Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15233
Revised: April 28, 2014
Accepted: June 12, 2014
Published online: November 7, 2014
Hepatitis C virus (HCV) infection is a common chronic liver disease worldwide. Non-alcoholic fatty liver disease and insulin resistance (IR) are the major determinants of fibrosis progression and response to antiviral therapy. The pathogenetic link between IR and chronic HCV infection is complex, and is associated with HCV genotype. Liver steatosis is the most common in the patients infected with genotype 3 virus, possibly due to direct effects of genotype 3 viral proteins. To the contrary, hepatic steatosis in the patients infected with other genotypes is thought to be mostly due to the changes in host metabolism, involving IR. In HCV genotype 3, liver steatosis correlates with viral load, reverts after reaching the sustained virologic response and reoccurs in the relapsers. A therapeutic strategy to improve IR and liver steatosis and subsequently the response to antiviral treatment in these patients is warranted.
Core tip: Three main types of steatosis in the patients with Hepatitis C virus (HCV) infection are known: a metabolic type associated with metabolic syndrome and two viral types: one that seems to be directly triggered by the virus and one that could originate from the interference of the virus in the mechanisms of insulin resistance. The first viral type is particularly widely considered to be predominant and, perhaps, strictly linked to HCV genotype 3 infection and its intra-hepatic viral load. This evidence is supported by the resolution of steatosis in most patients infected with genotype 3 virus after HCV eradication by antiviral therapy.
- Citation: Abenavoli L, Masarone M, Peta V, Milic N, Kobyliak N, Rouabhia S, Persico M. Insulin resistance and liver steatosis in chronic hepatitis C infection genotype 3. World J Gastroenterol 2014; 20(41): 15233-15240
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15233.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15233
Approximately 200 million of people worldwide are chronically infected with hepatitis C virus (HCV) which can result in progressive hepatic injury and fibrosis, culminating in cirrhosis and end-stage liver disease[1]. Many viral and host factors are involved in the disease progression and the response to antiviral treatment. Insulin resistance (IR) is in particular noteworthy. IR is defined as an impaired ability to clear glucose from the circulation at a given level of circulating insulin and is considered the main pathogenetic factor of the metabolic syndrome, a cluster of metabolic abnormalities (e.g., obesity, type 2 diabetes mellitus - T2DM, dyslipidemia), associated with an increased cardiovascular risk[2,3]. IR is related to the presence of non-alcoholic fatty liver disease (NAFLD), the most common cause of chronic liver disorder in the Western world. NAFLD is an umbrella term that embodies simple steatosis, non-alcoholic steatohepatitis (NASH) and advanced fibrosis or cirrhosis related to this pathological entity[4,5].
Several data have reported that liver steatosis is a common histological feature of chronic hepatitis C (CHC) infection. The prevalence of steatosis ranges from 40% to 86%[5,6] in these patients . Three types of steatosis have been defined in the HCV patients. The first is a metabolic type, associated with metabolic syndrome. The second is a viral steatosis without any known steatogenic co-factors and is directly linked to the cytopathic viral effect[6]. The third type can be considered a “middle ground” between the first and the second one: even if this entity is virus associated, it could be more appropriate to define it as a combination of viral and metabolic factors. This entity has been associated with a direct interference of HCV core protein in the intracellular, post-receptorial pathways of insulin. This evidence, mostly found in the HCV genotype 1b patients, convinced some authors to coin the term virus associated steatohepatitis (VASH)[7,8]. This finding is also supported by the reports enlightening this condition in the non-genotype 3 infected patients, to achieve the sustained virologic response (SVR) that can lead to the reduction or total disappearance of steatosis[9]. Nevertheless, virally induced steatosis is widely considered to be predominantly, and perhaps strictly, linked to HCV genotype 3 infection. Its severity correlates with the level of viral replication, and it disappears after successful antiviral therapy[10,11]. The biological mechanism of the underlying steatosis occurrence and the progression to the liver disease is not entirely understood and is probably due to a number of factors that are expressed in the context of genetic predisposition. In support to this assumption, there is evidence that in the genotype 3 infected patients a significant weight loss can also lead to a considerable reduction of liver steatosis[9] . This evidence demonstrates that in HCV genotype 3 infection steatosis may partially be related to the metabolic mechanisms. In this complex repertoire, a multi-step hypothesis, in which IR is still the key risk factor for the development of NAFLD, has been proposed[12-14].
The main treatment goal in the patients with chronic HCV infection is the SVR. A rapid virologic response (RVR, at week 4) predicts the SVR, although not all the patients with the RVR achieve the SVR. The therapeutic standard of care regimen for the untreated HCV-patients is the combination of pegylated interferon-α (PEG-IFN) with ribavirin (RBV)[15]. This therapy is highly successful in the patients with genotype 2 and 3 infections with the SVR that is defined by undetectable serum HCV-RNA and by quantitative PCR 24 wk after the end of treatment, ranges between of 76% and 82%[15,16]. International guidelines have been established that the PEG-IFN should be used at the dose of 180 μg/wk for PEG-IFN-α2a and 1.5 μg/kg per week for PEG-IFN-α2b respectively in the genotype 3 patients. The RBV dose should be administered with a flat dose of 800 mg/d[17]. However, IR is associated with a poor response to anti-viral treatment in the patients with HCV genotype 1, 2, and 3[18,19]. The recent introduction in clinical practice of protease inhibitors to treat CHC genotype 1 have increased the treatment response rate, which does not seem to be influenced by IR[20,21]. However, these drugs are not recommended to non-1 genotypes, and the triple therapy will not be available for all patients in many countries.
The aim of the present review is to summarize recent data on the relationship between HCV and IR, and in particular the role of the genotype 3 in the development of steatosis and its clinical management.
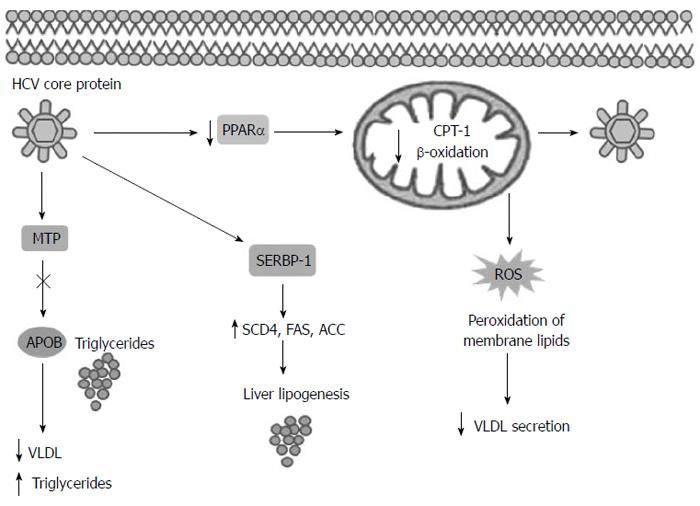
The pathogenetic link between IR and CHC is complex. IR promotes lipid accumulation, inflammation and fibrosis deposition in the liver with disease progression. On the other hand, HCV may itself induce a IR state, with T2DM promotion and lipid metabolism derangement[4]. Both NAFLD and HCV are associated with increased gluconeogenic drive and IR, as shown by the impaired suppression of hepatic glucose output by insulin[22,23]. Steatosis occurs frequently in the CHC patients, with a prevalence between 40% and 86% according to the genotype. The majority of the patients show simple steatosis, but features of NASH have been found in approximately 10% of the CHC patients[24]. In these subjects, macrovesicular steatosis is found in the periportal region of the liver, different from the centrilobular distribution characteristic of the NASH patients. This observation is genotype specific. In the CHC genotype 1 and 4, liver steatosis is associated with IR and is either virus-mediated, the above mentioned VASH, or is due to the host metabolic factors such as visceral obesity[14,19,25]. Conversely, hepatic steatosis in the HCV genotype 3 infection is predominantly a direct effect of the virus, occurring in the absence of other metabolic risk factors, such as the presence of NAFLD[26,27]. HCV genotype 3 modulates lipid metabolism and signaling via three distinct non-mutually exclusive mechanisms: by impared lipoprotein secretion or fatty acid degradation (β-oxidation) and by increased lipogenesis[28] (Figure 1). HCV core protein has been shown to inhibit microsomal triglyceride transfer protein (MTP) activity[29]. MTP is an enzyme that plays an important role in very-low-density lipoprotein (VLDL) assembly and apolipoprotein B (ApoB) secretion, its inhibition causes accumulation of triglycerides and steatosis[30]. HCV has been reported to upregulate sterol regulatory element binding protein (SREBP-1c) signaling pathway. SREBP-1c in the nucleus leading to activation of the enzymes involved in lipogenesis such as acetyl-CoA carboxylase (ACC), sterol CoA dehydrogenase 4 (SCD4) and fatty acid synthase (FAS)[28,31]. Moreover it’s showed that in the patients infected with the genotype 3 PPAR-α mRNA levels are significantly lower in the liver than in those infected with the other HCV genotype[32]. The HCV core protein reduces the expression of peroxisome proliferators-activated receptor (PPAR)-α, that is an important transcription factor involved in the regulation of several genes responsible for fatty acid degradation, like mitochondrial carnitine palmitoyltransferase type 1 (CPT)-1, an important enzyme of mitochondrial β-oxidation[32,33]. Recent data showed that HCV core may accumulate and interact with mitochondria and endoplasmic reticulum, inducing production of reactive oxygen species (ROS)[34]. The ROS production causes mitochondria damage and peroxidation of membrane lipids and structural proteins that are involved in VLDL trafficking and secretion, this mechanism leads to steatosis[14,34]. Okuda et al[35] have proposed that the core protein induces oxidative stress by the cytoepathic effect of high titre of intra-cytoplasmic negative strand HCV-RNA. Moreover, the patients infected with the genotype 3 present significantly lower homeostatic model assessment (HOMAR-IR) values than the patients infected with other genotypes, providing the support for a widely held view that hepatic steatosis is virally induced in this genotype infection and metabolically induced infections by other genotypes[36].
To support the role of different genotypes on lipid metabolism in the liver cells, Abid et al[37] developed an in vitro model to study the effect of the core protein belonging to several viral genotypes, in particular 1b, 2a, 3a, 3h, 4h and 5a. They concluded that the genotype 3a-derived core protein was about three times more potent than the corresponding protein from the genotype 1b at inducing triglycerides accumulation in transfected cells[37,38]. This evidence is supported by Pazienza et al[26,39] who using microarray analysis, showed that several genes, involving lipid transport and metabolism, were up- or down-regulated in a genotype-specific manner. In addition, some differences in the amino acid sequence in the core protein could explain, at least partially, that genotype 3a induces steatosis more efficiently than other genotypes. A specific polymorphism in the core protein from genotype 3 has been associated with a lipid accumulation in the hepatocytes. The evaluation of viral sequences, responsible for the genotype 3 core protein-steatogenic effects, has found the identification of a single amino-acid change, at the position 164[40]. Another study reported two amino-acid substitutions at the positions 182 and 186, specifically associated with lipid accumulation in hepatic cells and steatosis development[41]. However, the recent data have suggested that the polymorphism of various host genes, including the peroxisome proliferator-activated receptor-γ (PPAR-γ), interleukin-28 (IL-28)B, adiponutrin and microsomal triglyceride transfer protein (MTP) genes, may influence the development of more severe steatosis in the CHC patients. This phenomenon seems to concern the patients principally infected with non-genotype 3 viruses[42]. Indeed, steatosis often disappeared in the genotype 3 patients who had a SVR to standard PEG-IFN plus RBV treatment and recurred when HCV relapsed[43]. This phenomenon was not observed in other HCV genotypes.
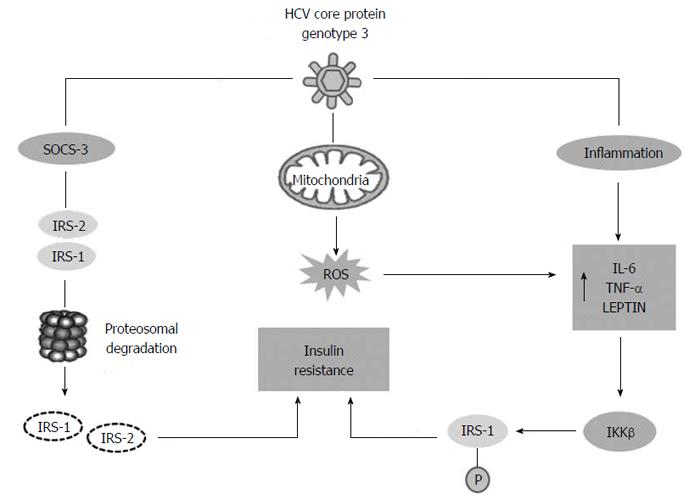
Molecular studies demonstrated the capacity of HCV genotype 3 to promote IR and type 2 diabetes (T2DM). HCV can induce insulin resistance in two different ways: the viral proteins can directly interfere with intra-cellular insulin signalling or the chronic inflammatory response can induces IR indirectly (Figure 2). In the directly mechanism HCV core protein stimulates increased levels of the molecule suppressor of cytokine signaling SOCS-3, that were demonstrated to be over-expressed in the insulin resistant, and causes proteasomal degradation of IRS-1 and IRS-2, two different molecules involved in insulin signalling. Chronic inflammation due to HCV plays an important role in IR, increased levels of IL-1, tumour necrosis factor (TNF)-α, IL-6 and leptin stimulate the expression of IKKβ, a protein kinase, that induces IR by inhibitory phosphorylation of IRS-1 at Serine 312[44]. Finally it was demonstrated that oxidative stress due to HCV infection may contribute to IR. High levels of ROS can activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) that is a transcription factor. As a consequence of NF-κB activation, expression of a variety of cytokines is increased, including tumour necrosis factors (TNF-α and TNF-β), interleukins (IL) 1, 6 and interferon-γ[45] .
All these changes can block the transactivation of glucose transporters (GLUT)-4 in the cells, suppressing glucose uptake, leading to a hyperinsulinism state. Increasing hepatic SOCS-3 expression has predictive a value for the outcome of anti-viral therapy in the patients with HCV infection[19]. Another steatogenic mechanism of genotype 3, is the down-regulation of PPAR-γ and up-regulation of SOCS-7, mediated by core protein[46]. However, Steatosis does not appear to predict the occurrence of fibrosis in CHC genotype 3[47]. In fact, even if it was demonstrated that, also in the genotype 3 patients, a significant weight loss could lead to a considerable reduction of liver steatosis[9], in a cohort study analysing the fibrosis progression rate in 1189 patients with known date of infection steatosis was not identified as an independent factor for accelerated fibrogenesis in genotype 3[11]. This suggests that viral steatosis contribute to the development of fibrosis, if a metabolic steatosis associated with IR and metabolic syndrome are present[47,48], which also may be likely present and active in the genotype 3 infected patients.
It is important to evaluate the impact of IR and liver steatosis on the antiviral treatment response in the patients with different genotypes. There is no consensus for the specific recommendations regarding diagnostic workup or therapy in the patients with CHC and IR. The IR development in the CHC patients should be assessed regularly by the HOMA-IR. The presence of IR and NAFLD reduces the probability of achieving the SVR after standard therapy (PEG-IFN and RBV) in genotype 3, especially if steatosis involves more than 33% of the liver[18,49]. The current data on this topic are summarized below.
Poustchi et al[50] demonstrated that IR was an independent predictor of lower SVR in 83 patients infected by genotypes 2 or 3 (or no-response with HOMA-IR > 2 OR = 6.5; 95%CI: 1.3-31.9; P = 0.02). Considering that steatosis development in CHC genotype 3 and its pathogenic mechanisms might be different to that one involved in the patients with genotype 2, the authors performed separate analyses, including only 67 genotype 3 subjects. The data were similar to the SVR rates of 93% in those with a HOMA-IR of < 2, while in those with HOMA-IR ≥ 2 was 64% (P < 0.01). Similarly, steatosis was not a predictor of the SVR among the patients with genotype 3. Another study on 62 subjects with different HCV genotypes (1, 2 and 3) demonstrated that SVR was reduced in the patients with HOMA-IR > 3, but this difference was not sustained when analysed for each genotype individually[51]. Also, the RVR was reduced in the patients with genotype 3, when HOMA-IR was > 3, although the difference was not statistically significant in relation to the small numbers of the patients included in the cohort study. A meta-analysis, aiming to evaluate the impact of IR on the SVR in CHC genotypes 1, 2, 3 and 4, reported that the elevated HOMA-IR was associated with a lower cure rate of the HCV patients treated with PEG-INF and RBV, independently of viral genotype[52]. However, the authors reported the differences on the determination and calculation of HOMA-IR in the considered studies. The same study group, with the purpose to investigate the effect of IR on SVR in 263 HCV subjects, subsequently demonstrated that the SVR rate was significantly reduced among the patients with HOMA-IR ≥ 2 for each genotype analyzed (1, 3, and 4)[53]. This difference was maintained even when different cut-off values of HOMA-IR were used to define IR (> 3 and > 4). In addition, the presence of IR, measured by the HOMA-IR, presents also a prognostic value in the standard therapy of the patients with genotype 2 or 3. However, despite these data, the SVR variation in HOMA-IR values and HCV genotype remain controversial. Fattovich et al[54] reported the association between HOMA-IR and rapid virologic response (RVR). In particular, the subgroup of the univariate analysis, according to infecting genotype, showed that a baseline HOMA-IR ≤ 2 was the only predictive factor of the RVR in the genotype 3 patients (P = 0.02), and the multivariate analysis, according to infecting genotype, confirmed the HOMA-IR ≤ 2 as an independent predictor of the RVR, but only in genotype 3 (OR = 5.3; 95%CI: 1.0-27.7; P = 0.05). Finally, the RVR was also detected as a predictive factor of the SVR in the genotype 3 patients (P = 0.02), in addition to lower body mass index (P = 0.004). It was clear that the presence of the IR was associated with reduced the SVR rates in the patients infected with HCV genotype 3, and that HOMA-IR index could be used as a predictive factor for the SVR in these patients. This presents a debating point on the recent meta-analyses, as there is no consensus regarding the HOMA-IR value cut-off that defines the IR, because different values (i.e., 2, 2.5, 2.7 or 3) were employed in literature[55]. Shah et al[56] reported that even in HCV genotype 3, steatosis was the independent predictor of relapse, independently of viral load, in the patients who achieved the RVR with PEG-IFN plus RBV regimen after 24 wk (OR = 3.0; 95%CI: 1.5-6.1; P = 0.003). These data were subsequently confirmed by Restivo et al[57] in the study where the therapy was reduced to 12 wk (OR = 0.988; 95%CI: 0.981-0.993; P < 0.001). Given these results, it is easy to assume that in genotype 3 steatosis might also be linked, not only to the direct viral mechanisms, but also to the insulin resistance that might originate from the genetic characteristics of the host and the activity of the virus itself on it, which may influence the response to the antiviral therapy. For this reason, the guidelines of European Association for the Study of the Liver (EASL) on the choice of the treatment duration reported that in the patients with HCV genotypes 3 who presented advanced fibrosis, cirrhosis or cofactors as well IR should not be considered for shortening of the treatment duration, even if they had low baseline HCV-RNA and RVR[15].
In the recent years, several potential direct-acting antiviral drugs (DAAs) targeting viral proteins (NS3/4A protease, NS5B nucleos(t)idic and non-nucleos(t)idic polymerase, NS5A viral replication complex) have been developped. However, the sensitivity of HCV genotype 3 to those new drugs remains relative and have raised questions on how to achieve universal cure. First generation protease inhibitors in combination with PEG-IFN/RBV are not efficient in genotype 3-infected patients. The combination of PEG-IFN/RBV with the oral nucleotidic polymerase inhibitor sofosbuvir (GS-7977) for 12 wk in naïve patients results in a SVR in more than 95%[58]. The results of the combination of sofosbuvir and RBV for 12 wk in genotype 3-infected patients have been rather disappointing with a slightly lower SVR than after 24 wk of PEG-IFN: around 60%, and only 30% in patients with cirrhosis. Extending treatment from 12 to 16 wk in treatment experienced patients doubled the SVR rate and an 80% SVR rate is expected by extending treatment to 24 wk[59,60]. There is no data on the influence of baseline insulin resistance on SVR in HCV genotype 3 patients treated with DAAs[61].
Steatosis development and CHC infection are clearly linked. HCV induces IR by several pathogenetic mechanisms that are also implicated in a treatment resistance and an increase in fibrosis progression. In this way, the antiviral responsiveness remains the major clinical problem in the eradication of HCV, even with the use of new drugs. The value of HOMA-IR index has been largely discussed. HOMA-IR index, measured before the start of the antiviral therapy, could be useful in clinical practice to improve the basic evaluation and to predict the treatment response. Defining the reliable HOMA-IR cut-off is probably the first step to determine the presence of IR, in particular, in the CHC patients infected with genotype 3 whose liver steatosis is induced by direct viral action. The 2014 EASL clinical practice guideline for the management of hepatitis C indicate that for HCV genotype 3 with the RVR and low viral load shortening of the treatment duration should be considered[15]. However, before starting this therapeutic approach it is also necessary to evaluate the presence of steatosis and its degree that is associated with significantly higher rates of relapse, irrespective of viral load, especially in the patients infected with HCV genotype 3 who have an RVR[56,57]. Further studies are needed to determine a new therapeutic strategy in this subgroup of the patients, especially, if the emergence of direct-acting antiviral drugs is near to come.
P- Reviewer: Grasso A, Larrubia JR, Takaki A S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1770] [Cited by in F6Publishing: 1796] [Article Influence: 163.3] [Reference Citation Analysis (0)] |
| 2. | Asselah T, Estrabaud E, Bieche I, Lapalus M, De Muynck S, Vidaud M, Saadoun D, Soumelis V, Marcellin P. Hepatitis C: viral and host factors associated with non-response to pegylated interferon plus ribavirin. Liver Int. 2010;30:1259-1269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Kosterin SA, Bratkova NF, Kurskiĭ MD. [Possible mechanism of the regulation of smooth muscle relaxation by calcium ions]. Biofizika. 1987;32:327-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 32] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Abenavoli L, Rouabhia S. Type 2 diabetes mellitus in chronic hepatitis C virus infection: risk factor or consequence? Expert Rev Gastroenterol Hepatol. 2013;7:295-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, Gasbarrini A, Loguercio C, Lonardo A, Marchesini G. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 6. | Persico M, Iolascon A. Steatosis as a co-factor in chronic liver diseases. World J Gastroenterol. 2010;16:1171-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Koike K, Moriya K. Metabolic aspects of hepatitis C viral infection: steatohepatitis resembling but distinct from NASH. J Gastroenterol. 2005;40:329-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Masarone M, La Mura V, Bruno S, Gaeta GB, Vecchione R, Carrino F, Moschella F, Torella R, Persico M. Steatohepatitis is associated with diabetes and fibrosis in genotype 1b HCV-related chronic liver disease. J Viral Hepat. 2007;14:714-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Poynard T, Ratziu V, McHutchison J, Manns M, Goodman Z, Zeuzem S, Younossi Z, Albrecht J. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 10. | Negro F. Steatosis and insulin resistance in response to treatment of chronic hepatitis C. J Viral Hepat. 2012;19 Suppl 1:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Bochud PY, Cai T, Overbeck K, Bochud M, Dufour JF, Müllhaupt B, Borovicka J, Heim M, Moradpour D, Cerny A. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51:655-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E, Ventura V, Greco M, Abenavoli L, Belfiore A. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond). 2010;34:1255-1264. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Adinolfi LE, Restivo L, Marrone A. The predictive value of steatosis in hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2013;7:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127-2133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 191] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 16. | Sarin SK, Kumar CK. Treatment of patients with genotype 3 chronic hepatitis C--current and future therapies. Liver Int. 2012;32 Suppl 1:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Abenavoli L, Mazza M, Almasio PL. The optimal dose of ribavirin for chronic hepatitis C: From literature evidence to clinical practice: The optimal dose of ribavirin for chronic hepatitis C. Hepat Mon. 2011;11:240-246. [PubMed] [Cited in This Article: ] |
| 18. | Sanyal AJ. Role of insulin resistance and hepatic steatosis in the progression of fibrosis and response to treatment in hepatitis C. Liver Int. 2011;31 Suppl 1:23-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Crocè L, La Mura V, Moschella F, Masutti F. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Serfaty L, Forns X, Goeser T, Ferenci P, Nevens F, Carosi G, Drenth JP, Lonjon-Domanec I, DeMasi R, Picchio G. Insulin resistance and response to telaprevir plus peginterferon α and ribavirin in treatment-naive patients infected with HCV genotype 1. Gut. 2012;61:1473-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Chou R, Hartung D, Rahman B, Wasson N, Cottrell EB, Fu R. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: a systematic review. Ann Intern Med. 2013;158:114-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Arrese M, Riquelme A, Soza A. Insulin resistance, hepatic steatosis and hepatitis C: a complex relationship with relevant clinical implications. Ann Hepatol. 2010;9 Suppl:112-118. [PubMed] [Cited in This Article: ] |
| 23. | Mancone C, Steindler C, Santangelo L, Simonte G, Vlassi C, Longo MA, D’Offizi G, Di Giacomo C, Pucillo LP, Amicone L. Hepatitis C virus production requires apolipoprotein A-I and affects its association with nascent low-density lipoproteins. Gut. 2011;60:378-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Bedossa P, Moucari R, Chelbi E, Asselah T, Paradis V, Vidaud M, Cazals-Hatem D, Boyer N, Valla D, Marcellin P. Evidence for a role of nonalcoholic steatohepatitis in hepatitis C: a prospective study. Hepatology. 2007;46:380-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Fukasawa M, Tanaka Y, Sato S, Ono Y, Nitahara-Kasahara Y, Suzuki T, Miyamura T, Hanada K, Nishijima M. Enhancement of de novo fatty acid biosynthesis in hepatic cell line Huh7 expressing hepatitis C virus core protein. Biol Pharm Bull. 2006;29:1958-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Pazienza V, Vinciguerra M, Andriulli A, Mangia A. Hepatitis C virus core protein genotype 3a increases SOCS-7 expression through PPAR-{gamma} in Huh-7 cells. J Gen Virol. 2010;91:1678-1686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Hwang SJ, Lee SD. Hepatic steatosis and hepatitis C: Still unhappy bedfellows? J Gastroenterol Hepatol. 2011;26 Suppl 1:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Khan M, Jahan S, Khaliq S, Ijaz B, Ahmad W, Samreen B, Hassan S. Interaction of the hepatitis C virus (HCV) core with cellular genes in the development of HCV-induced steatosis. Arch Virol. 2010;155:1735-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 445] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 30. | Mirandola S, Realdon S, Iqbal J, Gerotto M, Dal Pero F, Bortoletto G, Marcolongo M, Vario A, Datz C, Hussain MM. Liver microsomal triglyceride transfer protein is involved in hepatitis C liver steatosis. Gastroenterology. 2006;130:1661-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 1665] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 32. | de Gottardi A, Pazienza V, Pugnale P, Bruttin F, Rubbia-Brandt L, Juge-Aubry CE, Meier CA, Hadengue A, Negro F. Peroxisome proliferator-activated receptor-alpha and -gamma mRNA levels are reduced in chronic hepatitis C with steatosis and genotype 3 infection. Aliment Pharmacol Ther. 2006;23:107-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Tsutsumi T, Suzuki T, Shimoike T, Suzuki R, Moriya K, Shintani Y, Fujie H, Matsuura Y, Koike K, Miyamura T. Interaction of hepatitis C virus core protein with retinoid X receptor alpha modulates its transcriptional activity. Hepatology. 2002;35:937-946. [PubMed] [Cited in This Article: ] |
| 34. | Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 35. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 690] [Cited by in F6Publishing: 668] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 36. | Aghemo A, Prati GM, Rumi MG, Soffredini R, D’Ambrosio R, Orsi E, De Nicola S, Degasperi E, Grancini V, Colombo M. Sustained virological response prevents the development of insulin resistance in patients with chronic hepatitis C. Hepatology. 2012;56:1681-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | Abid K, Pazienza V, de Gottardi A, Rubbia-Brandt L, Conne B, Pugnale P, Rossi C, Mangia A, Negro F. An in vitro model of hepatitis C virus genotype 3a-associated triglycerides accumulation. J Hepatol. 2005;42:744-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 132] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: a way out for the virus? World J Gastroenterol. 2009;15:5014-5019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 42] [Cited by in F6Publishing: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Pazienza V, Clément S, Pugnale P, Conzelmann S, Pascarella S, Mangia A, Negro F. Gene expression profile of Huh-7 cells expressing hepatitis C virus genotype 1b or 3a core proteins. Liver Int. 2009;29:661-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Hourioux C, Patient R, Morin A, Blanchard E, Moreau A, Trassard S, Giraudeau B, Roingeard P. The genotype 3-specific hepatitis C virus core protein residue phenylalanine 164 increases steatosis in an in vitro cellular model. Gut. 2007;56:1302-1308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Jhaveri R, McHutchison J, Patel K, Qiang G, Diehl AM. Specific polymorphisms in hepatitis C virus genotype 3 core protein associated with intracellular lipid accumulation. J Infect Dis. 2008;197:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Cai T, Dufour JF, Muellhaupt B, Gerlach T, Heim M, Moradpour D, Cerny A, Malinverni R, Kaddai V, Bochud M. Viral genotype-specific role of PNPLA3, PPARG, MTTP, and IL28B in hepatitis C virus-associated steatosis. J Hepatol. 2011;55:529-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 43. | Castéra L, Hézode C, Roudot-Thoraval F, Lonjon I, Zafrani ES, Pawlotsky JM, Dhumeaux D. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009;15:4356-4364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Wright E, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006;60:308-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 46. | Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 47. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 48. | Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010;59:1279-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Abenavoli L, Almasio PL. Chronic hepatitis C infection and insulin resistance: two best friends. Expert Rev Anti Infect Ther. 2011;9:555-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 51. | Bortoletto G, Scribano L, Realdon S, Marcolongo M, Mirandola S, Franceschini L, Bonisegna S, Noventa F, Plebani M, Martines D. Hyperinsulinaemia reduces the 24-h virological response to PEG-interferon therapy in patients with chronic hepatitis C and insulin resistance. J Viral Hepat. 2010;17:475-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Eslam M, Aparcero R, Kawaguchi T, Del Campo JA, Sata M, Khattab MA, Romero-Gomez M. Meta-analysis: insulin resistance and sustained virological response in hepatitis C. Aliment Pharmacol Ther. 2011;34:297-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Eslam M, Aparcero R, Mousa YI, Grande L, Shaker Y, Ali A, Del Campo JA, Khattab MA, Romero-Gomez M. Insulin resistance impairs viral dynamics independently of ethnicity or genotypes. J Clin Gastroenterol. 2012;46:228-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Fattovich G, Covolo L, Pasino M, Perini E, Rossi L, Brocco G, Guido M, Cristofori C, Belotti C, Puoti M. The homeostasis model assessment of the insulin resistance score is not predictive of a sustained virological response in chronic hepatitis C patients. Liver Int. 2011;31:66-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Laurito MP, Parise ER. Association between insulin resistance and sustained virologic response in hepatitis C treatment, genotypes 1 versus 2 and 3: systematic literature review and meta-analysis. Braz J Infect Dis. 2013;17:555-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Shah SR, Patel K, Marcellin P, Foster GR, Manns M, Kottilil S, Healey L, Pulkstenis E, Subramanian GM, McHutchison JG. Steatosis is an independent predictor of relapse following rapid virologic response in patients with HCV genotype 3. Clin Gastroenterol Hepatol. 2011;9:688-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Restivo L, Zampino R, Guerrera B, Ruggiero L, Adinolfi LE. Steatosis is the predictor of relapse in HCV genotype 3- but not 2-infected patients treated with 12 weeks of pegylated interferon-α-2a plus ribavirin and RVR. J Viral Hepat. 2012;19:346-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Pol S, Vallet-Pichard A, Corouge M. Treatment of hepatitis C virus genotype 3-infection. Liver Int. 2014;34 Suppl 1:18-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1322] [Cited by in F6Publishing: 1287] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 60. | Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 846] [Cited by in F6Publishing: 820] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 61. | Grasso A, Malfatti F, Testa R. Are metabolic factors still important in the era of direct antiviral agents in patients with chronic hepatitis C? World J Gastroenterol. 2013;19:6947-6956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |