Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13556
Revised: June 11, 2014
Accepted: June 25, 2014
Published online: October 7, 2014
Processing time: 180 Days and 20.6 Hours
AIM: To test a new safe and simple technique for circular-stapled esophagojejunostomy in laparoscopic total gastrectomy (LATG).
METHODS: We selected 26 patients with gastric cancer who underwent LATG and Roux-en-Y gastrointestinal reconstruction with semi-end-to-end esophagojejunal anastomosis.
RESULTS: LATG with semi-end-to-end esophagojejunal anastomosis was successfully performed in all 26 patients. The average operation time was 257 ± 36 min, with an average anastomosis time of 51 ± 17 min and an average intraoperative blood loss of 88 ± 46 mL. The average postoperative hospital stay was 8 ± 3 d. There were no complications and no mortality in this series.
CONCLUSION: The application of semi-end-to-end esophagojejunal anastomosis after LATG is a safe and feasible procedure, which can be easily performed and has a short operation time in terms of anastomosis.
Core tip: Digestive tract reconstruction after laparoscopic total gastrectomy is always a challenge for surgeons. Traditional Roux-en-Y anastomosis is difficult through the small incision in laparoscopic gastrectomy and severe complications, such as anastomotic stenosis, often occur when the operation is performed improperly. The method of semi-end-to-end esophagojejunal anastomosis that we developed is easy to operate and can also prevent anastomotic stenosis and other complications effectively.
- Citation: Zhao YL, Su CY, Li TF, Qian F, Luo HX, Yu PW. Novel method for esophagojejunal anastomosis after laparoscopic total gastrectomy: Semi-end-to-end anastomosis. World J Gastroenterol 2014; 20(37): 13556-13562
- URL: https://www.wjgnet.com/1007-9327/full/v20/i37/13556.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i37.13556
Compared with conventional open radical gastrectomy (OG), laparoscopic radical gastrectomy (LG) is a minimally invasive surgical approach with the significant advantages of a short recovery time and fewer postoperative complications. Therefore, LG has been gradually adopted worldwide and has achieved a relatively good clinical efficacy[1-4]. With improved operative experience of the surgeons, the laparoscopy-assisted gastric radical total gastrectomy has also been more commonly performed in clinical practice[5-7]. Esophagojejunal Roux-en-Y anastomosis can leave patients with a relatively good nutritional status and ideal body weight after surgery; therefore, it has been widely used in gastrointestinal reconstruction after total gastrectomy[8,9], consisting of end-to-side esophagojejunal anastomosis combined with side-to-side jejunojejunal anastomosis. However, it is difficult to perform laparoscopic anastomosis, especially if the end-to-side esophagojejunal anastomosis is at a high level, which requires a longer operating time. In addition, severe complications, such as anastomotic stenosis, can occur if the operation is improperly performed[10]. Therefore, we developed a simple and safe method of laparoscopic semi-end-to-end esophagojejunal anastomosis that can be performed easily and requires a short operative time for anastomosis. This method can also prevent anastomotic stenosis and other complications effectively.
This group included 26 patients with gastric cancer. The characteristics of the patients are shown in Table 1. All patients underwent preoperative routine examinations, including upper gastrointestinal tract X-ray (barium-meal), electronic gastroscopy or endoscopic ultrasonography, to identify the tumor location and pathological type. The diagnosis of gastric cancer was confirmed by pathological examination. The tumor was located at the gastric cardia in seven cases, at the bottom of the stomach in 10 cases, and in the body of the stomach in 9 cases. No hepatic, pulmonary, or other distant metastases were found in the preoperative examinations.
| Characteristic | Data |
| Gender (male/female) | 15/11 |
| Age (yr) | 58.6 ± 13.4 |
| Body mass index (kg/m2) | 23.5 ± 5.1 |
| Operation time (min) | 257 ± 36 |
| Anastomotic time (min) | 51 ± 17 |
| Blood loss (mL) | 88 ± 46 |
| Postoperative hospital stay (d) | 8 ± 3 |
| Complication | 0 |
The patients were placed in the supine position with their legs spread. Five trocars were used routinely. The surgeon stood on the left side of the patient, the surgeon assistant stood on the right side, and the laparoscope-holder assistant stood between the patient’s legs. After a pneumoperitoneum was established and a lymphadenectomy was performed by laparoscopy, the esophagus was isolated for at least 5 cm over the upper edge of the tumor mass to ensure the edge of the dissection was tumor tissue-free. After a complete isolation, the duodenum was dissected using an intracorporeal linear cutter stapler[2].
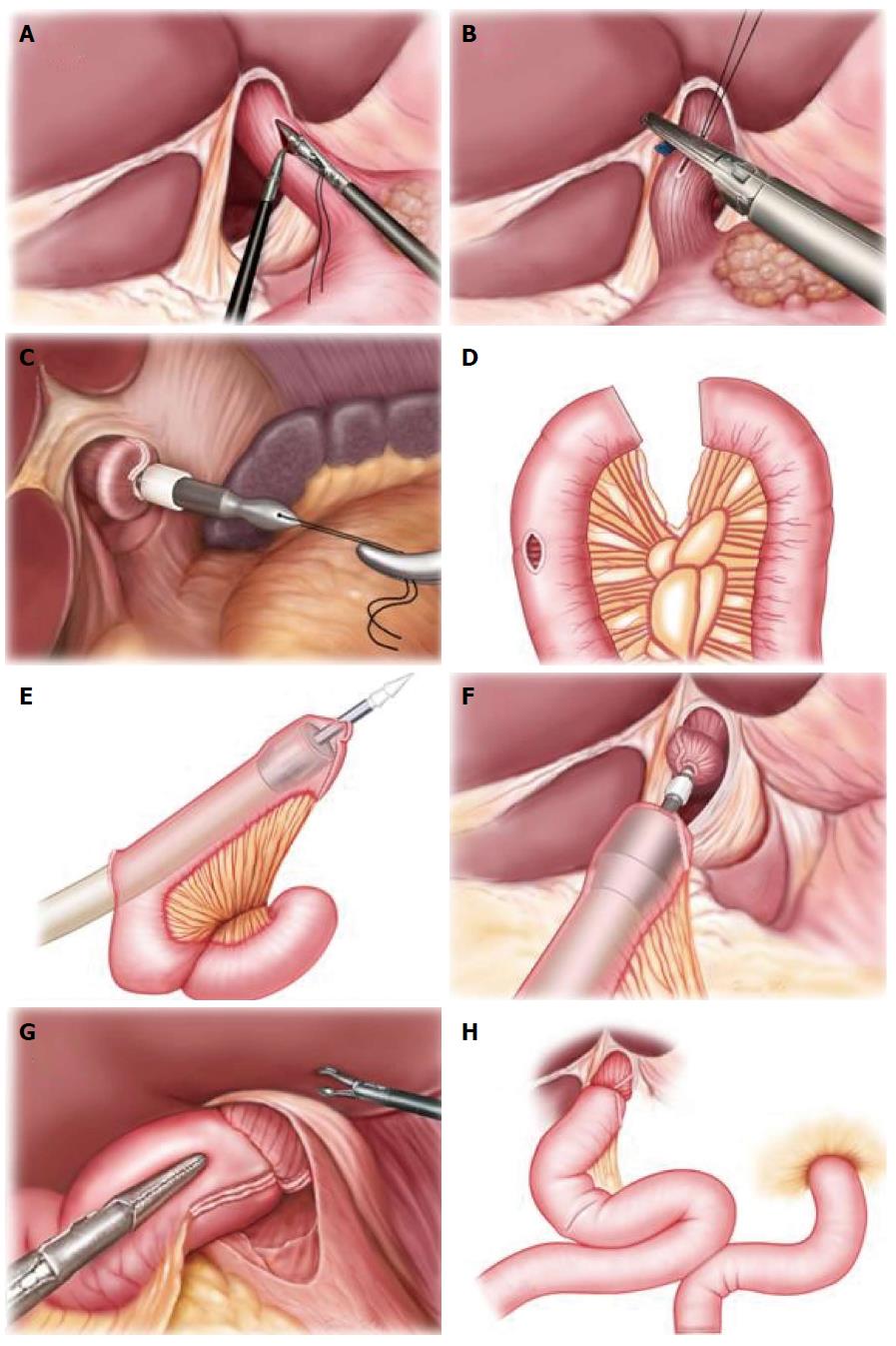
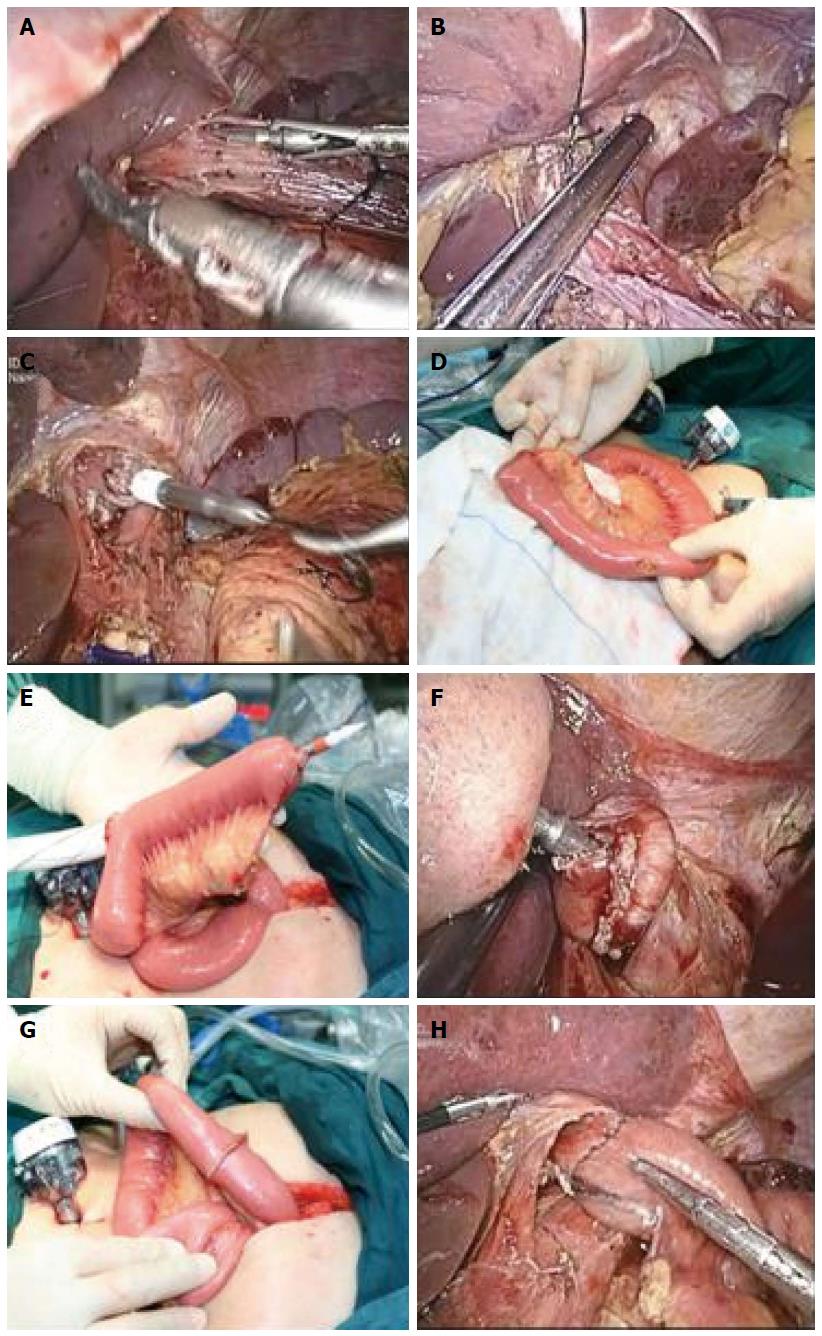
Before gastrointestinal reconstruction, a small incision of 5-7 cm was made at the subxiphoid. A 21- or 25-mm circular stapler (DST EEA, Covidien llc, Mansfield, MA, United States) was selected, and a thread was passed through the small hole at the center rod tip of the anvil as the retraction thread. The anastomosis anvil, with the thread attached, was inserted into the abdominal cavity for future use. After the incision, the wound was protected by an incision closure device, and the pneumoperitoneum was re-established. Under laparoscopy, the stomach was pulled down. The longitudinal incision of the esophagus was made 3 cm above the tumor with an ultrasonic harmonic scalpel, and the anvil with the thread was completely inserted into the proximal end of the esophagus. The retraction thread was then lifted close to the esophagus incision edge, and a linear cutter stapler was used to dissect the esophagus. The center rod of the anvil was then pulled out to complete the anvil placement (Figure 1A-C and Figure 2A-C).
The jejunum was extracted through a small incision of the abdominal wall. A small mesenteric incision was made at approximately 20 cm to the ligament of Treitz. Without the isolation of the small intestinal mesentery, the jejunum was dissected with the linear stapler. A 3-cm longitudinal incision was made at the contralateral side of the mesentery, which was approximately 10-15 cm from the distal jejunum end. The circular stapler was then inserted through this incision, and the center puncture rod of the circular stapler was passed through the contralateral side of the jejunal mesentery (Figure 1D and E; Figure 2D and E). The circular stapler was placed into the abdominal cavity. After the abdominal cavity was closed with the incision closure device, the pneumoperitoneum was re-established. The center cone of the anvil and the circular stapler were connected and rotated to approach each other to complete the semi-end-to-end esophagojejunal anastomosis under laparoscopic view (Figure 1F and G; Figure 2F and G). The jejunum was again brought out of the abdominal cavity, and the longitudinal incision of the distal jejunum was closed with a linear cutter stapler transversely (Figure 2H). Then, a similar approach was used to perform side-to-side anastomosis of the proximal and distal end of the jejunum. The linear cutter stapler was used 40-50 cm adjacent to the esophagojejunal anastomosis site to complete the Roux-en-Y anastomosis (Figure 1H).
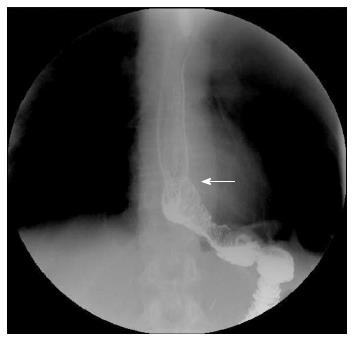
Twenty-six patients successfully underwent laparoscopic total gastrectomy (LATG) without the need for conversion to open abdominal surgery during the operation (Table 1). The semi-end-to-end esophagojejunal anastomosis was also successfully performed. No residual tumor was detected at the margin of the anastomosis. The average operation time was 257 ± 36 min (range, 217-356 min), with an average anastomosis time of 51 ± 17 min and an average intraoperative blood loss of 88 ± 46 mL. A liquid diet was administered on postoperative d3, and a semi-liquid diet was administered on postoperative d5. The average hospital stay was 8 ± 3 d after surgery. No postoperative complications, such as anastomotic bleeding or leakage, were found. Barium meal examination performed after the surgery demonstrated normal perfusion, without anastomosis-site stenosis (Figure 3).
Esophagojejunal Roux-en-Y anastomosis is generally performed for digestive tract reconstruction after total gastrectomy, and the circular stapler is routinely used for end-to-side esophagojejunal anastomosis[11-14]. In conventional open abdominal surgery, it is relatively easy to complete esophagojejunal anastomosis because of the large incision and hand-guided assistance. However, it is much more difficult to perform this procedure through a small incision with the assistance of laparoscopy. Therefore, laparoscopic esophagojejunal anastomosis after LATG has become a challenge for surgeons in laparoscopic surgery[15-18].
There are generally two technical difficulties in esophagojejunal anastomosis after LATG: anvil insertion, which has been successfully resolved using various placement methods[8,19-22], and esophagojejunal anastomosis. One method for end-to-side esophagojejunal anastomosis has been commonly applied in the literature, although this approach has several disadvantages. First, if end-to-side esophagojejunal anastomosis is performed through an incision window, the assisting incision should be selected below the subxiphoid, which requires an incision with a sufficient length because it is difficult to safely complete anastomosis through a small incision[23]. Second, because the circular stapler is inserted from the distal end of jejunum to perform end-to-side esophagojejunal anastomosis, the wall of the distal jejunum could potentially be stapled during anastomosis if the operation is not performed properly, which could cause subsequent anastomosis-site stenosis[24,25]. The above incident is more likely to occur when the anastomosis procedure is performed through a small incision or under laparoscopy because of occlusion of the liver and intestine. In addition, the operation time for this procedure is relatively long. Third, when the esophagojejunal anastomosis must be performed at a higher position, the anastomotic site will be above the diaphragmatic hiatus. In that case, there is a greater risk of anastomosis leaks from high anastomosis tension when the jejunum and mesentery near the anastomotic site can block the esophagus hiatus, resulting in postoperative symptoms such as obstruction-like sensations during eating and other type of discomfort. Furthermore, all of the above-mentioned disadvantages are more likely to present in obese patients, patients with narrow costal arch, and patients receiving esophageal dissection at a high plane.
For these reasons, we developed a method for semi-end-to-end anastomosis. The key concept in this surgical operation is that the circular stapler is inserted through a small incision of the jejunum, which is located 10-15 cm from the distal end on the contralateral side of the mesentery. In addition, the center puncture rod of the circular stapler must pass through to the jejunal end, which is also on the contralateral side of the mesentery. In such an anastomosis procedure, the anastomosis at the jejunum side is connected between the sidewall of the jejunum and the jejunal end. We therefore termed this procedure semi-end-to-end anastomosis. The anastomosis at the esophagojejunal site is at some distance from the jejunal mesentery, which can reduce the risk of anastomotic bleeding. We have also attempted to perform complete end-to-end esophagojejunal anastomosis, although if the isolated jejunal end is relatively long, a complete end-to-end anastomosis can lead to poor anastomotic vascular circulation, thereby increasing the risk of anastomotic leaks. If the mesentery is not isolated, anastomotic bleeding can easily occur after the anastomosis is completed. In our operation procedure, the jejunal mesentery is not isolated when the jejunum is dissected; however, the anastomotic site remains a certain distance from the mesentery, and it therefore does not cause anastomotic bleeding.
There are three main advantages to this semi-end-to-end esophagojejunal anastomosis. First, the procedure is simple, and the anastomosis time is significantly reduced. The entire procedure only requires a small incision, and the semi-end-to-end esophagojejunal anastomosis can be performed either through a small incision opening or under laparoscopy. As long as the stapler is connected to the center rod of the anvil, the anastomosis can be completed through rotation and tightening, which does not require repeated adjusting of the intestinal or mesenterial location under the operation window, and has no risk of anastomosis-site stenosis. The small incision in the distal jejunum can be brought out of the abdominal cavity for easy closure. Compared with the conventional end-to-side anastomosis operation, the semi-end-to-end esophagojejunal anastomosis is easy to perform and significantly reduces the anastomosis time, and the whole procedure can be successfully completed through a small incision. Second, the semi-end-to-end esophagojejunal anastomosis can effectively reduce the risk of anastomosis-site stenosis. In conventional esophagojejunal anastomosis, a stapler is required for insertion through the jejunal end, and the size of the anastomosis-site does not reflect the size of the channel for food passage after esophagojejunal anastomosis. As a result, anastomotic stenosis frequently occurs. However, by performing semi-end-to-end esophagojejunal anastomosis, the size of the anastomosis-site is equivalent to the size of the channel for food passage, which is also the actual size of the stapler. Therefore, anastomotic stenosis will not occur as long as the anastomosis is completed successfully. Third, semi-end-to-end esophagojejunal anastomosis reduces the anastomotic tension, which reduces the risk of anastomotic leaks. The anastomosis site can also be easily retracted to the esophagus hiatus after the esophagojejunal anastomosis. Therefore, this method of semi-end-to-end esophagojejunal anastomosis can effectively reduce the anastomotic tension in obese patients and patients receiving esophageal dissection at a high plane, thereby reducing the risk of anastomotic leaks. However, our study also has some limitations. This study enrolled relatively few patients and it lacks a proper control group to assess the clinical efficacy of this new method. Despite these, we still believe this is an important basis for subsequent studies and large-scaled, prospective, randomized, controlled trials are needed to evaluate the short-term and long-term outcome of this new method.
In conclusion, the application of semi-end-to-end esophagojejunal anastomosis after LATG is a safe and feasible procedure, which can be easily performed and has a short operation time for anastomosis. In addition, this procedure can effectively reduce the incidence of anastomotic stenosis and other postoperative complications.
Digestive tract reconstruction after laparoscopic total gastrectomy (LATG) is a problem for surgeons. The traditional reconstruction method is difficult and severe complications, such as anastomotic stenosis, often occur if the operation is performed improperly.
Digestive tract reconstruction after total gastrectomy is a clinical research focus. There are a few different approaches for esophagojejunal anastomosis, such as end-to-side esophagojejunal anastomosis, side-to-side esophagojejunal anastomosis and semi-end-to-end esophagojejunal anastomosis.
Conventional esophagojejunal Roux-en-Y anastomosis in open abdominal surgery is relatively difficult to perform through a small incision with the assistance of laparoscopy and often results in surgical complications. However, the method that we developed is not only easy to perform through a small incision, but can also effectively avoid anastomotic stenosis and reduce the risk of anastomotic leaks.
This study may represent a future strategy for digestive tract reconstruction after LATG in gastric cancer.
The number of the patients is relatively small, and would be better with a larger number of participants. The discussion part is good.
P- Reviewer: He SB S- Editor: Nan J L- Editor: Stewart G E- Editor: Ma S
| 1. | Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc. 2009;23:1759-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Zhao Y, Yu P, Hao Y, Qian F, Tang B, Shi Y, Luo H, Zhang Y. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc. 2011;25:2960-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Sakuramoto S, Kikuchi S, Kuroyama S, Futawatari N, Katada N, Kobayashi N, Watanabe M. Laparoscopy-assisted distal gastrectomy for early gastric cancer: experience with 111 consecutive patients. Surg Endosc. 2006;20:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Inokuchi M, Kojima K, Kato K, Motoyama K, Sugita H, Sugihara K. Feasibility of laparoscopy-assisted gastrectomy for patients with chronic obstructive pulmonary disease. Surg Endosc. 2013;27:2102-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Yoshikawa T, Murai S, Imai S, Oto I, Kitasato K, Shimizu H, Yabe N, Kitagawa Y. [Short-term outcomes of reduced port laparoscopy-assisted total gastrectomy]. Gan To Kagaku Ryoho. 2013;40:2295-2297. [PubMed] |
| 6. | Kwon Y, Cho SI, Kwon YJ, Yang KS, Jang YJ, Kim JH, Park SH, Mok YJ, Park S. Safety of transorally-inserted anvil for esophagojejunostomy in laparoscopic total gastrectomy. Eur J Surg Oncol. 2014;40:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kim KH, Kim YM, Kim MC, Jung GJ. Is laparoscopy-assisted total gastrectomy feasible for the treatment of gastric cancer? A case-matched study. Dig Surg. 2013;30:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Omori T, Oyama T, Mizutani S, Tori M, Nakajima K, Akamatsu H, Nakahara M, Nishida T. A simple and safe technique for esophagojejunostomy using the hemidouble stapling technique in laparoscopy-assisted total gastrectomy. Am J Surg. 2009;197:e13-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Tsujimoto H, Uyama I, Yaguchi Y, Kumano I, Takahata R, Matsumoto Y, Yoshida K, Horiguchi H, Aosasa S, Ono S. Outcome of overlap anastomosis using a linear stapler after laparoscopic total and proximal gastrectomy. Langenbecks Arch Surg. 2012;397:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Eom BW, Kim YW, Lee SE, Ryu KW, Lee JH, Yoon HM, Cho SJ, Kook MC, Kim SJ. Survival and surgical outcomes after laparoscopy-assisted total gastrectomy for gastric cancer: case-control study. Surg Endosc. 2012;26:3273-3281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Kinoshita T, Oshiro T, Ito K, Shibasaki H, Okazumi S, Katoh R. Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc. 2010;24:2908-2912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Wada N, Kurokawa Y, Takiguchi S, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Mori M, Doki Y. Feasibility of laparoscopy-assisted total gastrectomy in patients with clinical stage I gastric cancer. Gastric Cancer. 2014;17:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Ishigami S, Natsugoe S, Hokita S, Aoki T, Kashiwagi H, Hirakawa K, Sawada T, Yamamura Y, Itoh S, Hirata K. Postoperative long-term evaluation of interposition reconstruction compared with Roux-en-Y after total gastrectomy in gastric cancer: prospective randomized controlled trial. Am J Surg. 2011;202:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Lee MS, Lee JH, Park do J, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Haverkamp L, Weijs TJ, van der Sluis PC, van der Tweel I, Ruurda JP, van Hillegersberg R. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Azagra JS, Goergen M, Arru L, Facy O. Total gastrectomy for locally advanced cancer: the pure laparoscopic approach. Gastroenterol Rep (Oxf). 2013;1:119-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Lee SR, Kim HO, Son BH, Shin JH, Yoo CH. Laparoscopic-assisted total gastrectomy versus open total gastrectomy for upper and middle gastric cancer in short-term and long-term outcomes. Surg Laparosc Endosc Percutan Tech. 2014;24:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Jung YJ, Kim DJ, Lee JH, Kim W. Safety of intracorporeal circular stapling esophagojejunostomy using trans-orally inserted anvil (OrVil) following laparoscopic total or proximal gastrectomy - comparison with extracorporeal anastomosis. World J Surg Oncol. 2013;11:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Chong-Wei K, Dan-Lei C, Dan D. A modified technique for esophagojejunostomy or esophagogastrostomy after laparoscopic gastrectomy. Surg Laparosc Endosc Percutan Tech. 2013;23:e109-e115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Nunobe S, Hiki N, Tanimura S, Kubota T, Kumagai K, Sano T, Yamaguchi T. Three-step esophagojejunal anastomosis with atraumatic anvil insertion technique after laparoscopic total gastrectomy. J Gastrointest Surg. 2011;15:1520-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Wang ZQ, Cai ZM, Chen J, Lei X, Luo HX, Yu PW. A modified method of laparoscopic side-to-side esophagojejunal anastomosis: report of 14 cases. Surg Endosc. 2008;22:2091-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Kim J, Kim S, Min YD. Consideration of cardia preserving proximal gastrectomy in early gastric cancer of upper body for prevention of gastroesophageal reflux disease and stenosis of anastomosis site. J Gastric Cancer. 2012;12:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Lim SG, Lee KM, Kim SS, Kim JS, Hwang JC, Shin SJ, Han SU, Kim JH, Cho SW. Endoscopic approach for postoperative complications following laparoscopic-assisted gastrectomy in early gastric cancer: literature review. Hepatogastroenterology. 2012;59:1308-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |