Published online Feb 7, 2014. doi: 10.3748/wjg.v20.i5.1248
Revised: November 5, 2013
Accepted: December 12, 2013
Published online: February 7, 2014
Processing time: 167 Days and 10.4 Hours
The inflammatory bowel diseases (IBDs) are chronic incurable conditions that primarily present in young patients. Being incurable, the IBDs may be part of the patient’s life for many years and these conditions require therapies that will be effective over the long-term. Surgery in Crohn’s disease does not cure the disease with endoscopic recurrent in up to 70% of patients 1 year post resection. This means that, the patient will require many years of medications and the goal of the treating physician is to induce and maintain long-term remission without side effects. The development of the anti-tumour necrosis factor alpha (TNFα) agents has been a magnificent clinical advance in IBD, but they are not always effective, with loss of response overtime and, at times, discontinuation is required secondary to side effects. So what options are available if of the anti-TNFα agents can no longer be used? This review aims to provide other options for the physician, to remind them of the older established medications like azathioprine/6-mercaptopurine and methotrexate, the less established medications like mycophenolate mofetil and tacrolimus as well as newer therapeutic options like the anti-integins, which block the trafficking of leukocytes into the intestinal mucosa. The location of the intestinal inflammation must also be considered, as topical therapeutic agents may also be worthwhile to consider in the long-term management of the more challenging IBD patient. The more options that are available the more likely the patient will be able to have tailored therapy to treat their disease and a better long-term outcome.
Core tip: Overall the physician must keep an open mind when treating inflammatory bowel disease. These patients have a long-term incurable condition than can significantly impact on all aspects of their life. Surgery does not cure the disease and thus medications may be required for many decades in order to give the patients a decent quality of life. Both the patient and the physician, therefore, need to remember the “oldies but goodies” but also keep the door open to new innovations and novel therapies.
- Citation: Lawrance IC. What is left when anti-tumour necrosis factor therapy in inflammatory bowel diseases fails? World J Gastroenterol 2014; 20(5): 1248-1258
- URL: https://www.wjgnet.com/1007-9327/full/v20/i5/1248.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i5.1248
The chronic inflammatory bowel diseases, Crohn’s disease (CD) and ulcerative colitis (UC), are a huge challenge for the treating physician as these are life-long incurable conditions that frequently present in the 2nd or 3rd decade of life, a stage in the patient’s life where education, social integration and personal identity are key aspects being developed. There is no doubt that the anti-tumour necrosis factor alpha (TNFα) medications are efficacious in the management of both conditions[1-8] but they are, however, not a panacea as they are not effective in all patients and even in those in whom a remission is achieved, the effect may be lost over time.
The efficacy of maintenance therapy in CD with the anti-TNFα medication, certolizumab pegol, has been investigated out to 18 mo. This observed that slightly more than 60% of the original 60% of patients who responded to induction therapy continued to respond, which is encouraging[9,10]. This suggests, however, that by 18 mo only 40% of patients are still getting benefit from this medication. This is similar to the findings for adalimumab where 24% of all patients in the CHARM study remained in response at week 26[3] and after 2 years of adalimumab therapy, between 37% and 50% of these patients were in clinical remission[11]. Published data for infliximab out to 54 wk would also appear to be similar[12]. Additional long-term data, out to 4.5 years, suggest that although efficacy for certolizumab in CD is still present[13], the number of patients continuing to benefit falls with time. The problem for both the patient and physician is that the IBDs are life-long conditions and arguably the best medication options for these patients have only a limited subset of patients in whom they will be of long-term benefit.
In addition to a loss of effect over time, like all medications, there are potential side effects to the use of the anti-TNFα agents. As TNF is involved in the immune-mediated response to infection it is not unexpected that anti-TNF medications are associated with an increased risk of serious and opportunistic infections[14,15], including tuberculosis[16], Pneumocystis jirovecii pneumonia[17], and various viral, fungal and bacterial infections[18]. Deramatological side effects are also possible with new onset cutaneous eruptions observed in 20% of CD patients treated with infliximab[19], and immune-mediated cutaneous reactions seen in 11% of patients[20]. This risk is also present with the fully humanised antibody, adalimumab[21]. In addition, the potential risks of medication-induced skin cancers and lymphomas need to be considered[22].
Thus these long-term chronic inflammatory diseases, which may be part of a patient’s life anywhere from 10 and 70 years, require medications that will be effective over the long-term with minimal side effects. The development of the anti-TNFα agents has been a magnificent clinical advance in this management of these conditions, but what options are available if they lose effect or side effects necessitate cessation of the therapy?
An oldie but a goodie. We must never forget about the older medications that have stood the test of time as they are still frequently used and with more innovative thinking may be able to either enhance the effects of the anti-TNFα agents, or be a backstop if, or when, they are no longer of benefit. Through their effects on the synthesis of nucleic acids, the thiopurines reduce intracellular purine metabolism, induce T lymphocyte apoptosis, cause a reduction in the number of circulating B and T lymphocytes[23], decrease immunoglobulin synthesis[24] and reduce the production of interleukin (IL)-2[25] with the desired effect of reducing inflammation.
In many IBD centres, the measurement of thiopurine methyltransferase (TPMT) activity is frequently undertaken as this is the primary determinant of azathioprine (AZA)/6-mercaptopurine (6-MP) metabolism. For patients with moderate enzymic activity (5-12 U/mL), they are likely to achieve 6-thioguanine nucleotides (6-TGN) levels at standard drug dosing (AZA 1.5 mg/kg/6-MP 1.0 mg/kg), while patients with high enzyme activity (usually > 12 U/mL) may require higher doses than normal. TPMT activity, however, does fluctuate, and TPMT enzymic activity can be induced by AZA/6-MP therapy, while 5-aminosalicylates may cause a mild, but reversible, inhibition of TPMT activity.
The measurement of 6-TGN and 6-methylmercaptopurine (6-MMP) levels are now also frequently undertaken as these levels can correlate with the therapeutic response. A 6-TGN level of between 230-400 pmol/8 × 108 RBC has been associated with clinical response, although this data needs to be re-examined in a larger patient cohort. Of note is that 6-TGN levels > 400 pmol/8 × 108 RBC are often associated with myelosuppression, while 6-MMP levels of > 5700 pmol/8 × 108 RBC can be a cause of hepatotoxicity and other AZA/6-MP-induced side effects[26-29].
Of particular note is that the 6-TGN and 6-MMP levels can be used to determine a patient’s compliance and may indicate high TMPT activity with shunting of thiopurine metabolism towards the 6-MMP metabolite and away from 6-TGN. If shunting is observed with high 6-MMP and low 6-TGN levels, the addition of allopurinol (100 mg/d) appears to increase the activity of hypoxanthine-guanine phosphoribosyltransferase, which is the first step in the metobolism of the thiopurines to 6-TGN, resulting in increased 6-TGN levels[30-32]. If allopurinol is used then the AZA/6-MP dose must be markedly reduced, generally the author would reduce it to 25% of the original dose until rechecking of the metabolite profile[33].
If there is loss of response to anti-TNFα therapy then the combination of AZA/6-MP with the anti-TNFα agent could also be of benefit. It is now accepted that the combination of the thiopurines with the anti-TNFαs is more effective for the induction and maintenance of steroid-free remission, and mucosal healing in CD than with the use of either drug alone for up to 1 year in patients who are naïve to both agents[34,35]. The evidence for reclaiming a response to anti-TNFα therapy once lost is not clear, but it is a least a viable option for consideration. The evidence of the combined use of these agent in UC, however, is not as strong as in CD, but as there is a role for AZA/6-MP in mucosal healing, and protection against the development of colorectal cancer the combination of the two agents would again seem to be reasonable to consider[36].
Mycophenolate mofetil (MMF) is a immunosuppressing agent with similar anti-metabolite and pharmacodynamic properties to the thiopurines, which has primarily been used for preventing the rejection of solid organ transplantants. Its role as an immunosuppresant in the management of IBD has to date been fairly limited with several open labelled studies[37-39] and only a few randomized trials that have been limited by low patient numbers[40-42]. Consideration of its use in the management of difficult IBD cases, however, should not be ignored.
Most early studies investigating the use of MMF were undertaken in CD patients who had failed, or were intolerant to, AZA, and these demonstrated good efficacy[40,42,43]. Unfortunately, these findings were not always reproduced by later studies. These later studies suggested that there was both a low initial response rate as well as a high relapse rate. It was also noted that there was frequently a high medication discontinuation rate secondary to side effects[37,38,41,44,45]. Additional studies comparing the efficacy of AZA to MMF, however, observed that MMF could be more effective in AZA intolerant, rather than refractory patients, while being non-inferior to AZA in the management of UC, for the induction and maintenance of remission at 6 mo[40,46,47]. A longer-term study in a cohort of AZA resistant/intolerant patients, however, observed that although MMF was initially effective, the relapse rates were high, with the suggestion that MMF may be potentially effective but not a long-term solution[48]. There has also been suggested that the MMF dose needs to be increased over time in order to maintain an effect. This has not been the experience of the author as our data demonstrate that MMF was efficacious and well tolerated in treating refractory IBD who are intolerant to AZA/6-MP without the problems of an early disease flare, or the need for dose escalation over time[39].
As many of the studies suggest that MMF is potentially as effective as conventional immunosuppressants when these medications fail, or cannot be used due to hypersensitivity reactions including pancreatitis, then it is a potential alternative that is worthwhile for consideration[46,47]. Further evaluation of its role needs to be undertaken in larger randomized, double-blind studies comparing it to conventional immunosuppressants, however, such studies are expensive and not easy to undertake.
Methotrexate (MTX) exerts its activity at the DNA level. It inhibits the conversion of dihydrofolic acid to folinic acid, its active metabolite, through the competitive inhibition of dihydrofolate reductase. As folonic acid is required for purine and pyrimidine metabolism and amino acid synthesis, MTX alters their incorporation in the DNA and reduces cellular proliferation, increases T cell apoptosis and endogenous adenosine with alteration of the expression of cellular adhesion molecules and the production of proinflammatory cytokines. The resultant effect is a reduction on systemic inflammation.
Unfortunately, there have been only limited studies investigating MTX in IBD. The largest trial investigated the use of 25 mg/wk intramuscular (im), or placebo, in combination with 20 mg/d prednisolone[49]. At 16 wk, significantly more patients receiving MTX were in remission off steroids compared to placebo (P = 0.025), however, adverse events were significantly more common with MTX. Two other small trials examining oral MTX 15 mg/wk[50] for 3 mo compared to placebo and oral MTX 12.5 mg/wk in combination with 50 mg/d 6-MP or placebo for 9 mo[51] demonstrated no significant differences between the groups. The second study, however, used suboptimal doses of the immunomodulators, did not have well defined steroid reduction protocols and included patients with known thiopurine-resistant disease.
The use of MTX has been further examined in two open-label studies in CD, the first compared 25 mg/wk MTX intravenously for 3 mo followed by 25 mg/wk MTX orally, with 2 mg/kg per day oral AZA[52] for 6 mo. At 3 and 6 mo there was no difference between the percentage of patients in remission between the MTX and AZA groups, but there were significantly more adverse events with MTX. The second study examined patients naïve to immunomodulator therapy[53] and compared MTX 15 mg/wk orally with 6-MP 1.5 mg/kg per day and 5-ASA 3 g/d for 30 wk. Remission was achieved in 80% of patients on MTX and 94% on 6-MP, but this was not statistically different.
The combination of MTX and the anti-TNF medications has been suggested to be beneficial in paediatric patients with one retrospective analysis[54], and the findings are similar to those seen with the combination of thiopurines and an anti-TNF agent in CD. Expert opinion is also that the combination MTX and an anti-TNF agent can be of benefit in the adult IBD population[55], particularly when the anti-TNF therapy is used episodically. In addition, although the data on the effect that MTX has on mucosal healing is very limited with only a single case series of in CD patients, it does suggest that MTX does have the potential for mucosal healing[56].
Despite the limited number of studies of MTX in the induction and maintenance of remission in CD the conclusion of the Cochran review was that MTX was useful in steroid dependent CD and should be commenced at 25 mg/wk im of subcutaneous (SC) and continued for 16 wk[57-59]. Maintenance of remission could then be continued with MTX at 15 mg/wk im or SC but with no evidence to recommend the use of oral MTX[60]. The evidence for MTX in UC is even more limited with a single retrospective case series suggesting some benefit, and only a single prospective randomised trial[51]. The efficacy in UC thus appears to be primarily based on anecdotal experience alone and this is reflected in the Cochran review which stated that there was no evidence for MTX treatment in UC[61-63].
Tacrolimus is a macrolide immunosuppressant that is frequently used to prevent the rejection of renal and hepatic allografts. It has the ability to inhibit T cell activation through the formation of an intracellular complex with immunophilins[64] and these bind to, and inhibit, calcineurin, an enzyme involved in the regulation of transcription factors. Tacrolimus thus does not exert its effect through the inhibition of DNA synthesis but instead inhibits T lymphocyte proliferation through the inhibition of proliferative cytokine production like IL-2[65].
Its role in the management of IBD has been investigated in a number of studies, but unfortunately the majority of these are small, retrospective and uncontrolled[66-78]. In general there would appear to be some efficacy with remission rates ranging from 7%-69% in patients with luminal CD[79] and 9%-74%[70,80] in those with UC. The durability of the clinical response, however, is something that may not be optimal[72] with significant variability in the findings and this, in combination with the potential adverse effects of headache, tremor, parasaethesia, insomnia, gastrointestinal upset, arthralgia and particularly nephrotoxity[73,81], makes the use of tacrolimus in IBD somewhat controversial and its use is not wide spread.
Tacrolimus was initially used in the management of perianal CD where for 10 wk patients received tacrolimus 0.1 mg/kg per day or placebo[82]. There was a significant reduction in fistulae drainage in the tacrolimus-treated group of 43% compared to the placebo group of 8% (P = 0.01), however there was no difference in the remission rates between the groups.
The rest of the data for luminal CD comes primarily from retrospective case series that focus on patients who have failed, or are intolerant, to AZA/6-MP. There are also studies that have included patients failing anti-TNFα therapy, but the proportion of these patients is generally low and are in the range from 13% to 47%[67,70,83]. These studies, however, do suggest some efficacy in both the induction and maintenance of remission[68,70,72] with most patients commencing on 0.1 mg/kg tacrolimus twice a day with the aim to get the tacrolimus trough level within the therapeutic range of 5-20 ng/L. Better response and remission rates would appear to be associated trough levels of > 10 ng/L, and these can reach a response rate of between 68% to 83%[73] and a remission rate of 64% in CD patients[72].
In UC patients hospitalised with moderate/severe steroid refractory disease[81], not on AZA/6-MP, the use of tacrolimus after two weeks was associated with a clinical improvement that was statistically significant, and dose dependent, suggesting that high serum concentrations (10-15 ng/L) are more efficacious that low concentrations (5-10 ng/L) or placebo. Tacrolimus was also noted to achieved mucosal healing in 78.9% (15/19) of patients with the high trough levels compared to 44.4% (8/18) of patients with low trough levels and placebo 12.5% (2/16)[81]. The most recent Cochran review[84] also concluded that tacrolimus was effective in inducing a clinical improvement in a dose-dependent manner in treatment-resistant UC with the number needed to treat being 3.
More recently the long-term efficacy of tacrolimus in both CD and UC patients who had failed standard immunosuppressive and anti-TNFα therapy was assessed in a retrospective study with a trough level targeted between 8-12 ng/mL[85]. Clinical response, remission and surgery were then assessed at 30-d, 90-d and 1-year. This paper identified that 65.7% of patients had a clinical response at 30 d, 60% at 90 d and 31.4% at 1 year while 40% of patients were in remission at 30 d, 37.1% at 90 d and 22.9% at 1 year. The risk of surgery was significantly reduced in patients who achieved and maintain a clinical response at 90-d (P = 0.004). The risk of surgery at 1 year was still very high at 40% and almost 60% by 2 years, but the figures were similar to the 50% three year colectomy rate observed in steroid refractory UC patients treated with infliximab as rescue therapy[86]. The findings thus suggest that tacrolimus could induce both a clinical response and clinical remission in medically refractory IBD patients with long-term benefits.
In all conditions the disease location, severity, patient preferences and allergies need to be considered when prescribing any treatment. Topical tacrolimus has been effective in the treatment of both the perioral and perineal inflammation present in paediatric CD, with resolution of symptoms in up to 75% of patient[87]. More aggressive or novel topical therapy may also be of benefit in distal UC[88,89]. Distal ulcerative colitis (DC), also known as left-sided colitis or E2 disease under the Montreal classification[90], is disease confined to the colon distal to the splenic flexure, while proctitis, or E1 disease (Montreal classification), is disease localized to the rectum. These occur in over 50% of UC patients and, although these result in distressing symptoms, including increased stool frequency, tenesmus, urgency and bleeding, they can often be managed within the community. Resistant disease, however, can be extremely difficult to manage and when there is failure of disease control with routine topical 5-aminosalicylic acid (5-ASA) and steroid therapy, oral agents including the 5ASAs, AZA/6-MP, steroids or an anti-TNFα medication may be use. Unfortunately they do not always help and clinical remission with anti-TNFα therapy only occurs in at most a third of patients[7,91].
It is in these patients that the investigation of other agents is required. To date there have been numerous topical agents proposed for left-sided disease and these have been investigated primarily by open-labelled studies including tacrolimus suppositories[92,93], as well as tacrolimus[93], ecabet sodium[94,95], acetarsol[96] and thromboxane enemas[97]. Unfortunately none of these have undergone blinded randomised studies as yet, although tacrolimus suppositories are currently being investigated in a double-blind placebo-controlled trial. There are, fortunately, several other agents that have undergone randomised studies, and these include butyrate[98-100], cyclosporine[101] and nicotine enemas[102], however, none have demonstrated better efficacy than placebo in left-sided disease or proctitis. In addition, despite impressive evidence for epidermal growth factor enemas in one small randomized study, the finding has never been reexamined or reproduced[103]. It does appear, however, that the mucosal medication concentration and/or contact time may be important for the topical agents to work[104]. This suggests that enemas are not the best method of administration for patients with proctitis. Further investigation is still required, however, before any of these agents can be considered as routine in the management of DC or proctitis, but the need is great and hopefully further work will be undertaken.
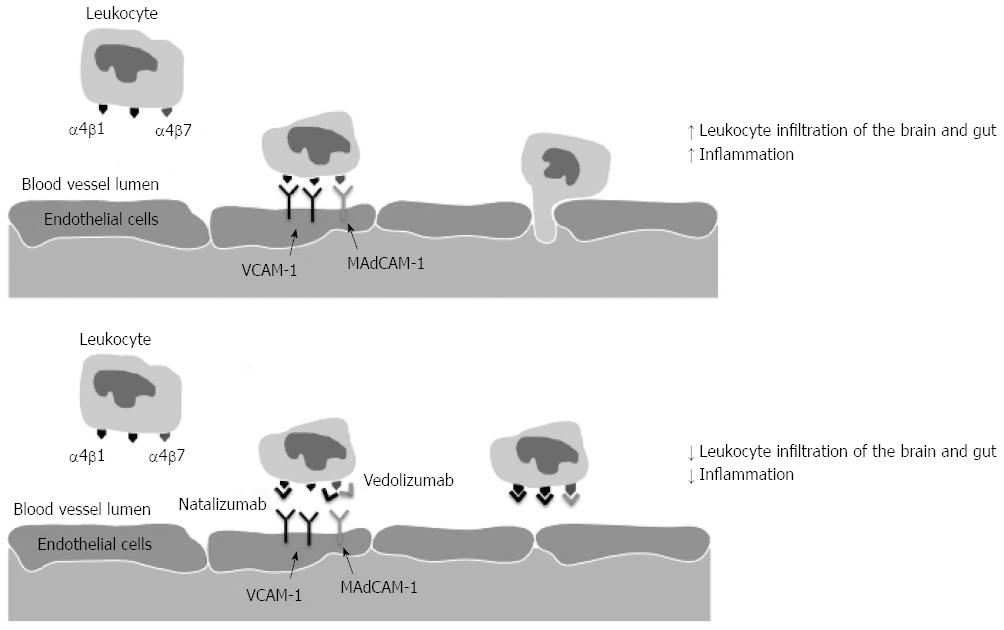
As inflammation in IBD is thought to result from inappropriate activation of the mucosal immune system by intestinal luminal antigens in genetically susceptible individuals[20,105], the trafficking of leukocytes into the intestinal mucosa would appear to be central to the induction, and maintenance, of the intestinal inflammation in IBD. Trafficking of leukocytes is mediated via the recognition of specific adhesion molecules, or integrins that are heterodimeric glycoproteins located on the cellular membrane[106]. These transmembrane receptors consist of an α- and β-subunit with at least 24 different combinations already identified allowing for a wide range of receptor specificity[107]. The α subunit determines the specificity of the interaction between the leukocyte and the endothelial cell and the α4 integrin is widely expressed in both the intestine and brain, and is able to form two different heterodimers with either the β1 or β7-subunit (Figure 1)[107].
The α4β1 integrin is primarily expressed on lymphocytes and monocytes[108], and binds with vascular cell adhesion molecule-1 that is located on vascular endothelial cells allowing cellular migration into the tissue matrix of the brain109. The α4β7 integrin demonstrates some overlapping specificity with the α4β1, but also recognises mucosal addressin cell adhesion molecule-1 (MAdCAM-1) that is important in trafficking of lymphocytes into the gut[109,110]. Of particular note is that MAdCAM-1 expression levels are know to be upregulated in association with chronic inflammation in both the small and large intestine of patients with both CD and UC[111,112] and that the α4β7 heterodimer is highly expressed on memory T cells within the intestine[113].
In addition to the integrins there are other proteins that are found on the cell surface of circulating lymphocytes. One of these is chemokine receptor 9 (CCR9) and it is the only known ligand for CCL25, which is expressed by gastrointestinal tract epithelial cells[114]. When CCR9 is expressed on circulating lymphocytes these cells are able to traffic to the intestine[115,116] and thus CCR9 has been implicated in the development and maintenance of the inflammation observed in IBD[117]. Thus modifying the trafficking of these cells may also impact on the development and progression of IBD inflammation.
The first of the medications to test the concept of altering leukocyte trafficking was Natalizumab (Tysabri, Elan Pharmaceuticals and Biogen Idec), which is a humanised anti-integrin to IgG4 monoclonal antibody that bound to, and inhibited that binding of the α4 integrin to its target proteins in the brain and the gut and it was shown to be effective in the treatment of multiple sclerosis[118,119]. In the 12-wk induction trial in moderate to severe CD patients, patients were randomly assigned in a 4:1 ratio to receive Natalizumab or placebo with the primary endpoint at week 10 and this was defined as a clinical response with a drop in the CD activity index (CDAI) of ≥ 70 points[120]. Although the primary end point was not met (P = 0.05) post hoc analysis identified that if a CDAI drop of > 100 was used or if patients were stratified for an elevated C-reactive protein at baseline, significance was detected between the groups. In the maintenance study, Natalizumab, however, demonstrated an ability to maintain a clinical response (P < 0.001) and remission (P = 0.003) compared to placebo. Unfortunately the emergence of the life threatening side effect, progressive multifocal leukoencephalopathy (PML), was associated with the use of Natalizumab and due to this the FDA added the criteria that Natalizumab must not be used in combination with immunosuppressants or inhibitors of TNF-α, and the use of Natalizumab for the management of CD has never been approved in many countries.
Natalizumab demonstrated that altering lymphocyte trafficking could effect site-specific inflammation[121,122]. But as this anti-α4 monoclonal antibody targeted both the α4β1 and α4β7, and was associated with an increased risk of PML, the potential of targeting the β7 subunit, or both the α4 and β7 subunits was considered. This would improve antibody specificity by only affecting those leukocytes homing to the intestine, and potentially would have less systemic side effects.
The humanised monoclonal antibody Vedolizumab (Millennium: The Takeda Oncology Company, Cambridge, MA, United States) was developed as a highly selective adhesion molecule antagonist that blocked the interaction between the α4β7 integrin and its ligand thus preventing lymphocyte migration into the gut[123]. Recently the phase III induction and maintenance studies for both CD and UC have been presented with very encouraging results. In UC there were response rates at 6 wk of 47.1% in the treatment arm [300 mg intravenously (iv) at weeks 0 and 2] compared to 25.5% of patients receiving placebo (P < 0.001), while maintenance therapy with iv Vedolizumab at either 4 of 8 weekly was compared to placebo and the percentage of patients who were in clinical remission at week 52 was 41.8, 44.8 and 15.9 respectively (P < 0.001 both treatment arms to placebo).
The use of Vedolizumab in CD has also been encouraging. The induction phase was the same as for the UC study and at week 6, 31.4% of patients on Vedolizumab had a clinical response compared to 25.7% of patients on placebo (P = 0.23) but 14.5% of patients on active treatment were in remission compare to 6.8% receiving placebo (P = 0.02). At week 52, however, the percentage of patients who were in clinical remission was 39.0% (4 weekly infusion), 36.4% (8 weekly infusion) compared to 21.6% receiving placebo (P < 0.0001 and P = 0.004 respectively). There was noted to be a higher risk of adverse events for patients receiving Vedolizumab, but there were no cases of PML, compared to those getting placebo suggesting that further experience and data collection will be required.
Vercirnon (CCX282-B) is a selective antagonist of CCR9 with the advantage of being orally active[124] that was initially synthesised by ChemoCentryx Inc, but was subsequently investigated by GlaxoSmithKline where it has just completed the pivotal induction study and was to continue investigation in the SHEID studies for the management of CD. The preclinical studies demonstrated that this molecule inhibits the CCL25-induced chemotaxis[125] and in animal models of colitis, was shown to reduce the severity of intestinal inflammation in the TNF∆ARE murine model of colitis[125].
In the two Phase II/III parallel studies, Vercirnon was examined in moderate to severe active CD. The percentage of patients achieved a clinical response (CDAI decrease ≥ 70 points from baseline), or remission (CDAI < 150), at 12 wk CD was 61% and 29.9% compared to those getting a placebo of 47.2% (P = 0.039) 27.1% (not significant) respectively. The percentage of patients in remission at 52 wk was 47% in the treatment arm and 31% with placebo (P = 0.012) suggesting that there was potentially some efficacy of the medication.
Further studies, however, have been unimpressive with the SHIELD-1 study undertaken by GSK determining that in adult patients with moderately-to-severely active CD, Vercirnon did not achieve the primary endpoint of improvement in clinical response nor the key secondary endpoint of clinical remission. Of note was that although the rates of serious adverse events, and withdrawals due to adverse events, were similar among the groups, there was a trend for a dose-dependent increase in overall adverse event rates with Vercirnon. Consequently, GSK has cease all clinical trials into the use of Vercirnon in management of CD until there have been further analysis of the SHIELD-1 findings.
There is no doubt that the anti-TNFα medications have been a great addition to the treatment options for both UC and CD with many promoting at “top down” therapeutic approach that commences with an anti-TNFα medication in the management of CD, or a rapid “step up” approach when this is not feasible. These medications, however, do not always induce remission and loss of response over time, or the development of side effects, may also limit their long-term efficacy.
In all cases the location and severity of the intestinal inflammation with determine which medications are required and the best mode of administration. For the left sided and distal colidities, and perianal CD, topical agents may be the best choice. Particular thought should be put into this by the physician as these often have less systemic side effects than other agents and can be every effective. Unfortunately further investigation is required before many of these will be part of routine management.
The thiopurines have demonstrated long-term efficacy and by measurement of their metabolities and modification of their activity of hypoxanthine-guanine phosphoribosyltransferase with allopurinol, their efficacy may be increased. Use in combination with the anti-TNFα medications is also of benefit and should be considered in all patients in order to prolong and improve the long-term outcomes with these medications. Methotrexate may also be able to be used in a similar manner to the thiopurines and improved patients outcomes. Less recognized are MMF and tacrolimus as medications for use in IBD but these should also be considered when conventional therapies fail or patient intolerances limit the use of conventional therapies.
There are now newer therapies that have been developed to target leukocyte trafficking to the intestine and these have, fortunately, demonstrated clinical efficacy. The most recent is Vedolizumab, which blocks the α4β7 integrin and it achieved demonstrated impressive efficacy for the induction and maintenance of remission in UC and also has a long-term effect on the maintenance of remission in CD. It would thus be expected that very soon this medication will be under consideration by the various regulatory authorities around the world for use in the IBDs thus allow a further therapeutic option.
Overall the physician must keep an open mind when treating IBD. These patients have a long-term incurable condition than can significantly impact on all aspects of their life. Surgery does not cure the disease and thus medications may be required for many decades in order to give the patients a decent quality of life. Both the patient and the physician, therefore, need to remember the “oldies but goodies” but also keep the door open to new innovations and novel therapies.
P- Reviewers: Manes G, Slomiany BL, Xu H S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Arts J, D’Haens G, Zeegers M, Van Assche G, Hiele M, D’Hoore A, Penninckx F, Vermeire S, Rutgeerts P. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Duerr RH, Barmada MM, Zhang L, Achkar JP, Cho JH, Hanauer SB, Brant SR, Bayless TM, Baldassano RN, Weeks DE. Evidence for an inflammatory bowel disease locus on chromosome 3p26: linkage, transmission/disequilibrium and partitioning of linkage. Hum Mol Genet. 2002;11:2599-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1620] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 4. | Mantzaris GJ, Christidou A, Sfakianakis M, Roussos A, Koilakou S, Petraki K, Polyzou P. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis. 2009;15:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Trinder MW, Lawrance IC. Efficacy of adalimumab for the management of inflammatory bowel disease in the clinical setting. J Gastroenterol Hepatol. 2009;24:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Pearce CB, Lawrance IC. Careful patient selection may improve response rates to infliximab in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22:1671-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1194] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 8. | Willert RP, Lawrance IC. Use of infliximab in the prevention and delay of colectomy in severe steroid dependant and refractory ulcerative colitis. World J Gastroenterol. 2008;14:2544-2549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Lichtenstein GR, Thomsen OØ, Schreiber S, Lawrance IC, Hanauer SB, Bloomfield R, Sandborn WJ. Continuous therapy with certolizumab pegol maintains remission of patients with Crohn‘s disease for up to 18 months. Clin Gastroenterol Hepatol. 2010;8:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, Bloomfield R, Sandborn WJ. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 730] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 11. | Panaccione R, Colombel JF, Sandborn WJ, Rutgeerts P, D’Haens GR, Robinson AM, Chao J, Mulani PM, Pollack PF. Adalimumab sustains clinical remission and overall clinical benefit after 2 years of therapy for Crohn’s disease. Aliment Pharmacol Ther. 2010;31:1296-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 13. | Lawrance I. Certolizumab pegol in the treatment of CD, evidence from the PRECiSE clinical Trials. J Clin Inves. 2011;1:459-465. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Walsh AJ, Weltman M, Burger D, Vivekanandarajah S, Connor S, Howlett M, Radford-Smith G, Selby W, Veillard AS, Grimm MC. Implementing guidelines on the prevention of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2013;7:e449-e456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Lawrance IC, Radford-Smith GL, Bampton PA, Andrews JM, Tan PK, Croft A, Gearry RB, Florin TH. Serious infections in patients with inflammatory bowel disease receiving anti-tumor-necrosis-factor-alpha therapy: an Australian and New Zealand experience. J Gastroenterol Hepatol. 2010;25:1732-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2608] [Cited by in RCA: 2490] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 17. | Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52:1481-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Shale MJ, Seow CH, Coffin CS, Kaplan GG, Panaccione R, Ghosh S. Review article: chronic viral infection in the anti-tumour necrosis factor therapy era in inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31:20-34. [PubMed] |
| 19. | Fidder H, Schnitzler F, Ferrante M, Noman M, Katsanos K, Segaert S, Henckaerts L, Van Assche G, Vermeire S, Rutgeerts P. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501-508. [PubMed] |
| 20. | Alfakih K, Brown B, Lawrance RA, Warburton P, Maqbool A, Walters K, Samani NJ, Ball SG, Balmforth AJ, Hall AS. Effect of a common X-linked angiotensin II type 2-receptor gene polymorphism (-1332 G/A) on the occurrence of premature myocardial infarction and stenotic atherosclerosis requiring revascularization. Atherosclerosis. 2007;195:e32-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Salama M, Lawrance IC. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Mill J, Lawrance IC. Prevention of cancer in IBD - a balancing act. Minerva Gastroenterol Dietol. 2013;59:261-272. [PubMed] |
| 23. | Trotter JL, Rodey GE, Gebel HM. Azathioprine decreases suppressor T cells in patients with multiple sclerosis. N Engl J Med. 1982;306:365-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Oger JJ, Antel JP, Kuo HH, Arnason BG. Influence of azathioprine (imuran) on in vitro immune function in multiple sclerosis. Ann Neurol. 1982;11:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Bacon PA, Salmon M. Modes of action of second-line agents. Scand J Rheumatol Suppl. 1987;64:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Mardini HE, Arnold GL. Utility of measuring 6-methylmercaptopurine and 6-thioguanine nucleotide levels in managing inflammatory bowel disease patients treated with 6-mercaptopurine in a clinical practice setting. J Clin Gastroenterol. 2003;36:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 28. | Wright S, Sanders DS, Lobo AJ, Lennard L. Clinical significance of azathioprine active metabolite concentrations in inflammatory bowel disease. Gut. 2004;53:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Goldenberg BA, Rawsthorne P, Bernstein CN. The utility of 6-thioguanine metabolite levels in managing patients with inflammatory bowel disease. Am J Gastroenterol. 2004;99:1744-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Ragab AH, Gilkerson E, Myers M. The effect of 6-mercaptopurine and allopurinol on granulopoiesis. Cancer Res. 1974;34:2246-2249. [PubMed] |
| 31. | Chevaux JB, Peyrin-Biroulet L, Sparrow MP. Optimizing thiopurine therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1428-1435. [PubMed] |
| 32. | Seinen ML, de Boer NK, Smid K, van Asseldonk DP, Bouma G, van Bodegraven AA, Peters GJ. Allopurinol enhances the activity of hypoxanthine-guanine phosphoribosyltransferase in inflammatory bowel disease patients during low-dose thiopurine therapy: preliminary data of an ongoing series. Nucleosides Nucleotides Nucleic Acids. 2011;30:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, Hanauer SB. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005;22:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Allez M, Vermeire S, Mozziconacci N, Michetti P, Laharie D, Louis E, Bigard MA, Hébuterne X, Treton X, Kohn A. The efficacy and safety of a third anti-TNF monoclonal antibody in Crohn’s disease after failure of two other anti-TNF antibodies. Aliment Pharmacol Ther. 2010;31:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | D’Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, Hanauer SB, Herfarth H, Hommes DW, Kamm M. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199-212; quiz 213. [PubMed] |
| 36. | Actis GC, Pellicano R, David E, Sapino A. Azathioprine, mucosal healing in ulcerative colitis, and the chemoprevention of colitic cancer: a clinical-practice-based forecast. Inflamm Allergy Drug Targets. 2010;9:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Hafraoui S, Dewit O, Marteau P, Cosnes J, Colombel JF, Modigliani R, Cortot A, Lémann M. Mycophenolate mofetil in refractory Crohn’s disease after failure of treatments by azathioprine or methotrexate. Gastroenterol Clin Biol. 2002;26:17-22. [PubMed] |
| 38. | Armuzzi A, Ahmad T, Ling KL, de Silva A, Cullen S, van Heel D, Orchard TR, Welsh KI, Marshall SE, Jewell DP. Genotype-phenotype analysis of the Crohn’s disease susceptibility haplotype on chromosome 5q31. Gut. 2003;52:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Tan T, Lawrance IC. Use of mycophenolate mofetil in inflammatory bowel disease. World J Gastroenterol. 2009;15:1594-1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Neurath MF, Wanitschke R, Peters M, Krummenauer F, Meyer zum Büschenfelde KH, Schlaak JF. Randomised trial of mycophenolate mofetil versus azathioprine for treatment of chronic active Crohn‘s disease. Gut. 1999;44:625-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Fellermann K, Steffen M, Stein J, Raedler A, Hämling J, Ludwig D, Loeschke K, Stange EF. Mycophenolate mofetil: lack of efficacy in chronic active inflammatory bowel disease. Aliment Pharmacol Ther. 2000;14:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Fickert P, Hinterleitner TA, Wenzl HH, Aichbichler BW, Petritsch W. Mycophenolate mofetil in patients with Crohn’s disease. Am J Gastroenterol. 1998;93:2529-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Miehsler W, Reinisch W, Moser G, Gangl A, Vogelsang H. Is mycophenolate mofetil an effective alternative in azathioprine-intolerant patients with chronic active Crohn’s disease? Am J Gastroenterol. 2001;96:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Hassard PV, Vasiliauskas EA, Kam LY, Targan SR, Abreu MT. Efficacy of mycophenolate mofetil in patients failing 6-mercaptopurine or azathioprine therapy for Crohn’s disease. Inflamm Bowel Dis. 2000;6:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Palaniappan S, Ford AC, Greer D, Everett SM, Chalmers DM, Axon AT, Hamlin PJ. Mycophenolate mofetil therapy for refractory inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Papay P, Reinisch W, Ho E, Gratzer C, Lissner D, Herkner H, Riss S, Dejaco C, Miehsler W, Vogelsang H. The impact of thiopurines on the risk of surgical recurrence in patients with Crohn’s disease after first intestinal surgery. Am J Gastroenterol. 2010;105:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Baudouin-Legros M, Brouillard F, Cougnon M, Tondelier D, Leclerc T, Edelman A. Modulation of CFTR gene expression in HT-29 cells by extracellular hyperosmolarity. Am J Physiol Cell Physiol. 2000;278:C49-C56. [PubMed] |
| 48. | Wenzl HH, Hinterleitner TA, Aichbichler BW, Fickert P, Petritsch W. Mycophenolate mofetil for Crohn’s disease: short-term efficacy and long-term outcome. Aliment Pharmacol Ther. 2004;19:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 608] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 50. | Arora S, Katkov W, Cooley J, Kemp JA, Johnston DE, Schapiro RH, Podolsky D. Methotrexate in Crohn’s disease: results of a randomized, double-blind, placebo-controlled trial. Hepatogastroenterology. 1999;46:1724-1729. [PubMed] |
| 51. | Oren R, Moshkowitz M, Odes S, Becker S, Keter D, Pomeranz I, Shirin C, Reisfeld I, Broide E, Lavy A. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol. 1997;92:2203-2209. [PubMed] |
| 52. | Ardizzone S, Bollani S, Manzionna G, Imbesi V, Colombo E, Bianchi Porro G. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’s disease: a randomised, investigator-blind study. Dig Liver Dis. 2003;35:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Maté-Jiménez J, Hermida C, Cantero-Perona J, Moreno-Otero R. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Absah I, Faubion WA. Concomitant therapy with methotrexate and anti-TNF-α in pediatric patients with refractory crohn’s colitis: a case series. Inflamm Bowel Dis. 2012;18:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Rutgeerts P, Van Assche G, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 56. | Mañosa M, Naves JE, Leal C, Cabré E, Moreno V, Lorenzo-Zuñiga V, Boix J, Domènech E. Does methotrexate induce mucosal healing in Crohn’s disease? Inflamm Bowel Dis. 2010;16:377-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Alfadhli AA, McDonald JW, Feagan BG. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2005;CD003459. [PubMed] |
| 58. | Balis FM, Mirro J, Reaman GH, Evans WE, McCully C, Doherty KM, Murphy RF, Jeffries S, Poplack DG. Pharmacokinetics of subcutaneous methotrexate. J Clin Oncol. 1988;6:1882-1886. [PubMed] |
| 59. | Egan LJ, Sandborn WJ, Tremaine WJ, Leighton JA, Mays DC, Pike MG, Zinsmeister AR, Lipsky JJ. A randomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Patel V, Macdonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009;CD006884. [PubMed] |
| 61. | Chande N, MacDonald JK, McDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;CD006618. [PubMed] |
| 62. | Nathan DM, Iser JH, Gibson PR. A single center experience of methotrexate in the treatment of Crohn‘s disease and ulcerative colitis: a case for subcutaneous administration. J Gastroenterol Hepatol. 2008;23:954-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | El-Matary W, Vandermeer B, Griffiths AM. Methotrexate for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2009;CD007560. [PubMed] |
| 64. | Klee CB, Draetta GF, Hubbard MJ. Calcineurin. Adv Enzymol Relat Areas Mol Biol. 1988;61:149-200. [PubMed] |
| 65. | Dumont FJ, Staruch MJ, Koprak SL, Melino MR, Sigal NH. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144:251-258. [PubMed] |
| 66. | Fellermann K, Tanko Z, Herrlinger KR, Witthoeft T, Homann N, Bruening A, Ludwig D, Stange EF. Response of refractory colitis to intravenous or oral tacrolimus (FK506). Inflamm Bowel Dis. 2002;8:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Baumgart DC, Pintoffl JP, Sturm A, Wiedenmann B, Dignass AU. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease--a long-term follow-up. Am J Gastroenterol. 2006;101:1048-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 68. | Baumgart DC, Wiedenmann B, Dignass AU. Rescue therapy with tacrolimus is effective in patients with severe and refractory inflammatory bowel disease. Aliment Pharmacol Ther. 2003;17:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Ng SC, Arebi N, Kamm MA. Medium-term results of oral tacrolimus treatment in refractory inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Benson A, Barrett T, Sparberg M, Buchman AL. Efficacy and safety of tacrolimus in refractory ulcerative colitis and Crohn‘s disease: a single-center experience. Inflamm Bowel Dis. 2008;14:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Bousvaros A, Kirschner BS, Werlin SL, Parker-Hartigan L, Daum F, Freeman KB, Balint JP, Day AS, Griffiths AM, Zurakowski D. Oral tacrolimus treatment of severe colitis in children. J Pediatr. 2000;137:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Tamaki H, Nakase H, Matsuura M, Inoue S, Mikami S, Ueno S, Uza N, Kitamura H, Kasahara K, Chiba T. The effect of tacrolimus (FK-506) on Japanese patients with refractory Crohn’s disease. J Gastroenterol. 2008;43:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Sandborn WJ, Present DH, Isaacs KL, Wolf DC, Greenberg E, Hanauer SB, Feagan BG, Mayer L, Johnson T, Galanko J. Tacrolimus for the treatment of fistulas in patients with Crohn’s disease: a randomized, placebo-controlled trial. Gastroenterology. 2003;125:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 208] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 74. | Fellermann K, Ludwig D, Stahl M, David-Walek T, Stange EF. Steroid-unresponsive acute attacks of inflammatory bowel disease: immunomodulation by tacrolimus (FK506). Am J Gastroenterol. 1998;93:1860-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 112] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Högenauer C, Wenzl HH, Hinterleitner TA, Petritsch W. Effect of oral tacrolimus (FK 506) on steroid-refractory moderate/severe ulcerative colitis. Aliment Pharmacol Ther. 2003;18:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Ierardi E, Principi M, Francavilla R, Pisani A, Rendina M, Ingrosso M, Guglielmi FW, Panella C, Francavilla A. Oral tacrolimus long-term therapy in patients with Crohn’s disease and steroid resistance. Aliment Pharmacol Ther. 2001;15:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Ziring DA, Wu SS, Mow WS, Martín MG, Mehra M, Ament ME. Oral tacrolimus for steroid-dependent and steroid-resistant ulcerative colitis in children. J Pediatr Gastroenterol Nutr. 2007;45:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Yamamoto S, Nakase H, Mikami S, Inoue S, Yoshino T, Takeda Y, Kasahara K, Ueno S, Uza N, Kitamura H. Long-term effect of tacrolimus therapy in patients with refractory ulcerative colitis. Aliment Pharmacol Ther. 2008;28:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 79. | McSharry K, Dalzell AM, Leiper K, El-Matary W. Systematic review: the role of tacrolimus in the management of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:1282-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Gisbert JP, González-Lama Y, Maté J. Systematic review: Infliximab therapy in ulcerative colitis. Aliment Pharmacol Ther. 2007;25:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 81. | Ogata H, Matsui T, Nakamura M, Iida M, Takazoe M, Suzuki Y, Hibi T. A randomised dose finding study of oral tacrolimus (FK506) therapy in refractory ulcerative colitis. Gut. 2006;55:1255-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 328] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 82. | Hart AL, Plamondon S, Kamm MA. Topical tacrolimus in the treatment of perianal Crohn’s disease: exploratory randomized controlled trial. Inflamm Bowel Dis. 2007;13:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Aberra FN, Stettler N, Brensinger C, Lichtenstein GR, Lewis JD. Risk for active tuberculosis in inflammatory bowel disease patients. Clin Gastroenterol Hepatol. 2007;5:1070-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Baumgart DC, Macdonald JK, Feagan B. Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis. Cochrane Database Syst Rev. 2008;CD007216. [PubMed] |
| 85. | Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis. 2013;19:1490-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Gustavsson A, Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, Vilien M, Ström M, Verbaan H. Clinical trial: colectomy after rescue therapy in ulcerative colitis - 3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010;32:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 87. | Casson DH, Eltumi M, Tomlin S, Walker-Smith JA, Murch SH. Topical tacrolimus may be effective in the treatment of oral and perineal Crohn’s disease. Gut. 2000;47:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Lawrance IC. Topical agents for idiopathic distal colitis and proctitis. J Gastroenterol Hepatol. 2011;26:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Lawrance IC. Novel topical therapies for distal colitis. World J Gastrointest Pharmacol Ther. 2010;1:87-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 91. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 92. | Lawrance IC, Copeland TS. Rectal tacrolimus in the treatment of resistant ulcerative proctitis. Aliment Pharmacol Ther. 2008;28:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | van Dieren JM, van Bodegraven AA, Kuipers EJ, Bakker EN, Poen AC, van Dekken H, Nieuwenhuis EE, van der Woude CJ. Local application of tacrolimus in distal colitis: feasible and safe. Inflamm Bowel Dis. 2009;15:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Kono T, Nomura M, Kasai S, Kohgo Y. Effect of ecabet sodium enema on mildly to moderately active ulcerative proctosigmoiditis: an open-label study. Am J Gastroenterol. 2001;96:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Lawrance IC. Ecabet sodium: a potential new agent in the management of distal colitis. J Gastroenterol Hepatol. 2010;25:1182-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 96. | Forbes A, Britton TC, House IM, Gazzard BG. Safety and efficacy of acetarsol suppositories in unresponsive proctitis. Aliment Pharmacol Ther. 1989;3:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Auwerda JJ, Zijlstra FJ, Tak CJ, van den Ingh HF, Wilson JH, Ouwendijk RJ. Ridogrel enemas in distal ulcerative colitis. Eur J Gastroenterol Hepatol. 2001;13:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 98. | Vernia P, Cittadini M, Caprilli R, Torsoli A. Topical treatment of refractory distal ulcerative colitis with 5-ASA and sodium butyrate. Dig Dis Sci. 1995;40:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 99. | Steinhart AH, Brzezinski A, Baker JP. Treatment of refractory ulcerative proctosigmoiditis with butyrate enemas. Am J Gastroenterol. 1994;89:179-183. [PubMed] |
| 100. | Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51-56. [PubMed] |
| 101. | Sandborn WJ, Tremaine WJ, Schroeder KW, Batts KP, Lawson GM, Steiner BL, Harrison JM, Zinsmeister AR. A placebo-controlled trial of cyclosporine enemas for mildly to moderately active left-sided ulcerative colitis. Gastroenterology. 1994;106:1429-1435. [PubMed] |
| 102. | Ingram JR, Thomas GA, Rhodes J, Green JT, Hawkes ND, Swift JL, Srivastava ED, Evans BK, Williams GT, Newcombe RG. A randomized trial of nicotine enemas for active ulcerative colitis. Clin Gastroenterol Hepatol. 2005;3:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. N Engl J Med. 2003;349:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 104. | van Dieren JM, Lambers ME, Kuipers EJ, Samsom JN, van der Woude CJ, Nieuwenhuis EE. Local immune regulation of mucosal inflammation by tacrolimus. Dig Dis Sci. 2010;55:2514-2519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1348] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 106. | Lawrance IC. Modifying T-cell trafficking to the intestinal as a potential management for inflammatory bowel disease. Expert Opin Investig Drugs. 2012;21:975-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 107. | Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6323] [Cited by in RCA: 6563] [Article Influence: 285.3] [Reference Citation Analysis (0)] |
| 108. | Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1272] [Cited by in RCA: 1279] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 109. | Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2222] [Cited by in RCA: 2145] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 110. | Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5269] [Cited by in RCA: 5167] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 111. | 1 Arihiro S, Ohtani H, Suzuki M, Murata M, Ejima C, Oki M, Kinouchi Y, Fukushima K, Sasaki I, Nakamura S. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol Int. 2002;52:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97-110. [PubMed] |
| 113. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2200] [Article Influence: 137.5] [Reference Citation Analysis (6)] |
| 114. | Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 516] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 115. | Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med. 1999;190:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 376] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 116. | Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069-5076. [PubMed] |
| 117. | Papadakis KA, Prehn J, Moreno ST, Cheng L, Kouroumalis EA, Deem R, Breaverman T, Ponath PD, Andrew DP, Green PH. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology. 2001;121:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 118. | Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 873] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 119. | Tubridy N, Behan PO, Capildeo R, Chaudhuri A, Forbes R, Hawkins CP, Hughes RA, Palace J, Sharrack B, Swingler R. The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology. 1999;53:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 212] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 120. | Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 664] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 121. | Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Palmer T, Donoghue S. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 617] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 122. | Gordon FH, Lai CW, Hamilton MI, Allison MC, Srivastava ED, Fouweather MG, Donoghue S, Greenlees C, Subhani J, Amlot PL. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha4 integrin in active Crohn’s disease. Gastroenterology. 2001;121:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 221] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 123. | Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of Vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 346] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 124. | Eksteen B, Adams DH. GSK-1605786, a selective small-molecule antagonist of the CCR9 chemokine receptor for the treatment of Crohn’s disease. IDrugs. 2010;13:472-781. [PubMed] |
| 125. | Walters MJ, Wang Y, Lai N, Baumgart T, Zhao BN, Dairaghi DJ, Bekker P, Ertl LS, Penfold ME, Jaen JC. Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2010;335:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |