Immunohistochemically, appendiceal GISTs are ubiquitously positive for CD117 and DOG1, whereas the staining of S100 protein is consistently negative. It is worthy to note that focal immunoreactivity of CD117 can be observed in a minority of microcystic/reticular schwannoma. However, the absence of KIT or PDGFRA gene mutation denies the diagnosis of GIST.
As abovementioned, most cases of microcystic/reticular schwannoma were well circumscribed. However, a few tumors showed focal infiltration between the smooth muscle fibers of the muscularis mucosa, between the colonic crypts, or extended into the mucosa[12-14]. The epithelioid morphology, signet ring cell appearance of the tumor cells in some cases together with a myxoid background may cause confusion with poorly differentiated adenocarcinoma or signet ring cell carcinoma[12,18]. Of note, the so-called signet ring cell gastric schwannoma described by Tozbikian et al[26] in 2008, in our opinion, represents a morphologic spectrum of microcystic/reticular schwannoma. Erroneous diagnosis of a benign microcystic/reticular schwannoma as a malignant signet ring cell carcinoma will lead to inappropriate treatment of the patients. The absence of nuclear atypia and negativity for epithelial markers allow the differentiation of microcystic/reticular schwannomas from carcinomas.
In summary, we reported for the first time a microcystic/reticular schwannoma arising primarily in the vermiform appendix. Although very rare, microcystic/reticular schwannoma represents a unique and distinctive morphological variant of schwannoma with a benign clinical course. Enhanced awareness of its characteristic features will facilitate the differential diagnosis from a wide variety of neoplasm with overlapping features.
The patient was incidentally found to have a pelvic mass by computed tomography (CT) scan during a check- up for mild abdominal pain.
The characteristic features in immunohistochemistry may facilitate the differentiation of microcystic/reticular schwannoma from a variety of tumors with overlapping features.
Immunohistochemically, the tumor cells showed diffuse and strong nuclear and cytoplasmic staining of S100 protein and glial fibrillary acid protein (GFAP), compatible with a peripheral nerve sheath tumor.
CT scan revealed an isodense mass in the right lower quadrant which was suspected to be a gastrointestinal stromal tumor (GIST) or a leiomyoma.
The well-circumscribed tumor was composed of relatively alternating fibrillary and myxoid areas, creating a distinctive lacelike and microcystic growth pattern.
At laparoscopic surgery, the solid mass of the meso-appendix was excised and no adjuvant treatment was applied after surgery.
Thirteen cases of gastrointestinal microcystic/reticular schwannoma have been reported, most of which are located in the wall of the gastroenterologic tract. This is a first case of a microcystic/reticular schwannoma arising primarily in the vermiform appendix.
Although termed as a schwannoma, the tumor lacks the distinctive features that are typically noted in a classic schwannoma, such as alternating areas of Antoni A and Antoni B, presence of palisading or Verocay bodies, aggregates of foamy histiocytes, and hyalinized blood vessels. The hallmark of this rare variant is the presence of a striking reticular and microcystic architecture which may cause confusion with other neoplasms.
This case was initially considered to be a GIST. However, the strong immunostaining of S100 protein and GFAP suggested a peripheral nerve sheath tumor. The striking microcystic/reticular arrangement of the tumor cells helped to recognize the lesion as a special variant of schwannoma which was recently described.
This article represents the first report of a microcystic/reticular schwannoma arising primarily in the vermiform appendix. Increased awareness of this special variant of schwannoma may help to avoid diagnostic pitfalls. More case reports are needed to further expand the clinical spectrum of the disease.
P- Reviewers: Roeb E, Sipos F S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Dahl I. Ancient neurilemmoma (schwannoma). Acta Pathol Microbiol Scand A. 1977;85:812-818. [PubMed] |
| 2. | Casadei GP, Scheithauer BW, Hirose T, Manfrini M, Van Houton C, Wood MB. Cellular schwannoma. A clinicopathologic, DNA flow cytometric, and proliferation marker study of 70 patients. Cancer. 1995;75:1109-1119. [PubMed] |
| 3. | Kao GF, Laskin WB, Olsen TG. Solitary cutaneous plexiform neurilemmoma (schwannoma): a clinicopathologic, immunohistochemical, and ultrastructural study of 11 cases. Mod Pathol. 1989;2:20-26. [PubMed] |
| 4. | Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol. 1990;14:206-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 223] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Font RL, Truong LD. Melanotic schwannoma of soft tissues. Electron-microscopic observations and review of literature. Am J Surg Pathol. 1984;8:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Kindblom LG, Meis-Kindblom JM, Havel G, Busch C. Benign epithelioid schwannoma. Am J Surg Pathol. 1998;22:762-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Feany MB, Anthony DC, Fletcher CD. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: a conceptual challenge. Histopathology. 1998;32:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Yang X, Zeng Y, Wang J. Hybrid schwannoma/perineurioma: report of 10 Chinese cases supporting a distinctive entity. Int J Surg Pathol. 2013;21:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hou YY, Tan YS, Xu JF, Wang XN, Lu SH, Ji Y, Wang J, Zhu XZ. Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology. 2006;48:536-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Fisher C, Chappell ME, Weiss SW. Neuroblastoma-like epithelioid schwannoma. Histopathology. 1995;26:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Plaza JA, Wakely PE, Suster S. Lipoblastic nerve sheath tumors: report of a distinctive variant of neural soft tissue neoplasm with adipocytic differentiation. Am J Surg Pathol. 2006;30:337-344. [PubMed] |
| 12. | Liegl B, Bennett MW, Fletcher CD. Microcystic/reticular schwannoma: a distinct variant with predilection for visceral locations. Am J Surg Pathol. 2008;32:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Lee SM, Goldblum J, Kim KM. Microcystic/reticular schwannoma in the colon. Pathology. 2009;41:595-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Agaimy A, Märkl B, Kitz J, Wünsch PH, Arnholdt H, Füzesi L, Hartmann A, Chetty R. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch. 2010;456:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Kienemund J, Liegl B, Siebert F, Jagoditsch M, Spuller E, Langner C. Microcystic reticular schwannoma of the colon. Endoscopy. 2010;42 Suppl 2:E247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Chetty R. Reticular and microcystic schwannoma: a distinctive tumor of the gastrointestinal tract. Ann Diagn Pathol. 2011;15:198-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Liegl B, Bodo K, Martin D, Tsybrovskyy O, Lackner K, Beham A. Microcystic/reticular schwannoma of the pancreas: a potential diagnostic pitfall. Pathol Int. 2011;61:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Trivedi A, Ligato S. Microcystic/reticular schwannoma of the proximal sigmoid colon: case report with review of literature. Arch Pathol Lab Med. 2013;137:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Lau PP, Yau DT, Lau WH, Mak LS, Chan JK. Multinodular reticular schwannoma in the head and neck region: a potential diagnostic pitfall. Int J Surg Pathol. 2013;21:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Li BZ, Wang JW, Wei HQ. Microcystic/reticular schwannoma occurring in cervical spine: report of a case with literature review. Zhonghua Binglixue Zazhi. 2010;39:396-399. [PubMed] |
| 21. | Pang JM, Mahar A, Shannon K, Kench J, Chan C, Gupta R. Reticular and microcystic schwannoma of the parotid gland. Pathology. 2013;45:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Hornick JL, Fletcher CD. Intestinal perineuriomas: clinicopathologic definition of a new anatomic subset in a series of 10 cases. Am J Surg Pathol. 2005;29:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Graadt van Roggen JF, McMenamin ME, Belchis DA, Nielsen GP, Rosenberg AE, Fletcher CD. Reticular perineurioma: a distinctive variant of soft tissue perineurioma. Am J Surg Pathol. 2001;25:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Agaimy A, Pelz AF, Wieacker P, Roessner A, Wünsch PH, Schneider-Stock R. Gastrointestinal stromal tumors of the vermiform appendix: clinicopathologic, immunohistochemical, and molecular study of 2 cases with literature review. Hum Pathol. 2008;39:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 26. | Tozbikian G, Shen R, Suster S. Signet ring cell gastric schwannoma: report of a new distinctive morphological variant. Ann Diagn Pathol. 2008;12:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |