Published online Oct 28, 2014. doi: 10.3748/wjg.v20.i40.14986
Revised: April 27, 2014
Accepted: June 12, 2014
Published online: October 28, 2014
Processing time: 245 Days and 18.7 Hours
AIM: To evaluate the effect of bursectomy on overall survival, recurrence-free survival and safety of patients with gastric cancer by performing a meta-analysis.
METHODS: A literature search was performed in PubMed, EMBASE, and the Cochrane Library databases for clinical research that compared bursectomy with non-bursectomy published before October 2013. Inclusion and exclusion criteria were established and applied. Overall survival, recurrence-free survival, complications, hospital stay, operative time and blood loss were compared using hazard ratios (HRs), relative risks and weighted mean differences. Stata 12.0 software was used for statistical analysis.
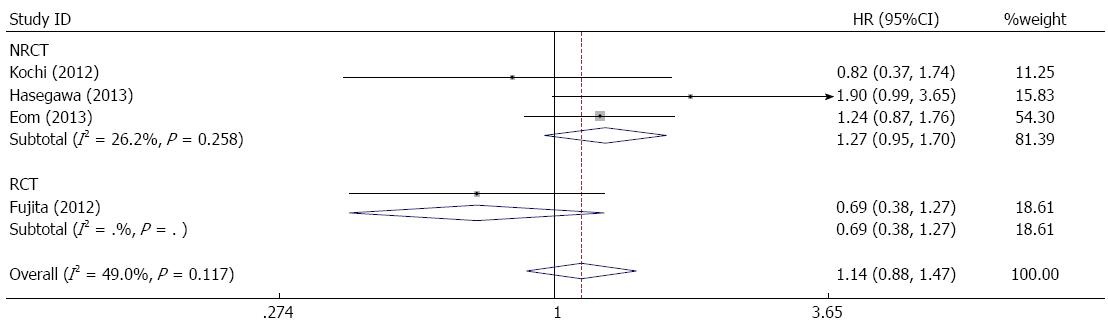
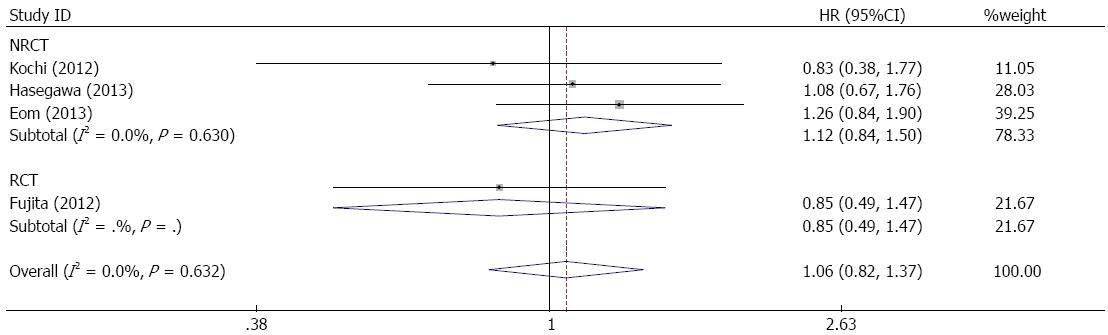
RESULTS: Four studies including 1130 patients were available for the analysis (430 in the bursectomy group, 700 in the non-bursectomy group). No statistically significant difference was observed in the rate of complications between the bursectomy group and the non-bursectomy group. Bursectomy did not have a significant effect (combined HR = 1.14, 95%CI: 0.88-1.47) on overall survival, and it was not a significant factor for recurrence-free survival (combined HR = 1.06, 95%CI: 0.82-1.37).
CONCLUSION: Gastrectomy with bursectomy is not superior to non-bursectomy in terms of survival. Bursectomy is not recommended as a routine procedure for the surgical treatment of gastric cancer.
Core tip: To our knowledge, no large-scale studies have investigated the survival benefit of bursectomy. Several studies have described the relationship between bursectomy and prognosis in gastric cancer. However, the results were inconsistent. The purpose of this study was to determine the survival benefit of bursectomy by meta-analysis comparing the prognosis in patients undergoing gastrectomy with bursectomy for gastric cancer with that in patients undergoing gastrectomy alone.
- Citation: Shen WS, Xi HQ, Wei B, Chen L. Effect of gastrectomy with bursectomy on prognosis of gastric cancer: A meta-analysis. World J Gastroenterol 2014; 20(40): 14986-14991
- URL: https://www.wjgnet.com/1007-9327/full/v20/i40/14986.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i40.14986
Gastric cancer is a common malignant tumor and a major health problem worldwide[1,2]. Although the incidence of gastric cancer has been declining in the West, it remains the second leading cause of cancer-related death in the world, especially in some Eastern countries, such as Japan, South Korea and China[3-5]. For curative purposes, surgery is considered the optimal treatment; however, the clinical value of bursectomy in addition to gastrectomy in curable gastric cancer is controversial. The Japanese Gastric Cancer Association’s gastric cancer treatment guidelines recommend bursectomy for tumors with invasion of the serosa, and should be avoided in T1/T2 tumors to prevent injury to the pancreas or adjacent blood vessels[6].
To the best of our knowledge, no large-scale studies have investigated the survival benefit of bursectomy. Several studies have described the relationship between bursectomy and prognosis in gastric cancer[7-11]. However, the results were inconsistent. Meta-analysis is a popular and powerful tool that overcomes the limitation of small sample sizes by combining results from several individual studies to generate a best assessment[12]. There is evidence that pooling of high-quality non-randomized comparative trials (NRCTs) is as credible as pooling of randomized controlled trials (RCTs) when comparing surgical outcomes[13]. In this study, we analyzed systematically high-quality clinical trials, compared bursectomy with non-bursectomy and performed a meta-analysis of combined RCTs and NRCTs.
The purpose of this meta-analysis was to determine the survival benefit of bursectomy by comparing the prognosis of patients undergoing gastrectomy with bursectomy for gastric cancer with that of patients undergoing gastrectomy alone.
We searched the electronic databases of PubMed, EMBASE, and the Cochrane Library up to October 2013. The language was restricted to English. We used the following search terms: gastric cancer, bursectomy, prognosis or prognostic or survival. The references of all relevant articles were evaluated to identify other related studies.
Eligibility criteria for inclusion in this meta-analysis were as follows: (1) studies that compared the outcomes of gastrectomy with non-bursectomy; (2) evaluation of the correlation between bursectomy and overall survival in gastric cancer patients; and (3) publication as a full text in the English language. Studies that included recurrent gastric cancer, gastrointestinal stromal tumors or benign gastric diseases were excluded unless the data were presented separately.
Quality assessment was peer-reviewed by two reviewers independently in each of the included studies. The Jadad Scale[14] assessed the methodological quality of the eligible RCTs, and the Methodological Index assessed that of the NRCTs for Nonrandomized Studies (MINORS)[15].
Two investigators (Shen WS, Xi HQ) independently extracted the data. The data extracted included the author’s name, year of publication, study location, number of patients and tumor characteristics. Univariate hazard ratios (HRs) and 95%CI were used to calculate the overall survival. Some of the studies provided HRs and 95%CI values. If HR and 95%CI values were not directly reported in the included studies, we assessed the values in the original studies using the methods illustrated by Parmar et al[16]. In addition, if the original studies included the median, range and the size of a sample, we estimated the mean and variance using the methods illustrated by Hozo et al[17].
Stata 12.0 software (StatCorp, College Station, TX, United States) was used for statistical analysis. Weighted mean differences (WMDs) were used to compare operative time, estimated blood loss and hospital stay. HRs were used to compare overall survival and recurrence-free survival. Relative risks (RRs) were used to compare complications. The Cochran Q and I2 statistics were used to assess heterogeneity and were considered significant when P < 0.1. If heterogeneity was found, the random effects model was used. Begg’s funnel plot and Egger’s linear regression test[18] were used to assess publication bias.
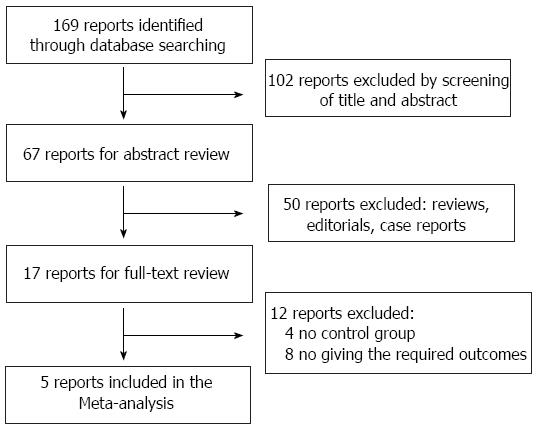
A total of 169 articles were retrieved from a search of the above databases using the described search strategies. After screening the titles and abstracts, 102 reports were excluded. After reading the abstracts, we excluded 50 reports as they were either a review, editorial, or case report. On full-text review, four reports were excluded that lacked a control group. Eight reports were excluded as the required outcomes were not stated in the studies. Fujita’s and Imamura’s reports, which were published by the same institute, were included as one study, and shared the same study number, as the separately published data was complementary[10,11]. Therefore, four studies were eligible for the meta-analysis (Figure 1). The characteristics of the four eligible studies are summarized in Table 1.
| Ref. | Year | Nation | Study type | Study year | TNM stage | Sample size | Outcomes | Quality score1 | |
| Bursectomy(+) | Bursectomy(-) | ||||||||
| Kochi et al[7] | 2014 | Japan | NRCT | 2004-2009 | pIA-IIIC | 121 | 133 | OS, RFS | 15/24 |
| Hasegawa et al[8] | 2013 | Japan | NRCT | 2000-2009 | pI-III | 98 | 98 | OS, RFS | 17/24 |
| Eom et al[9] | 2013 | South Korea | NRCT | 2001-2006 | cI-IV | 107 | 363 | OS, RFS | 13/24 |
| Fujita et al[10] | 2012 | Japan | RCT | 2002-2007 | cT2-T3 | 104 | 106 | OS, RFS | 3/5 |
| Imamura et al[11] | 2011 | Japan | RCT | 2002-2007 | cT2-T3 | 104 | 106 | - | 3/5 |
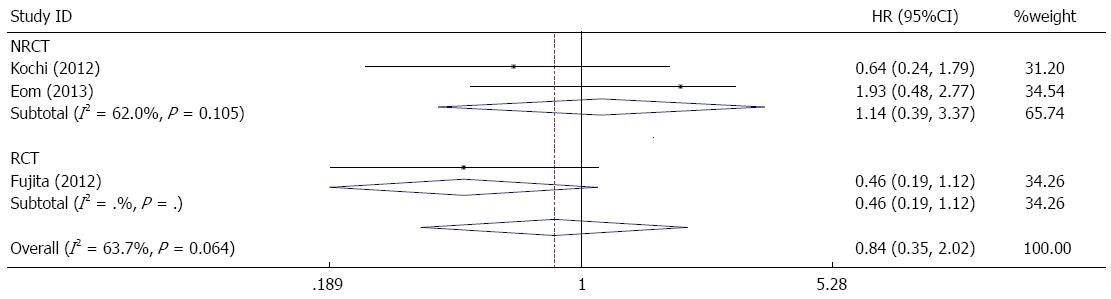
There was no significant difference in overall survival when a forest plot of the individual HRs was constructed (combined HR = 1.14, 95%CI: 0.88-1.47) (Figure 2). In the subgroup of serosa-positive patients, the difference in overall survival between the bursectomy and non-bursectomy groups was not statistically significant (combined HR = 0.84, 95%CI: 0.35-2.02) (Figure 3).
All studies reported recurrence-free survival of patients. Figure 4 shows a forest plot of recurrence-free survival and the results of the meta-analysis. Bursectomy did not have a significant association with increased recurrence-free survival of gastric cancer patients (combined HR = 1.06, 95%CI:0.82-1.37). In the subgroup of serosa-positive patients, no significant difference in recurrence-free survival was observed (combined HR = 0.81, 95%CI: 0.55-1.20) (Figure 5).
As indicated in Table 2, we assessed the association between bursectomy and postoperative complications in the patients. The difference in complications was not statistically significant between the groups (combined RR = 0.98, 95%CI: 0.80-1.21). Operative time was recorded in the four studies. In a forest plot, operative time was longer for the bursectomy group than for the non-bursectomy group (combined WMD = 105.61 min, 95%CI: 2.27-208.94). Data regarding blood loss was reported in three studies. The bursectomy group had more blood loss than the non-bursectomy group (combined WMD = 254.91 mL, 95%CI: 154.21-355.62). Two studies reported data on the adequacy of hospital stay, and there was no difference in hospital stay between the bursectomy group and the non-bursectomy group (combined WMD = -4.83, 95%CI:-12.96-3.30).
| Outcomes | Subgroup | n | Heterogeneity | Effect size | 95%CI of effect | P value | |
| I2 | P value | ||||||
| Complications | NRCT | 3 | 0.0% | 0.794 | RR = 0.98 | 0.78-1.22 | 0.838 |
| RCT | 1 | - | - | RR = 1.02 | 0.53-1.98 | 0.955 | |
| Overall | 4 | 0.0% | 0.926 | RR = 0.98 | 0.80-1.21 | 0.866 | |
| Operative time (min) | NRCT | 3 | 79.6% | 0.007 | WMD = 57.18 | 35.57-78.79 | 0.000 |
| RCT | 1 | - | - | WMD = 244.20 | 230.38-258.02 | 0.000 | |
| Overall | 4 | 99.4% | 0.000 | WMD = 105.61 | 2.27-208.94 | 0.045 | |
| Blood loss (mL) | NRCT | 2 | 78.9% | 0.029 | WMD = 229.63 | 106.77-352.49 | 0.000 |
| RCT | 1 | - | - | WMD = 338.00 | 184.21-491.79 | 0.000 | |
| Overall | 3 | 68.2% | 0.043 | WMD = 254.91 | 154.21-355.62 | 0.000 | |
| Hospital day (d) | NRCT | 2 | 96.3% | 0.000 | WMD = 4.83 | -12.96- 3.30 | 0.244 |
| RCT | 0 | - | - | - | - | - | |
| Overall | 2 | 96.3% | 0.000 | WMD = -4.83 | -12.96-3.30 | 0.244 | |
The assessment of publication bias showed that Egger’s test (P = 0.729) and Begg’s test (P = 1.0) were not significant (P > 0.05) in the analysis of overall survival in the included studies. A funnel plot analysis of the studies is shown in Figure 6.
Gastric cancer can be divided into two levels of severity, early gastric cancer and advanced gastric cancer. Advanced gastric cancer accounts for 92%-95% of cases in China, 40%-60% in Japan, and 80%-90% in Europe[19,20]. Radical gastrectomy is commonly accepted as the preferred treatment for advanced gastric cancer. The five-year survival rate in patients with advanced gastric cancer is 45% in Japan, 40% in China, only about 20% in Western countries and as low as 6% in sub-Saharan Africa[21-24]. Bursectomy, a procedure that allows radical gastrectomy and potentially decreases the incidence of recurrence, has been described in the Japanese Gastric Cancer Treatment Guidelines[25,26]. However, the effect of complete bursectomy on overall survival in gastric cancer patients remains unclear. In this study, we compared overall survival, recurrence-free survival, complications, operative time, blood loss, and hospital stay between the bursectomy and non-bursectomy groups.
Based on the present meta-analysis, overall survival was not statistically different between the two groups, and was slightly better in the non-bursectomy group. Bursectomy is unlikely to improve overall survival in gastric cancer patients at all T stages. In the subgroup of serosa-positive patients, the analysis revealed that the bursectomy group had better overall survival than the non-bursectomy group, although this difference was not statistically significant. Similarly, recurrence-free survival benefits were not found in the bursectomy group, and no difference was observed between the two groups (Figure 3). However, the bursectomy group had better recurrence-free survival than the non-bursectomy group in the subgroup of serosa-positive patients. Therefore, bursectomy is not considered an effective treatment for gastric cancer patients at all T stages, as it results in decreased overall survival and recurrence-free survival. However, in serosa-positive patients, bursectomy could improve overall survival and recurrence-free survival.
With regard to the safety of bursectomy, we found that complications and hospital stay were equivalent between the bursectomy and non-bursectomy groups. However, bursectomy tended to increase operative time and blood loss. Although surgical blood loss and operative time were significantly increased in the bursectomy group, overall, we concluded that gastrectomy with bursectomy is safe and acceptable.
We attempted to identify all related studies by performing a literature search using PubMed, Embase, and the Cochrane library databases. Our review only included fully published studies. Unpublished studies and conference abstracts were not included in our meta-analysis, as the required data were unavailable. In addition, our search was conducted with language restrictions. This analysis only included fully published studies in English. For those reasons, only four studies were included in this meta-analysis.
In conclusion, bursectomy did not show superiority to non-bursectomy in terms of survival in gastric cancer patients. Although the subgroup analyses suggested that bursectomy may improve survival in serosa-positive patients, this was not statistically significant and a definitive conclusion could not be made. Therefore, well-designed large-scale studies are required to obtain definitive data. Based on the current evidence, gastrectomy with bursectomy is not recommended as a routine procedure for the surgical treatment of gastric cancer.
Bursectomy, is a procedure that allows radical gastrectomy and potentially reduces the incidence of recurrence; however, the clinical value of bursectomy in addition to gastrectomy in curable gastric cancer is controversial.
Several studies have described the relationship between bursectomy and prognosis in gastric cancer. However, the results were inconsistent. The aim of this meta-analysis was to evaluate the effect of bursectomy on overall survival, recurrence-free survival and the safety of patients with gastric cancer.
Gastrectomy with bursectomy did not show superiority compared with non-bursectomy in terms of survival. Bursectomy is not recommended as a routine procedure for the surgical treatment of gastric cancer.
Based on current evidence, gastrectomy with bursectomy is not recommended as a routine procedure for the surgical treatment of gastric cancer. Well-designed large-scale studies are required to obtain definitive data.
This is nicely written manuscript providing important information about bursectomy as an advanced technique for the treatment of gastric cancer.
P- Reviewer: Mou YP, Shinohara T, Vix M S- Editor: Qi Y L- Editor: Stewart G E- Editor: Zhang DN
| 1. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2645] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Mansfield PF, Pisters PW, Feig BW, Janjan NA, Crane C, Ajani JA. Combined-modality therapy for gastric cancer. Semin Surg Oncol. 2003;21:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Jeung HC, Rha SY, Noh SH, Min JS, Kim BS, Chung HC. Adjuvant 5-fluorouracil plus doxorubicin in D2-3 resected gastric carcinoma: 15-year experience at a single institute. Cancer. 2001;91:2016-2025. [PubMed] |
| 4. | Parkin DM. International variation. Oncogene. 2004;23:6329-6340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 460] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 5. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [PubMed] |
| 6. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 7. | Kochi M, Fujii M, Kanamori N, Kaiga T, Mihara Y, Funada T, Tamegai H, Takayama Y, Yoshida N, Takayama T. D2 gastrectomy with versus without bursectomy for gastric cancer. Am J Clin Oncol. 2014;37:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Hasegawa S, Kunisaki C, Ono H, Oshima T, Fujii S, Taguri M, Morita S, Sato T, Yamada R, Yukawa N. Omentum-preserving gastrectomy for advanced gastric cancer: a propensity-matched retrospective cohort study. Gastric Cancer. 2013;16:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Eom BW, Joo J, Kim YW, Bae JM, Park KB, Lee JH, Ryu KW, Kook MC. Role of bursectomy for advanced gastric cancer: result of a case-control study from a large volume hospital. Eur J Surg Oncol. 2013;39:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Fujita J, Kurokawa Y, Sugimoto T, Miyashiro I, Iijima S, Kimura Y, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Survival benefit of bursectomy in patients with resectable gastric cancer: interim analysis results of a randomized controlled trial. Gastric Cancer. 2012;15:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Imamura H, Kurokawa Y, Kawada J, Tsujinaka T, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Influence of bursectomy on operative morbidity and mortality after radical gastrectomy for gastric cancer: results of a randomized controlled trial. World J Surg. 2011;35:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267-285. [PubMed] |
| 13. | Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. 2010;63:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [PubMed] |
| 15. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [PubMed] |
| 16. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [PubMed] |
| 17. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6895] [Article Influence: 344.8] [Reference Citation Analysis (0)] |
| 18. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 19. | Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000;3:9-18. [PubMed] |
| 20. | Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol. 2005;2:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Ito Y, Ohno Y, Rachet B, Coleman MP, Tsukuma H, Oshima A. Cancer survival trends in Osaka, Japan: the influence of age and stage at diagnosis. Jpn J Clin Oncol. 2007;37:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 669] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 23. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 24. | Chen L, Zhang Y, Wei B, Zhao XY, Li T. [Surgical treatment for patients with gastric cancer: report of 2335 cases]. Zhonghua Wei Chang Wai Ke Zazhi. 2007;10:421-424. [PubMed] |
| 25. | Groves EW. On the radical operation for cancer of the pylorus: with especial reference to the advantages of the two-stage operation and to the question of the removal of the associated lymphatics. Br Med J. 1910;1:366-370. [PubMed] |
| 26. | Hagiwara A, Sawai K, Sakakura C, Shirasu M, Ohgaki M, Yamasaki J, Togawa T, Takahashi T. Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepatogastroenterology. 1998;45:1922-1929. [PubMed] |