Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15691
Revised: March 25, 2014
Accepted: June 2, 2014
Published online: November 14, 2014
Processing time: 267 Days and 19.3 Hours
AIM: To investigate whether 5-hydroxytryptamine (serotonin; 5-HT) is involved in mediating abnormal motor activity in dogs after cisplatin administration.
METHODS: After the dogs had been given a 2-wk recovery period, all of them were administered cisplatin, and the motor activity was recorded using strain gauge force transducers. Blood and intestinal fluid samples were collected to measure 5-HT for 24 h. To determine whether 5-HT in plasma or that in intestinal fluids is more closely related to abnormal motor activity we injected 5-HT into the bloodstream and the intestinal tract of the dogs.
RESULTS: Cisplatin given intravenously produced abnormal motor activity that lasted up to 5 h. From 3 to 4 h after cisplatin administration, normal intact dogs exhibited retropropagation of motor activity accompanied by emesis. The concentration of 5-HT in plasma reached the peak at 4 h, and that in intestinal fluids reached the peak at 3 h. In normal intact dogs with resection of the vagus nerve that were administered kytril, cisplatin given intravenously did not produce abnormal motor activity. Intestinal serotonin administration did not produce abnormal motor activity, but intravenous serotonin administration did.
CONCLUSION: After the intravenous administration of cisplatin, abnormal motor activity was produced in the involved vagus nerve and in the involved serotonergic neurons via another pathway. This study was the first to determine the relationship between 5-HT and emesis-induced motor activity.
Core tip: Previous studies have investigated the motility change unique to cisplatin-induced emesis. This study is the first to investigate whether 5-hydroxytryptamine (serotonin; 5-HT) is involved in mediating abnormal motor activity in dogs after cisplatin administration. Cisplatin given intravenously produced abnormal motor activity that lasted up to 5 h. In normal dogs with resection of the vagus nerve that were administered 5-HT 3 receptor antagonist (kytril), cisplatin given intravenously did not produce abnormal motor activity. Abnormal motor activity was produced in the involved vagus nerve and in the involved serotonergic neurons via another pathway.
- Citation: Ando H, Mochiki E, Ohno T, Yanai M, Toyomasu Y, Ogata K, Tabe Y, Aihara R, Nakabayashi T, Asao T, Kuwano H. Mechanism of gastrointestinal abnormal motor activity induced by cisplatin in conscious dogs. World J Gastroenterol 2014; 20(42): 15691-15702
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15691
Cisplatin is a widely used chemotherapeutic drug in cancer treatment; however, it produces many side effects, including nephrotoxicity, emesis, and diarrhea[1]. Emesis is an especially common and severe side effect after cisplatin administration. The main mechanism of cancer chemotherapeutic induced acute emesis is thought to be the stimulation of the vomiting center of the medulla oblongata by 5-hydroxytryptamine (serotonin; 5-HT), which is released from intestinal enterochromaffin (EC) cells following cisplatin administration via afferent vagal nerve fibers[2]. Suppression of nausea appears to be due to an antagonistic action on the 5-HT3 receptors[3]. After the 5-HT receptors were classified by Bradley et al[4], selective antagonists of 5-HT3 receptors were developed. Many studies have confirmed the pharmacological role of 5-HT, and particularly 5-HT3 receptors, in the control of anticancer drug-induced emesis[5]. However, the mechanism of emesis-induced abnormal gastrointestinal motility remains unclear.
The gastrointestinal (GI) tract is the largest single store of 5-HT within the mammalian body due to the presence of EC cells in the intestinal mucosa[6] and serotonergic neurons in the enteric nervous system, such as the myenteric plexus[7]. Most of the experiments have been carried out in vitro[8-10]. One study has shown that 5-HT can be released from EC cells into the intestinal lumen[11] and portal circulation via mechanisms associated with cholinergic and adrenergic actions, intraluminal pressure, hypertonic glucose, or luminal acidification[10,12]. However, the precise role of 5-HT in the occurrence of vomiting has not been fully elucidated.
Since emesis is known to be accompanied by abnormal gastrointestinal motility[13-17], in the present study, we focused on both gastrointestinal motor activity and the concentrations of 5-HT in plasma and intestinal fluids of conscious dogs after intravenous administration of cisplatin. We investigated whether 5-HT is involved in mediating abnormal gastrointestinal motor activity in conscious dogs. We also studied the relationship between plasma and luminal concentrations of 5-HT in conscious dogs after intravenous administration of cisplatin. This study is the first to determine the relationship between 5-HT and emesis-induced gastrointestinal motor activity.
Healthy mongrel dogs of both sexes weighing 10-15 kg were used. All dogs were fasted overnight and then anesthetized by a single intravenous injection of thiopental sodium (Ravonal; Tanabe Pharmaceutical, Osaka, Japan; 20 mg/kg body weight). General anesthesia was maintained by intratracheal inhalation of halothane (Fluothane; Takeda Chemical Industries, Osaka, Japan) and oxygen. A silastic tube (Silastic 602-205; Dow Corning, Midland, MI) was inserted into the superior vena cava through a branch of the right external jugular vein (jugular tube) and used for the withdrawal of blood samples and injections. The jugular tube was brought out through a skin incision on the neck, and its outer end was fixed to the adjacent skin with silk sutures. The abdominal cavity was opened by a middle incision.
Force transducers were implanted on the serosal surfaces of the gastric body, antrum, pylorus, mid-duodenum, and jejunum 1 and 2 (20 and 40 cm distal to Treitz’s ligament, respectively). Another silastic tube (Silicone tube SR1554; Tigers Polymer, Osaka, Japan) was inserted into the duodenum through the duodenal wall (duodenal tube) and used for the injection of agents and the withdrawal of intestinal fluid samples. The lead wires of the force transducers and the duodenal tube were taken out of the abdominal cavity through a subcutaneous tunnel and brought out through a skin incision made between the right and left scapula. After closure of the abdominal cavity, a jacket-type protector was placed on each dog to protect the lead wires and tubes from being damaged if the dogs scratched themselves. The dogs were housed in individual experiment cages, maintained with intravenous drip infusions of Lactec G (Otsuka Pharmaceutical, Tokyo, Japan) for 6 d postoperatively, and gradually returned to normal dog food (Funabashi Farm, Funabashi, Japan; 15 g/kg body weight per day). This study was approved by the Review Committee on Animal Use at Gunma University, Maebashi, Japan (07-139).
The wires from the transducer were connected to a telemeter, and the data were transmitted to a recording system (Eight Star system, Star Medical, Tokyo, Japan). Recorded signals were used to determine the motility index (MI) values. The MI was the integrated area between the baseline (zero level) and the contractile wave expressed as motor units measured with the Eight Star system. In addition, the data were used to identify the phases of contractile activity. Each phase was visually determined according to the following criteria: the quiescent period was defined as phase I; phase II consisted of clusters of irregular contractions that followed phase I and preceded phase III; phase III was the period during which a group of strong contractions lasting more than 15 min occurred; and phase IV was a short period of subsiding contractions immediately following the phase III contractions.
Cisplatin and 5-HT (serotonin hydrochloride: Sigma-Aldrich Japan K.K., Tokyo, Japan) were dissolved in an injection solvent (Hikari Pharmaceutical, Tokyo, Japan) immediately before use. Cisplatin was donated by Nippon Kayaku (Tokyo, Japan). The dexamethasone sodium phosphate (decadron phosphate injection; Banyu Pharmaceutical, Tokyo, Japan), α-receptor antagonist phentolamine (regitin) (Novartis Pharma K.K., Tokyo, Japan), β-receptor antagonist propranolol (inderal) (AstraZeneca K.K., Osaka, Japan), 5-HT3 receptor antagonist (kytril) (Chugai Pharmaceutical, Tokyo, Japan), muscarinic receptor antagonist atropine (Tanabe Pharmaceutical, Osaka, Japan), and dopamine D2 receptor antagonist (prinperan) (Astellas Pharmaceutical, Tokyo, Japan) were purchased. The antagonist doses were determined according to previous studies[2,10,18-22]. Emesis did not occur in dogs receiving 1.0 mg/kg cisplatin; however, it did occur in dogs receiving 1.2 mg/kg cisplatin, which was the dose selected for the present study. In previous studies[19,20,22], dogs were usually given cisplatin at a dose of 3.0 mg/kg, about three times higher than the dose administered in the present low-dose study. Before each intravenous administration of cisplatin, we confirmed that there was no abnormal motor activity.
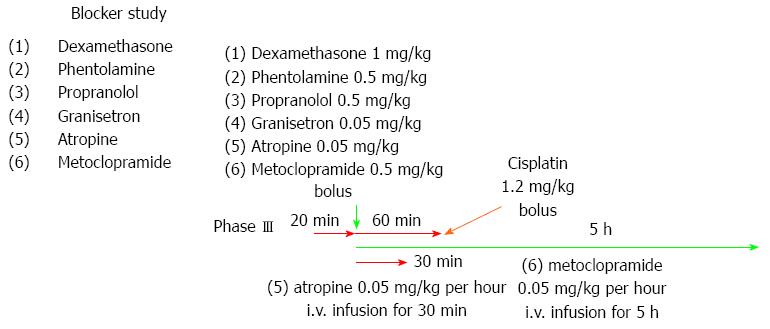
Effects of cisplatin and drugs on cisplatin-induced emesis: The experiments were started after all the dogs had been given a 2-wk recovery period. Before cisplatin administration, the interdigestive state of each dog was recorded for at least 6 h as a control study. After control data were obtained from each dog, the study with cisplatin and various other drugs commenced. After the interdigestive migrating motor contractions (IMCs) were confirmed to have occurred and 20 min after the spontaneous phase III contractions of the jejunum 2 (40 cm distal to Treitz’s ligament) had terminated, all the dogs were administered cisplatin (1.2 mg/kg). Blood and intestinal fluid samples were withdrawn from the jugular tube and duodenal tube 0, 1, 3, 4, 5, 7, 9, and 24 h later, and 5-HT was measured. For the 5-HT measurements, whole blood and intestinal fluids were collected in vacuum plastic tubes containing ethylenediaminetetraacetic acid (EDTA) 2Na. The fluids were then frozen and stored at -30 °C until assaying. The samples were subjected to high-performance liquid chromatography (HPLC) (BML, Kawagoe, Japan).
Dexamethasone (1 mg/kg), phentolamine (regitin; 0.5 mg/kg), propranolol (inderal; 0.5 mg/kg), or granisetron (kytril; 0.05 mg/kg) was given as a single-bolus injection 20 min after the spontaneous phase III contractions of the jejunum 2 had terminated. Granisetron (kytril; 0.05 mg/kg) was also given as a single-bolus injection in the dogs. Cisplatin (1.2 mg/kg) was given 1 h after the administration of each inhibitor.
Atropine (0.05 mg/kg + 0.05 mg/kg per hour) was given as a single-bolus injection. The injection was followed by a 30-min continuous intravenous infusion starting 20 min after the spontaneous phase III contractions of the jejunum 2 had terminated. Metoclopramide (0.5 mg/kg + 0.5 mg/kg per hour) was given as a single-bolus injection. This injection was followed by a 5-h continuous intravenous infusion starting 20 min after the spontaneous phase III contractions of the jejunum 2 had terminated. Cisplatin (1.2 mg/kg) was given 1 h after the start of the administration of each inhibitor.
Then, the dogs were divided into two groups: a normal intact dog group (ND) and a normal intact dog group with resection of the vagus nerve (NDRVN). The NDRVN (n = 4) underwent a truncal vagotomy, with the ventral and dorsal vagi cut immediately below the diaphragm. The dogs in the ND (n = 10) did not undergo surgery and served as the control group.
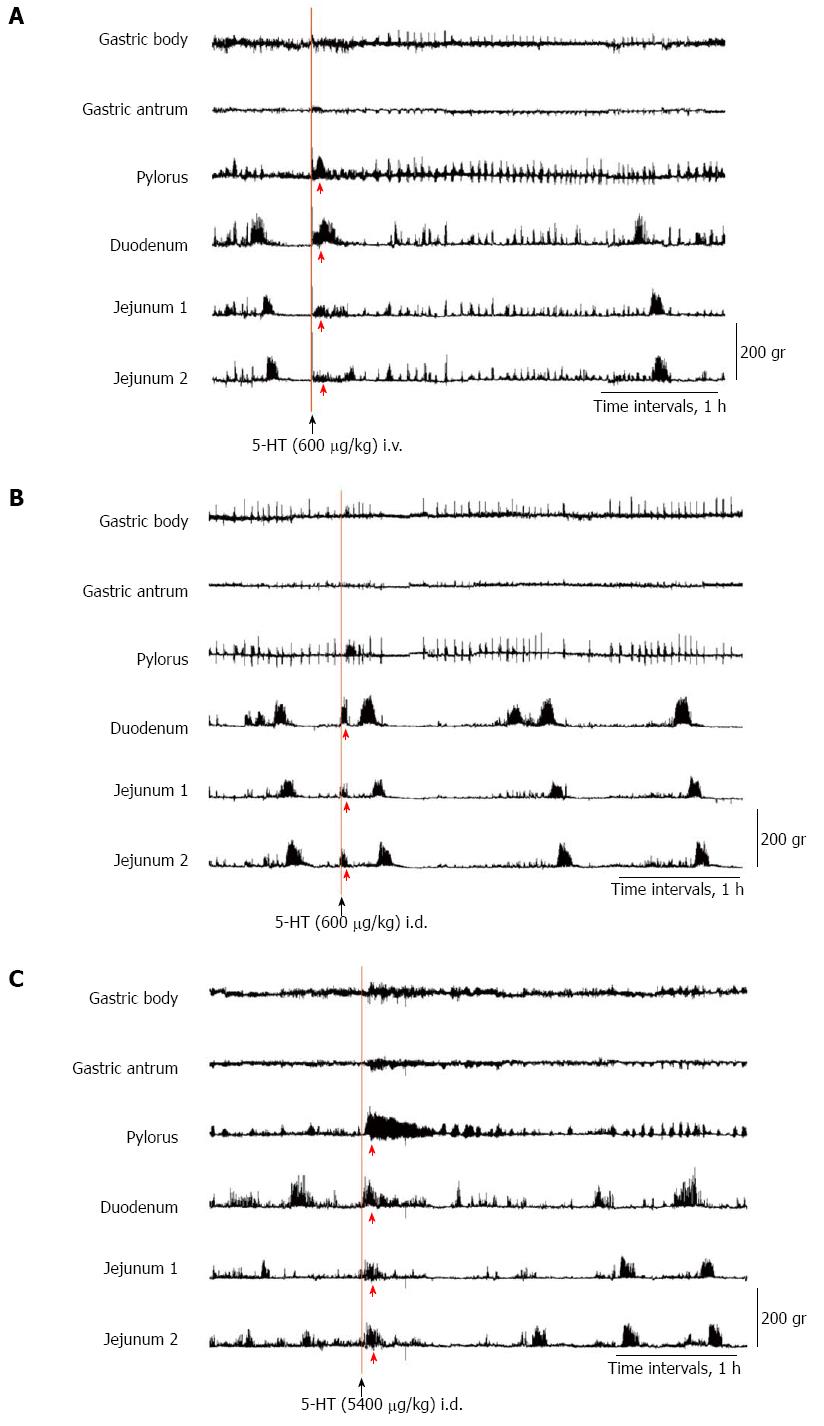
After the IMCs were confirmed to have occurred and 20 min after the spontaneous phase III contractions of the jejunum 2 had terminated, the ND and NDRVN were administered 5-HT intravenously through the jugular tube (600 μg/kg) and into the duodenum through the duodenal tube (600 and 5400 μg/kg) according to the procedures described in previous studies[2,10,18-22]. Zhu et al[10] reported that intraluminal infusion of 10-5 M 5-HT elicited increases in vagal afferent discharge by activating the 5-HT3 receptors in rats. Following the procedures described previously[10], we administered 5-HT (200 μg/kg) according to the conversion of the mol concentration. In the dogs, such doses of an intraluminal infusion of 5-HT did not elicit abnormal motor activity, but three-fold doses (600 μg/kg) did. The subsequent gastrointestinal motility was recorded for at least 4 h. The MI values during the 60 min following 5-HT administration (600 μg/kg) were calculated for the ND and NDRVN.
Gastrointestinal motility was quantified by calculating the MI, which was equivalent to the area under the curve. The MI was calculated using a computer-assisted system (Eight Star system, version 6.0, Star Medical, Tokyo, Japan). Gastrointestinal motility after 5-HT administration (600 μg/kg) was analyzed for 60 min.
Results are expressed as mean ± SE. The data were subjected to detailed statistical analyses using repeated measures of analysis of variance (ANOVA). When significant differences were detected, differences between means were checked using the Fisher’s protected least significant difference test. The Mann-Whitney U test was used to test the significance of differences among groups. P-values < 0.05 were considered significant. Statistical calculations were performed with the use of StatView® software (version 5.0; Abacus Concepts, Inc., Berkeley, CA).
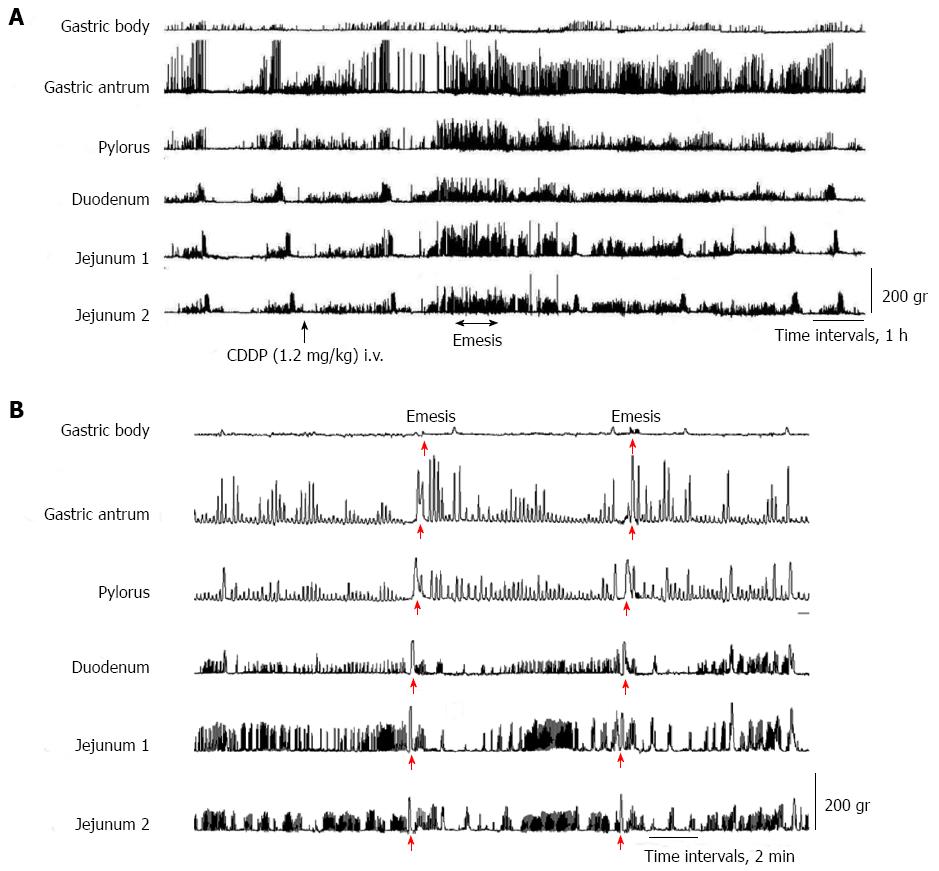
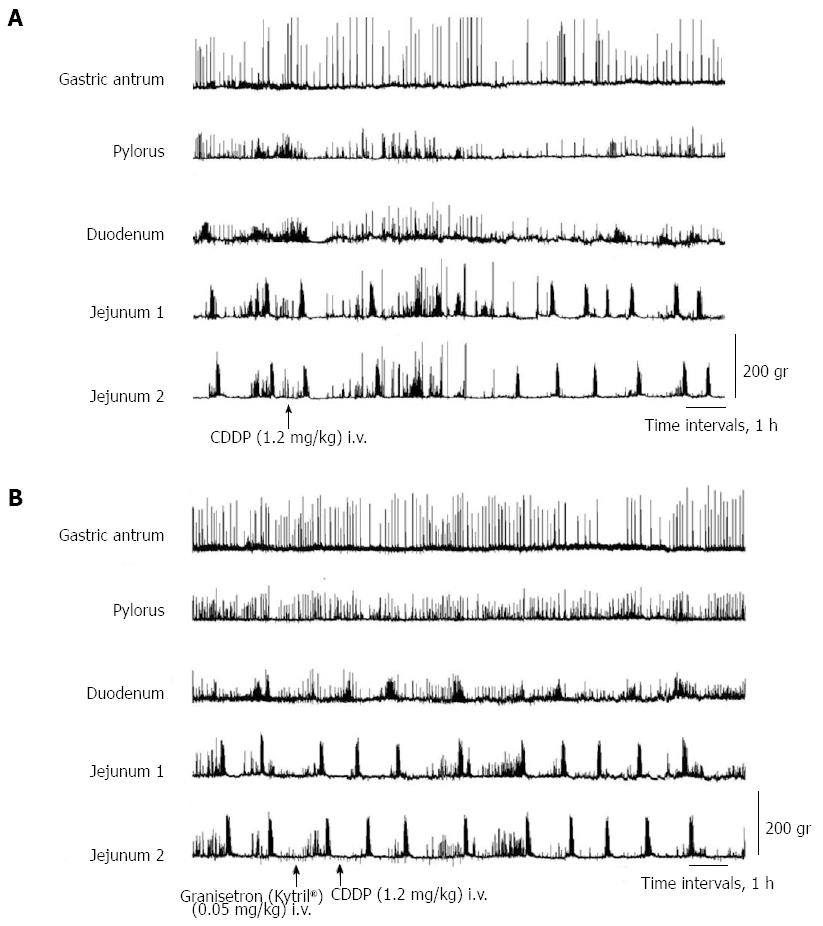
In all dogs in the fasted state, cyclic changes of contractions were detected, including a quiescence period followed by a group of strong contractions (phase III). Abnormal motor activity in the dogs developed from the duodenum, followed by jejunum 1 and 2 and the antrum and pylorus about 2.5 h after intravenous administration of cisplatin (Figure 1A). Cisplatin given intravenously resulted in the complete interruption of IMCs and predominantly produced abnormal motor activity which lasted up to 5 h (Figure 1A). From about 3 to 4 h after the intravenous administration of cisplatin, the dogs exhibited retropropagation of gastrointestinal motor contractions from jejunum 2 to the gastric body, accompanied by emesis (Figure 1B). The most frequent emetic episodes were observed from 3 to 4 h after cisplatin administration. Emesis developed in all the dogs.
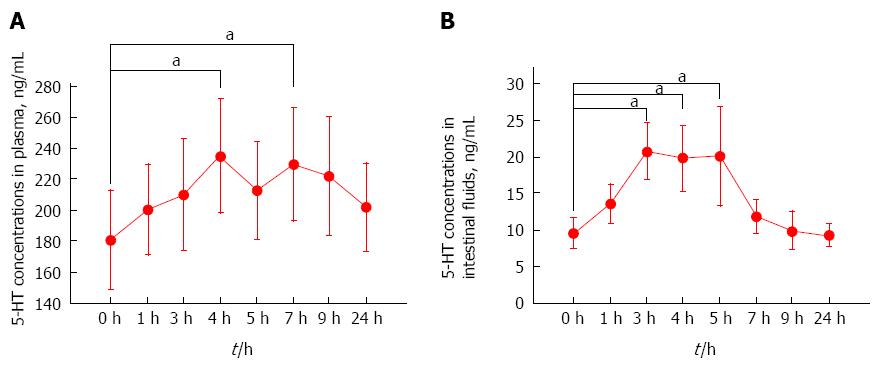
The concentrations of 5-HT in plasma and intestinal fluids determined in these experiments are shown in Figure 2. The plasma 5-HT concentration increased, reached the peak at 4 h after the intravenous administration of cisplatin, and then exhibited small fluctuations and declined (Figure 2A). The plasma 5-HT concentration at 0 h was significantly higher than those obtained at 4 and 7 h (P < 0.05). The 5-HT concentrations in intestinal fluids increased, reached the peak at 3 h after the intravenous administration of cisplatin, and then declined (Figure 2B). The 5-HT concentration in intestinal fluids at 0 h was significantly higher than those obtained at 3, 4, and 5 h (P < 0.05).
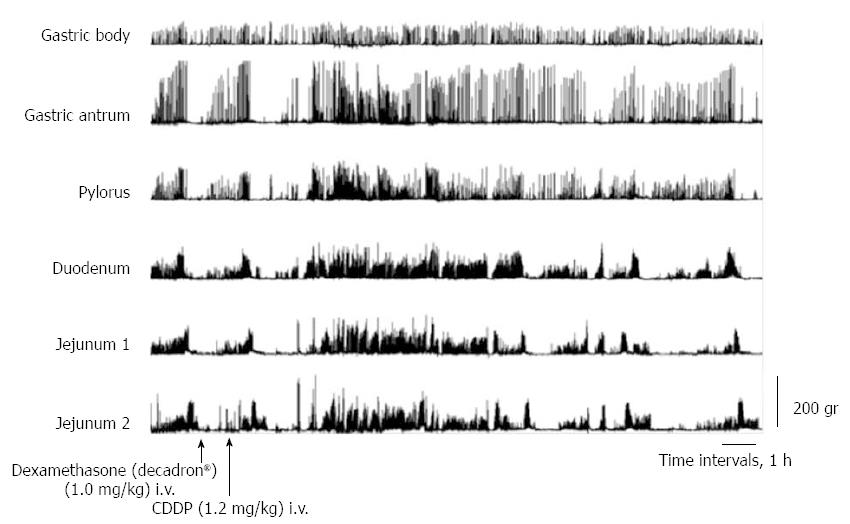
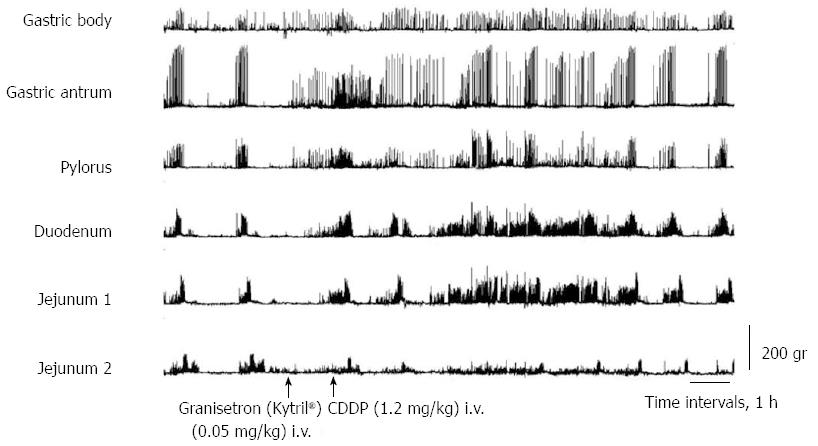
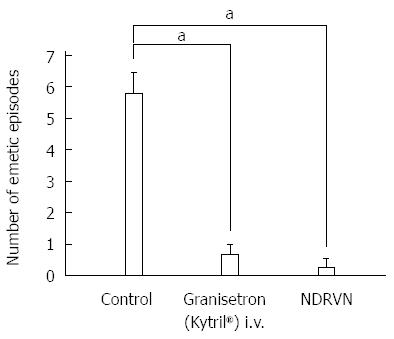
After dexamethasone (1 mg/kg), phentolamine (regitin; 0.5 mg/kg), propranolol (inderal; 0.5 mg/kg), atropine (0.05 mg/kg + 0.05 mg/kg per hour), or metoclopramide (0.5 mg/kg + 0.5 mg/kg per hour) administration, cisplatin given intravenously also resulted in the complete interruption of IMCs and predominantly produced abnormal motor activity that lasted up to about 5 h (Figures 3 and 4). However, in dogs that had received intravenous metoclopramide, emesis did not occur. In the NDRVN (n = 4) (Figure 5A), cisplatin given intravenously also produced abnormal motor activity that, however, lasted for a shorter duration. After the NDRVN (n = 4) was administered granisetron (kytril; 0.05 mg/kg), cisplatin given intravenously did not produce abnormal motor activity (Figure 5B). In the ND (n = 6) administered granisetron (kytril; 0.05 mg/kg) (Figure 6), cisplatin given intravenously also produced abnormal motor activity that, however, lasted for a shorter duration. In three of the four NDRVN dogs and three of the six ND dogs that received granisetron (kytril; 0.05 mg/kg), emesis did not occur. The emetic episodes in the dogs receiving cisplatin alone (control, n = 10), granisetron (kytril; 0.05 mg/kg, i.v., n = 6), and NDRVN dogs (n = 4) are shown in Figure 7. The emetic episodes were, respectively, 5.80 ± 0.66, 0.66 ± 0.33, and 0.25 ± 0.25 in the control group, granisetron (kytril) group, and NDRVN. Significant differences were detected among the control group, kytril group, and NDRVN (P < 0.05).
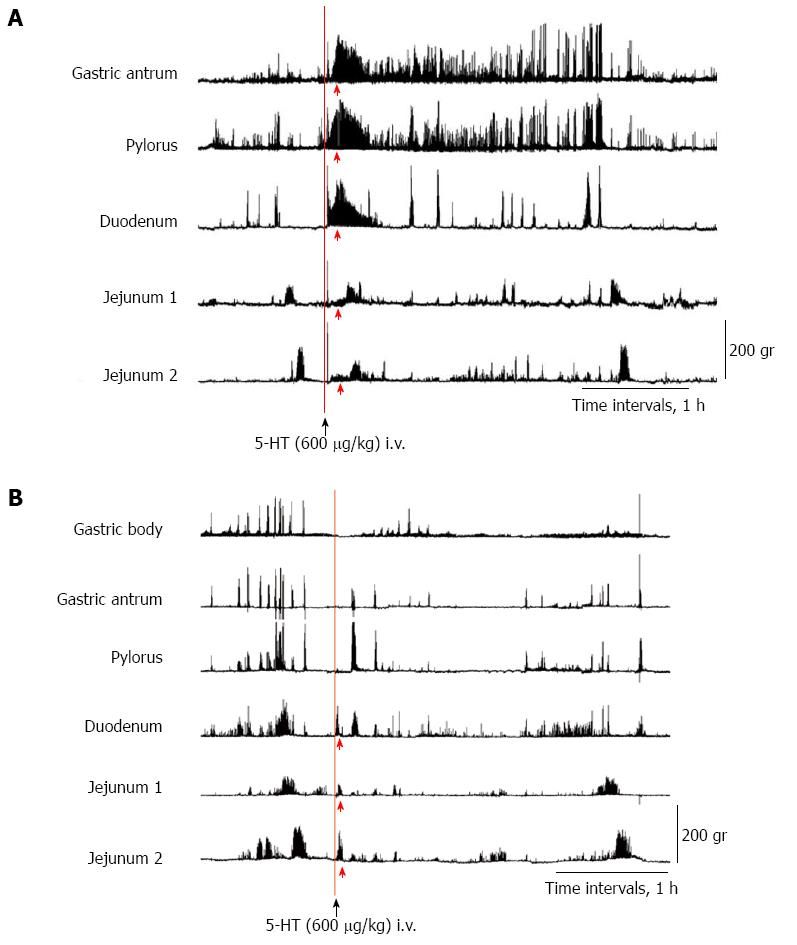
To determine whether 5-HT in plasma or in intestinal fluids causes abnormal motor activity, we administered 5-HT intravenously through the jugular tube (600 μg/kg) (Figures 8A and 9A) and into the duodenum through the duodenal tube (600 and 5400 μg/kg) (Figures 8B, 9B, and C), respectively, in the ND and NDRVN.
In the ND, after the intravenous administration of 5-HT (600 μg/kg), phasic contractions, such as abnormal motor activity lasting for about 30 min, were induced immediately in the antrum, pylorus, duodenum, and jejunum 1 and 2. The duration and amplitude of the motor activity in the antrum and pylorus were longer and stronger than those in the duodenum and jejunum (Figure 8A). In the NDRVN, after the intravenous administration of 5-HT (600 μg/kg), phasic contractions, such as abnormal motor activity lasting for 10 to 20 min, were immediately induced in the pylorus, duodenum, and jejunum 1 and 2. The duration and amplitude of motor activity were not longer or stronger than those in the ND (Figure 9A). In the ND and NDRVN, after the intraduodenal administration of 5-HT (600 μg/kg), phasic contractions, such as abnormal motor activity lasting for about 10 min, were immediately induced in the duodenum and jejunum 1 and 2 (Figures 8B and 9B). In the ND and NDRVN, contractions induced by the intraduodenal administration of 5-HT were obviously shorter and weaker than those resulting from intravenous administration. In the NDRVN, the duration and amplitude tended to increase as the dose of 5-HT was increased, and after the intraduodenal administration of 5-HT (5400 μg/kg), phasic contractions, such as phase II-like activity lasting for about 1 h, were immediately induced in the pylorus, duodenum, and jejunum 1 and 2 (Figure 9C). The pattern of these contractions induced by the intravenous administration of 5-HT was different from that of spontaneously occurring IMCs.
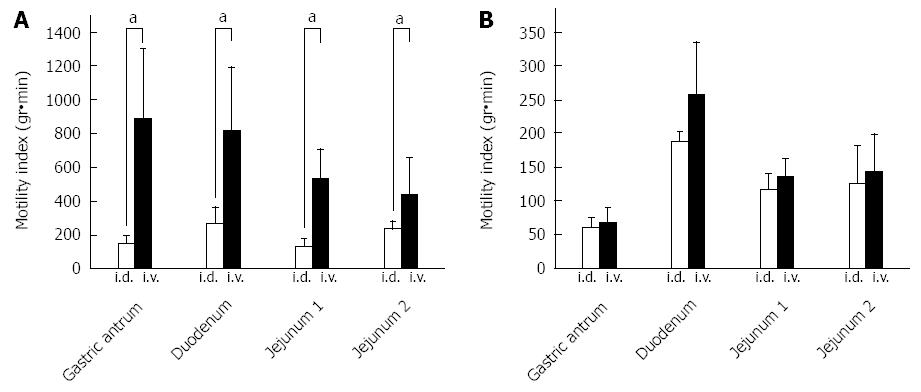
MI data were recorded for 60 min after the intravenous and intraduodenal administration of 5-HT (600 μg/kg) in the ND (n = 4) (Figure 10A) and the NDRVN (n = 4) (Figure 10B). The MI for the intravenous administration was higher than that for the intraduodenal administration in the antrum, duodenum, jejunum 1, and jejunum 2. In the MI of the antrum, duodenum, jejunum 1, and jejunum 2 in the ND showed significant differences between the intravenous and intraduodenal groups (P < 0.05) (Figure 10A).
In this study, when cisplatin was given intravenously, IMCs disappeared and were replaced predominantly by abnormal phase II-like motor activity. Thus, in all dogs, phase I, III, and IV activities either disappeared or markedly decreased in duration, and abnormal motor activity developed for about 5 h, starting at 2.5 h after cisplatin administration. Retropropagation of gastrointestinal motor activity originating from jejunum 2 occurred in all dogs in which emesis developed. This motility change, unique to emesis, was similar to that reported in other studies[14-17]. The present study is the first to characterize and quantify gastrointestinal motor responses during cisplatin administration.
When the concentrations of 5-HT in plasma and intestinal fluids increased, IMCs disappeared and were replaced predominantly by abnormal motor activity. To determine whether 5-HT in plasma or in intestinal fluids is more closely related to abnormal motor activity, we administered 5-HT into the vein and the intestinal tract of the dogs. Motor activity similar to cisplatin-induced abnormal motor activity was produced by intravenous serotonin administration. We administered the same amount of serotonin into the duodenum, but the reaction was negligible. The dose of intravenously administered serotonin was different from the maximal value of 5-HT, which we measured after cisplatin administration. We did not measure the 5-HT concentration in whole blood but in plasma because the platelets, which are in whole blood, include the most 5-HT. We assumed that the doses of intravenously administrated serotonin were different from the maximal value of 5-HT measured after cisplatin administration.
Most of the 5-HT from EC cells is secreted via the basolateral membrane into the portal circulation; a smaller portion is released from the apical membrane into the intestinal lumen[23]. The basal vascular release of serotonin was 10 times higher than the luminal release[24]. Serotonin released from the basolateral site of the EC cells may reach the connective tissue space of the lamina propria and exert paracrine effects on either endocrine cells or intrinsic and extrinsic nerve terminals[24]. It has been reported that exogenously applied serotonin inhibits acid secretion and that this effect is exerted by serotonin injected into blood vessels but not by serotonin injected into the gastric lumen[25]. Judging from the previous results, serotonin released from the basolateral site of the EC cells seems to be more important than serotonin released from the apical site of the EC cells. Our results are in good agreement with those of previous studies, confirming that abnormal motor activity is produced by intravenous serotonin administration.
We investigated the effects of various antagonists on abnormal activity induced by cisplatin administration. Cisplatin-induced responses, which are abnormal activities, were not inhibited by dexamethasone, phentolamine, propranolol, atropine, or metoclopramide but were partly inhibited by granisetron (kytril). Fukunaka et al[2] reported that dexamethasone is effective in cisplatin-induced emesis in the delayed phase. In the present study, dexamethasone did not inhibit cisplatin-induced responses, which are abnormal in acute emesis. Phentolamine and propranolol did not affect abnormal activity induced by cisplatin administration, which suggests that cisplatin administration exerts its effects independently of the sympathetic nervous system. Lang et al[17] reported that sympathetic splanchnic nerve denervation did not block vomiting in dogs. Supradiaphragmatic vagotomy eliminated the digestive tract responses associated with motion sickness-induced vomiting, but phasic contractions were not blocked by atropine[26]. These results coincided with our result showing no emesis in dogs receiving intravenous metoclopramide. It has been shown that metoclopramide blocks dopamine D2 receptors at conventional doses (0.1-0.4 mg/kg, i.v.)[27]; however, the drug also blocks 5-HT3 receptors at higher doses[5,27-29]. In fact, the results of the present study demonstrate that cisplatin-induced emesis was suppressed by the injection of a high dose of metoclopramide.
The action of cytotoxic drugs initiates within the GI tract, resulting in activation of the biosynthesis of 5-HT and an increase in the concentration of 5-HT[23]. 5-HT may stimulate the 5-HT3 receptors on the adjacent vagal afferent nerves[30-32]. Fukunaka et al[2] demonstrated that electrical stimulation of the abdominal vagal afferents induced an increase in the concentration of 5-HT in the ferret area postrema. Pretreatment with 5-HT3 receptor antagonists diminished the increase in area postrema 5-HT but did not alter the increase in ileal 5-HT levels[2]. Thus, the increased 5-HT levels induced by anticancer drugs in the vomiting center were inhibited by 5-HT3 receptor antagonists. Taken together, these results suggest that cytotoxic drugs induce acute emesis mainly through actions on the gastrointestinal tract. In ferrets, the area postrema contains a high concentration of 5-HT3 receptors and receives visceral afferent innervation from the gastrointestinal tract[2]. With regard to our results, in the NDRVN dogs receiving granisetron (kytril) intravenously, cisplatin given intravenously also produced abnormal motor activity that, however, lasted for a shorter duration. In three of the four NDRVN dogs and three of six ND dogs receiving granisetron (kytril), emesis did not occur. In the NDRVN dogs receiving intravenous granisetron (kytril) administration, cisplatin did not induce abnormal activity. Our results, which are in good agreement with those of previous studies[2,22], demonstrate that 5-HT3 receptors act dominantly on the visceral neuron, which projects from the gut to the central nervous system. Fukui et al[22] reported that cisplatin evokes emesis mainly by acting on the vagal afferent terminals through the release of 5-HT and that peripheral 5-HT3 receptors are involved in this action. The abnormal motor activity was produced in part in the involved vagus nerve and in the involved serotonergic neurons[33,34].
In conclusion, cisplatin-induced abnormal motor activity was produced by 5-HT in blood or in intestinal fluids. After the intravenous administration of cisplatin, abnormal motor activity was produced in part in the involved vagus nerve and in the involved serotonergic neurons. This study was the first to determine the relationship between 5-HT and emesis-induced abnormal motor activity, which is going on now.
5-hydroxytryptamine (5-HT; serotonin) is known to affect the control of anticancer drug-induced emesis. The mechanism of emesis-induced abnormal gastrointestinal motility remains unclear. Analyzing the relationship between 5-HT and emesis-induced gastrointestinal motor activity is important.
Previous studies have demonstrated that 5-HT3 blockers have an effect on acute emesis, but no effect on delayed emesis. Recently, palonosetron hydrochloride (a 5-HT3 blocker) has been demonstrated to have an effect on acute and delayed emesis.
Most of the experiments have been carried out in vitro. This study is the first to investigate the relationship between plasma and luminal concentrations of 5-HT in conscious dogs after intravenous administration of cisplatin.
In the normal intact dog group with resection of the vagus nerve (NDRVN) that was administered kytril, cisplatin given intravenously did not produce abnormal motor activity and emesis. For example, patients with gastric cancer receiving chemotherapy may undergo resection of the vagus nerve.
Motility index (MI) was defined as the integrated area between baseline (zero level) and the contractile wave expressed in motor units. The data were used to identify the phases of contractile activity. 5-HT is found within enterochromaffin cells, and it is released from intestinal enterochromaffin cells following cisplatin administration.
This is a good valuable study in which the authors analyze the relationship between 5-HT and emesis-induced gastrointestinal motor activity. They concluded that abnormal motor activity was produced in part in the involved vagus nerve and in the involved serotonergic neurons.
P- Reviewer: Kakizaki S, Roeb E S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Von Hoff DD, Schilsky R, Reichert CM, Reddick RL, Rozencweig M, Young RC, Muggia FM. Toxic effects of cis-dichlorodiammineplatinum(II) in man. Cancer Treat Rep. 1979;63:1527-1531. [PubMed] |
| 2. | Fukunaka N, Sagae S, Kudo R, Endo T, Hirafuji M, Minami M. Effects of granisetron and its combination with dexamethasone on cisplatin-induced delayed emesis in the ferret. Gen Pharmacol. 1998;31:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Miner WD, Sanger GJ. Inhibition of cisplatin-induced vomiting by selective 5-hydroxytryptamine M-receptor antagonism. Br J Pharmacol. 1986;88:497-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 243] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Bradley PB, Engel G, Feniuk W, Fozard JR, Humphrey PP, Middlemiss DN, Mylecharane EJ, Richardson BP, Saxena PR. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986;25:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 925] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 5. | Costall B, Domeney AM, Naylor RJ, Tattersall FD. Emesis induced by cisplatin in the ferret as a model for the detection of anti-emetic drugs. Neuropharmacology. 1987;26:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Erspamer V, Testini A. Observations on the release and turnover rate of 5-hydroxytryptamine in the gastrointestinal tract. J Pharm Pharmacol. 1959;11:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Gershon MD. Serotonin: its role and receptors in enteric neurotransmission. Adv Exp Med Biol. 1991;294:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Canfield SP, Spencer JE. The inhibitory effects of 5-hydroxytryptamine on gastric acid secretion by the rat isolated stomach. Br J Pharmacol. 1983;78:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Yamamoto I, Kuwahara A, Fujimura M, Kadowaki M, Fujimiya M. Involvement of 5-HT3 and 5-HT4 receptors in the motor activity of isolated vascularly perfused rat duodenum. Neurogastroenterol Motil. 1999;11:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 1997;108:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G916-G923. [PubMed] |
| 13. | Ehrlein HJ. Retroperistaltism and duodenogastric reflux in dogs. Scand J Gastroenterol Suppl. 1981;67:29-32. [PubMed] |
| 14. | Weisbrodt NW, Christensen J. Electrical activity of the cat duodenum in fasting and vomiting. Gastroenterology. 1972;63:1004-1010. [PubMed] |
| 15. | Akwari OE. The gastrointestinal tract in chemotherapy-induced emesis. A final common pathway. Drugs. 1983;25 Suppl 1:18-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Lee KY, Park HJ, Chey WY. Studies on mechanism of retching and vomiting in dogs. Effect of peripheral dopamine blocker on myoelectric changes in antrum and upper small intestine. Dig Dis Sci. 1985;30:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Lang IM, Sarna SK, Condon RE. Gastrointestinal motor correlates of vomiting in the dog: quantification and characterization as an independent phenomenon. Gastroenterology. 1986;90:40-47. [PubMed] |
| 18. | Shibata C, Jin XL, Naito H, Matsuno S, Sasaki I. Intraileal capsaicin inhibits gastrointestinal contractions via a neural reflex in conscious dogs. Gastroenterology. 2002;123:1904-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Eglen RM, Lee CH, Smith WL, Johnson LG, Clark R, Whiting RL, Hegde SS. Pharmacological characterization of RS 25259-197, a novel and selective 5-HT3 receptor antagonist, in vivo. Br J Pharmacol. 1995;114:860-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Yoshida N, Omoya H, Ito T. DAT-582, a novel serotonin3 receptor antagonist, is a potent and long-lasting antiemetic agent in the ferret and dog. J Pharmacol Exp Ther. 1992;260:1159-1165. [PubMed] |
| 21. | Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H. The peptide hormone xenin induces gallbladder contractions in conscious dogs. Neurogastroenterol Motil. 2007;19:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Fukui H, Yamamoto M, Sato S. Vagal afferent fibers and peripheral 5-HT3 receptors mediate cisplatin-induced emesis in dogs. Jpn J Pharmacol. 1992;59:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, Nemoto M, Saito H, Yoshioka M. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003;99:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Yu PL, Fujimura M, Hayashi N, Nakamura T, Fujimiya M. Mechanisms in regulating the release of serotonin from the perfused rat stomach. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1099-G1105. [PubMed] |
| 25. | Lepard KJ, Chi J, Mohammed JR, Gidener S, Stephens RL. Gastric antisecretory effect of serotonin: quantitation of release and site of action. Am J Physiol. 1996;271:E669-E677. [PubMed] |
| 26. | Lang IM, Sarna SK, Shaker R. Gastrointestinal motor and myoelectric correlates of motion sickness. Am J Physiol. 1999;277:G642-G652. [PubMed] |
| 27. | Gylys JA, Wright RN, Nicolosi WD, Buyniski JP, Crenshaw RR. BMY-25801, an antiemetic agent free of D2-dopamine receptor antagonist properties. J Pharmacol Exp Ther. 1988;244:830-837. [PubMed] |
| 28. | Costall B, Domeney AM, Naylor RJ, Tattersall FD. 5-Hydroxytryptamine M-receptor antagonism to prevent cisplatin-induced emesis. Neuropharmacology. 1986;25:959-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Miner WD, Sanger GJ, Turner DH. Evidence that 5-hydroxytryptamine3 receptors mediate cytotoxic drug and radiation-evoked emesis. Br J Cancer. 1987;56:159-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Ruff P, Paska W, Goedhals L, Pouillart P, Rivière A, Vorobiof D, Bloch B, Jones A, Martin C, Brunet R. Ondansetron compared with granisetron in the prophylaxis of cisplatin-induced acute emesis: a multicentre double-blind, randomised, parallel-group study. The Ondansetron and Granisetron Emesis Study Group. Oncology. 1994;51:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Bermudez J, Boyle EA, Miner WD, Sanger GJ. The anti-emetic potential of the 5-hydroxytryptamine3 receptor antagonist BRL 43694. Br J Cancer. 1988;58:644-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Higgins GA, Kilpatrick GJ, Bunce KT, Jones BJ, Tyers MB. 5-HT3 receptor antagonists injected into the area postrema inhibit cisplatin-induced emesis in the ferret. Br J Pharmacol. 1989;97:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Darmani NA, Johnson JC. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Chey RD, Lee KY, Asbury R, Chey WY. Effect of cisplatin on myoelectric activity of the stomach and small intestine in dogs. Dig Dis Sci. 1988;33:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |