Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15920
Revised: May 21, 2014
Accepted: June 14, 2014
Published online: November 14, 2014
Processing time: 262 Days and 17.5 Hours
The patient was an asymptomatic 43-year-old woman. Abdominal ultrasonography and enhanced computed tomography showed a tumor lesion accompanied by multiple cystic changes in the liver and the pancreatic tail. Endoscopic ultrasound-fine needle aspiration was performed on the pancreatic tumor lesion and revealed pancreatic neuroendocrine tumor (PNET). As it was unresectable due to multiple liver metastases, the decision was made to initiate treatment with everolimus and transcatheter arterial chemoembolization. The patient ceased menstruating after the start of everolimus administration. When the administration was discontinued due to interstitial lung disease, menstruation resumed, but then again stopped with everolimus resumption. An association between everolimus and amenorrhea was highly suspected. Amenorrhea occurred as a rare adverse event of everolimus. As the younger women might be included in PNETs patients, we should put this adverse event into consideration.
Core tip: This is the first case report of amenorrhea as a rare adverse event associated with everolimus treatment for pancreatic neuroendocrine tumor. As the younger women might be included in pancreatic neuroendocrine tumors patients, we should put this adverse event into consideration.
- Citation: Kawaguchi Y, Maruno A, Kawashima Y, Ito H, Ogawa M, Mine T. Amenorrhea as a rare drug-related adverse event associated with everolimus for pancreatic neuroendocrine tumors. World J Gastroenterol 2014; 20(42): 15920-15924
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15920
Pancreatic neuroendocrine tumors (PNETs) are becoming more frequent in both incidence and prevalence. PNETs at the time of diagnosis have reached an advanced stage, and the tumors are metastatic or unresectable, in 65% of cases. For PNETs at such a stage, prognosis is poor, mainly because few good treatments exist. A recent prospective study showed sunitinib and everolimus to exert antitumor activity against PNETs. The drug-related adverse events associated with everolimus were stomatitis, aphthous ulceration, lymphopenia, neutropenia or infections[1]. In this case, we experienced amenorrhea as a rare drug-related adverse event. Thia is the first case report of amenorrhea as a rare adverse event associated with everolimus treatment for PNETs.
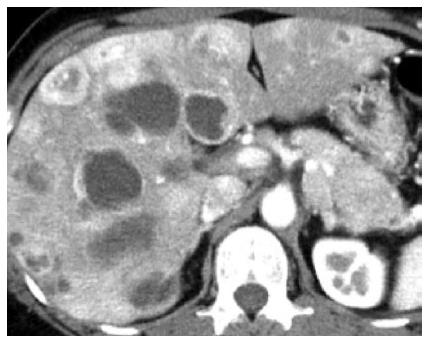
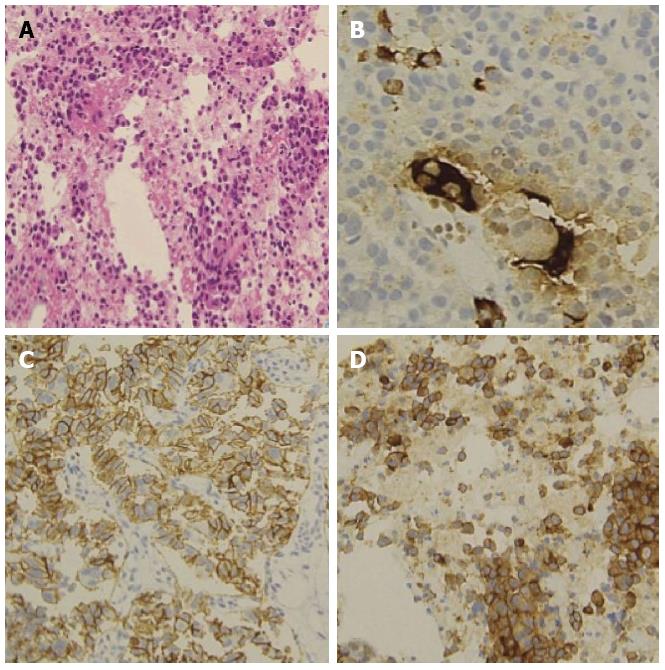
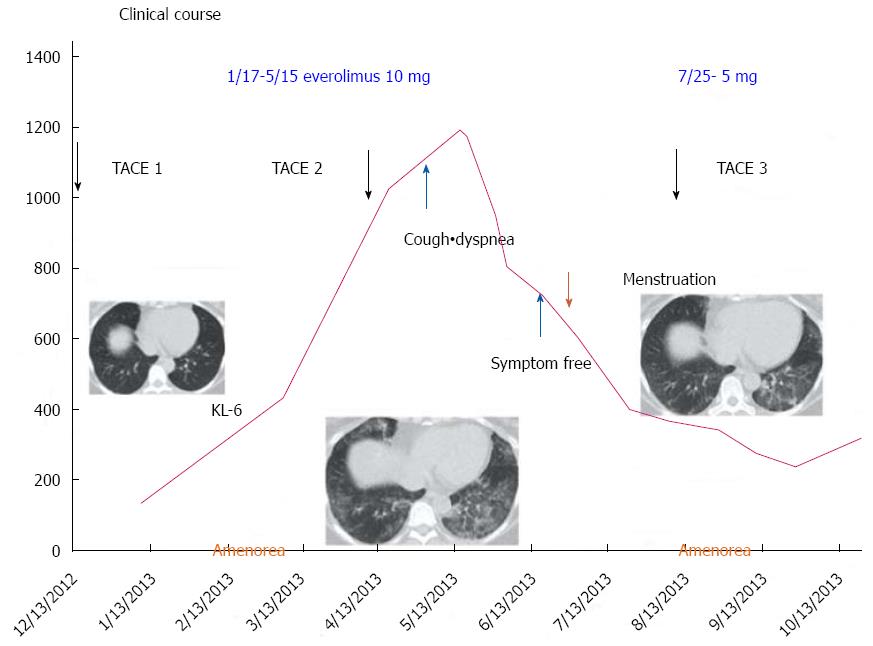
The patient was an asymptomatic 43-year-old woman. Her past medical and family histories were unremarkable. Because multiple hepatic tumors were identified at a routine health check-up, she was referred to our hospital. There were no marked physical findings. Blood biochemistry tests revealed no abnormalities in either tumor markers or endocrine data except for mildly elevated alkaline phosphatase and gamma-glutamyl transferase. Abdominal ultrasonography showed a tumor lesion accompanied by multiple cystic changes in the liver, and abdominal enhanced computed tomography identified a contrast-enhanced tumor lesion accompanied by multiple cystic changes in the liver (Figure 1). The pancreatic tail showed contrast enhancement of the same level as that in the pancreatic parenchyma, confirming a tumor lesion accompanied by cystic changes in a portion of the liver (Figure 1). Endoscopic ultrasound-fine needle aspiration was performed on the pancreatic tumor lesion and revealed a neuroendocrine tumor (NET) G2 (Figure 2). As it was unresectable due to multiple liver metastases, the decision was made to initiate treatment with everolimus and transcatheter arterial chemoembolization (TACE). Approximately 4 mo after starting everolimus administration, the patient developed interstitial lung disease (Grade 2). As symptoms disappeared and image findings improved after drug suspension for 2 mo and 10 d, treatment with everolimus was resumed with a dose reduction to 5 mg/d. At 2.5 mo after treatment resumption, there was no recurrence of interstitial lung disease. The KL-6 level reflected well the state of interstitial lung disease. The patient ceased menstruating after the start of everolimus administration. When the administration was discontinued due to interstitial lung disease, menstruation resumed, but then again stopped with everolimus resumption. As the patient had experienced no prior episodes of menstrual disorders or irregularities, an association with everolimus was highly suspected (Figure 3). At approximately 1 year after the start of treatment, she underwent TACE three times and has since had a favorable course, with the therapeutic effect of a partial response. We experienced a patient with a PNET accompanied by multiple liver metastases that responded to combination treatment with everolimus and TACE. After discontinuation of everolimus due to the development of interstitial lung disease, this drug could be resumed and continuously administered at a reduced dose without recurrence of the lung disease. However, amenorrhea occurred as a rare adverse event.
PNETs are becoming more frequent in both incidence and prevalence, and now account for about 1.3% of the incidence, and 10% of the prevalence, of pancreatic cancer[2-4]. PNETs at the time of diagnosis have reached an advanced stage, and the tumors are metastatic or unresectable, in 65% of cases. For PNETs at such a stage, prognosis is poor. Patients with distant metastatic disease have a median survival time of 24 mo[3], and few good treatments exist.
The majority of patients with PNETs receive chemotherapy, radiotherapy, somatostatin analogue therapy, or some combination of these treatments.
A recent prospective study showed sunitinib and everolimus to exert antitumor activity against PNETs[1,5]. Yao et al[1] showed that 10 mg/d everolimus significantly prolonged the survival of advanced PNETs in comparison with a placebo. Also, the rate of severe adverse events was lower in the everolimus group than in the placebo group.
Everolimus is an antitumor drug that inhibits mammalian target of rapamycin (mTOR) pathway. As mTOR pathway plays a role in proliferation and angiogenesis, everolimus has an important antitumor effect on PNETs[4,6-10].
The safety profile of everolimus has been examined in prior studies[1,11]. Stomatitis and aphthous ulceration were reported as the commonest drug-related adverse events in these studies. Lymphopenia, neutropenia and infections have also been reported as common adverse events associated with everolimus. However, as most of these adverse events were mild and manageable, there were few that this treatment failed to terminate[1]. Though severe adverse events of interstitial pneumonitis were also reported, in most cases, the treatment guidelines were effectual in eliminating the disease. The present patient developed interstitial pneumonitis during everolimus administration. However, it resolved with temporary suspension of drug administration, and there has been no recurrence, to date, since the resumption of everolimus at a reduced dose.
With regard to amenorrhea, our patient had no prior history of menstrual disorders. Menstruation ceased after the start of everolimus administration and resumed when the drug was suspended, then ceased again with re-administration. Therefore, an association between everolimus and amenorrhea was strongly suspected. There are no case reports, to our knowledge, describing amenorrhea associated with everolimus, and it was thus considered to be a rare drug-related adverse event. In the EXIST-2 study of angiomyolipoma, 11 amenorrhea-related events were reported in 8 of 52 female patients (15%) receiving everolimus[12]. When patients were followed without receiving treatment, amenorrhea disappeared in 7 and persisted in 4 until 28 d after the end of treatment. In the EXIT-1 study of subependymal giant cell astrocytomas, 3 amenorrhea-related events were reported in 3 of 29 female patients (10%) receiving everolimus[13]. Two patients were followed without treatment and one received medication. The amenorrhea disappeared in 1 patient and persisted in 2, though reportedly disappeared thereafter.
Everolimus-induced amenorrhea is a potential, albeit rare, drug-related adverse event. Unfortunately, the mechanism underlying the occurrence of amenorrhea has not been examined endocrinologically, and no treatment for amenorrhea has yet been established. Therefore, endocrinological studies are required in the future. As PNETs tend to develop in relatively young people, we should be aware that the administration of everolimus could result in amenorrhea.
An asymptomatic 43-year-old woman that multiple hepatic tumors were identified at a routine health check-up.
Multiple hepatic tumors, pancreatic tumor.
Blood biochemistry tests revealed no abnormalities in either tumor markers or endocrine data except for mildly elevated alkaline phosphatase and gamma-glutamyl transferase.
Abdominal enhanced computed tomography identified contrast-enhanced multiple tumor lesions accompanied by cystic changes in the liver and the pancreatic tail.
Endoscopic ultrasound-fine needle aspiration, which was performed on the pancreatic tumor lesion, revealed a neuroendocrine tumor (NET) G2.
The patient was treated with everolimus in combination with transcatheter arterial chemoembolization.
This is the first case report of amenorrhea as a rare adverse event associated with everolimus treatment for pancreatic NET.
Everolimus is an antitumor drug that inhibits mammalian target of rapamycin (mTOR) pathway. As mTOR pathway plays a role in proliferation and angiogenesis, everolimus has an important antitumor effect on PNETs.
As the younger women might be included in PNETs patients, we should put this adverse event “amenorrhea” into consideration.
This article is the first case report of amenorrhea as a rare adverse event associated with everolimus treatment for PNETs.
P- Reviewer: Shi BM, Trevisani L S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2113] [Article Influence: 150.9] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, Hassan M, Evans DB. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492-3500. [PubMed] |
| 3. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3239] [Article Influence: 190.5] [Reference Citation Analysis (0)] |
| 4. | Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, Hoosen S, St Peter J, Haas T, Lebwohl D. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 474] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 5. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1826] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 6. | Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 462] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 7. | O’Donnell A, Faivre S, Burris HA, Rea D, Papadimitrakopoulou V, Shand N, Lane HA, Hazell K, Zoellner U, Kovarik JM. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 387] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | von Wichert G, Jehle PM, Hoeflich A, Koschnick S, Dralle H, Wolf E, Wiedenmann B, Boehm BO, Adler G, Seufferlein T. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573-4581. [PubMed] |
| 9. | Moreno A, Akcakanat A, Munsell MF, Soni A, Yao JC, Meric-Bernstam F. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer. 2008;15:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A, delle Fave G. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 415] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 11. | Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2371] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 12. | Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 624] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 13. | Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |