Published online Oct 7, 2014. doi: 10.3748/wjg.v20.i37.13382
Revised: March 23, 2014
Accepted: May 29, 2014
Published online: October 7, 2014
Processing time: 252 Days and 16.4 Hours
Biliary lithiasis is an endemic condition in both Western and Eastern countries, in some studies affecting 20% of the general population. In up to 20% of cases, gallbladder stones are associated with common bile duct stones (CBDS), which are asymptomatic in up to one half of cases. Despite the wide variety of examinations and techniques available nowadays, two main open issues remain without a clear answer: how to cost-effectively diagnose CBDS and, when they are finally found, how to deal with them. CBDS diagnosis and management has radically changed over the last 30 years, following the dramatic diffusion of imaging, including endoscopic ultrasound (EUS) and magnetic resonance cholangiography (MRC), endoscopy and laparoscopy. Since accuracy, invasiveness, potential therapeutic use and cost-effectiveness of imaging techniques used to identify CBDS increase together in a parallel way, the concept of “risk of carrying CBDS” has become pivotal to identifying the most appropriate management of a specific patient in order to avoid the risk of “under-studying” by poor diagnostic work up or “over-studying” by excessively invasive examinations. The risk of carrying CBDS is deduced by symptoms, liver/pancreas serology and ultrasound. “Low risk” patients do not require further examination before laparoscopic cholecystectomy. Two main “philosophical approaches” face each other for patients with an “intermediate to high risk” of carrying CBDS: on one hand, the “laparoscopy-first” approach, which mainly relies on intraoperative cholangiography for diagnosis and laparoscopic common bile duct exploration for treatment, and, on the other hand, the “endoscopy-first” attitude, variously referring to MRC, EUS and/or endoscopic retrograde cholangiography for diagnosis and endoscopic sphincterotomy for management. Concerning CBDS diagnosis, intraoperative cholangiography, EUS and MRC are reported to have similar results. Regarding management, the recent literature seems to show better short and long term outcome of surgery in terms of retained stones and need for further procedures. Nevertheless, open surgery is invasive, whereas the laparoscopic common bile duct clearance is time consuming, technically demanding and involves dedicated instruments. Thus, although no consensus has been achieved and CBDS management seems more conditioned by the availability of instrumentation, personnel and skills than cost-effectiveness, endoscopic treatment is largely preferred worldwide.
Core tip: Common bile duct stones (CBDS) are not infrequent in patients with gallstones and should be treated. The concept of “risk of carrying CBDS”, based on symptoms, liver serology and ultrasound, is pivotal to identify the appropriate management. While “low risk” patients do not require further examination, “intermediate to high risk” patients may be offered intraoperative cholangiography (IOC) and laparoscopic choledochus exploration, or may be referred to magnetic resonance cholangiography (MRC), endoscopic ultrasound (EUS), endoscopic retrograde cholangiopancreatography and sphincterotomy. Whereas the results of IOC, MRC and EUS are similar in identifying CBDS, surgery seems superior to endoscopic sphincterotomy in choledochus clearance, although this latter is preferred worldwide.
- Citation: Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol 2014; 20(37): 13382-13401
- URL: https://www.wjgnet.com/1007-9327/full/v20/i37/13382.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i37.13382
The prevalence of gallbladder lithiasis is roughly 20.5 million (6.3 million men and 14.2 million women) in the United States[1], whereas in Europe it is reported to vary between 5.9% and 21.9% of the general population[2]. Eleven to 21% of patients with cholelithiasis also have concomitant common bile duct stones (CBDS) at the time of surgery[3-5].
The vast majority of CBDS form within the gallbladder and then migrate into the common bile duct (CBD), following gallbladder contractions. Once in the CBD, stones may reach the duodenum following the bile flow; otherwise, also owing to the smaller diameter of the distal CBD at the Vater papilla, they may remain in the choledochus. In this latter case, gallstones may be fluctuant, thus remaining mostly asymptomatic, or cause a variety of bile flow problems, including complete obstruction and jaundice. Bilostasis may be responsible for bile infection and consequent ascending cholangitis, whereas bile/ pancreatic juice flow problems at the merging of the CBD and the main pancreatic duct (Wirsung) are presumed to potentially trigger the intrapancreatic activation of pancreatic enzymes, thus causing acute biliary pancreatitis[6]. Thus, the clinical presentation of choledocholithiasis may vary widely, as CBDS may be asymptomatic (up to half of cases[7]), or associated with various symptoms and conditions, ranging from colicky pain to potentially life-threatening complications, such as ascending cholangitis or acute pancreatitis (see below).
CBDS diagnosis and management have radically changed over the last 30 years. Since the early eighties, the dramatic diffusion of endoscopic techniques, namely endoscopic retrograde cholangiography (ERC), has changed the approach to patients affected by cholelithiasis, thus potentially carrying CBDS. Until then, a first evaluation was made by clinical symptoms, preoperative ultrasound (US), liver laboratory examinations and systematic preoperative intravenous cholangiography[8], which had replaced oral cholecystography[9]; later, intravenous cholangiograpy became a pre-ERC examination aimed at defining CBDS risk[10,11], and is nowadays replaced by magnetic resonance cholangiography (MRC), endoscopic ultrasound (EUS) and ERC[12-14].
During the 1990s, the diffusion of laparoscopy changed biliary lithiasis management even more radically, by introducing laparoscopic cholecystectomy[15-17], intraoperative cholangiography[18,19] and, when needed, laparoscopic CBD exploration[20-22]. Concomitantly, other techniques were proposed for CBDS management, including lithotripsy[23-25].
During the 2000s, a critical appraisal of management options[26-28] and the diffusion of new diagnostic examinations led to a more cautious, patient-tailored preoperative workup, based on patient risk of carrying CBDS[3,29,30], and management, based on the perception that CBDS may be treated in a multidisciplinary way[31-33]. Moreover, the categorical diktat to treat any patient with CBDS stones (even asymptomatic), which was still incorporated in international guidelines at least until the late nineties[34], is nowadays challenged by a more conservative attitude, according to the belief that in over one third of cases CBDS will finally pass through without complications[31,35,36]. Interestingly, the 2006 European Society for Endoscopic Surgery (EAES) guideline update justified an expectant attitude in elderly patients[37], whereas, in 2008, the British Society of Gastroenterology guidelines[38] recommended that whenever patients have symptoms and investigations suggest ductal stones, extraction should be performed when possible (on the strength of supporting evidence grade III; recommendation grade B). In 2011, the American Society for Gastrointestinal Endoscopy (ASGE) guidelines[39] incorporated those recommendations with a “low quality of evidence”.
Such considerations gain importance for asymptomatic CBDS, which represent up to roughly one half of cases[7] and where aggressive procedures may appear unjustified[40]. Asymptomatic CBDS are actually the most challenging situation, since patients risk being “under-studied” by poor diagnostic work up or “over-studied” by excessively invasive examinations. Similarly, they can be under- or over-treated by inappropriate procedures. Two main open issues remain without a clear answer: how to cost-effectively diagnose CBDS and, eventually, how to deal with them. In this light, the concept of “risk of carrying CBDS” is pivotal to identifying the most appropriate algorithm of management of a specific patient[29,41-44].
Two main “philosophical approaches” face each other nowadays, at least for patients with an intermediate to high risk of carrying CBDS: on one hand, the “laparoscopy-first” approach, which mainly relies on intraoperative cholangiography and laparoscopic CBD exploration, and, on the other hand, the “endoscopy-first” attitude, variously referring to MRC or EUS for diagnosis and ERC followed by endoscopic sphincterotomy for CBD clearance. As no consensus has been achieved, CBDS management seems more conditioned by availability of instrumentation, personnel and skills than cost-effectiveness.
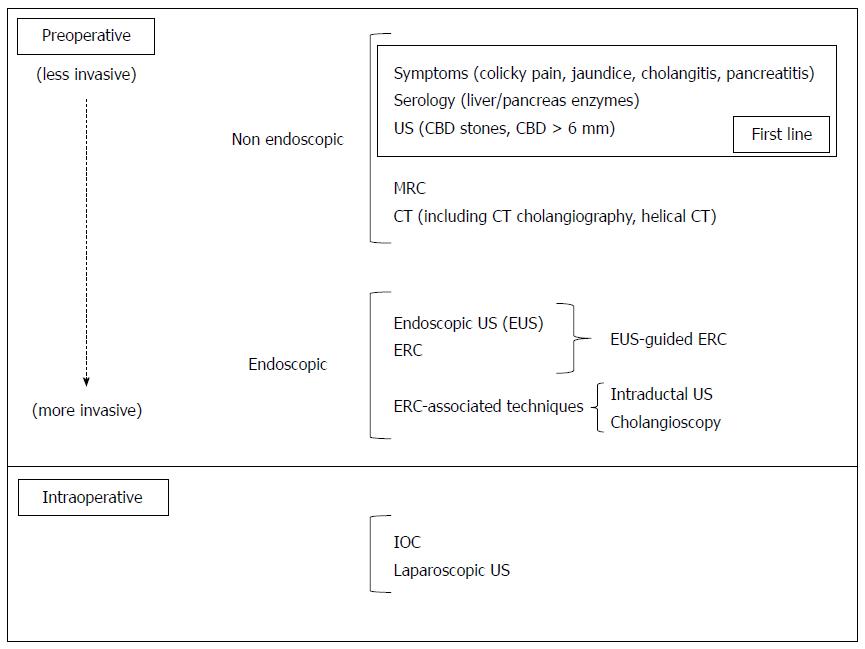
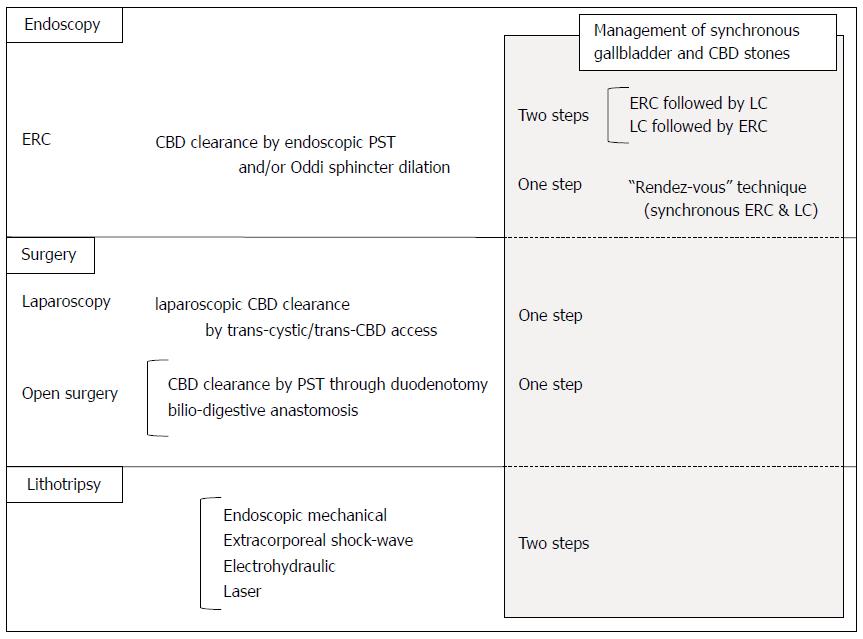
Here we propose a review of the most widely diffused approaches to CBDS diagnosis (Figure 1) and management (Figure 2).
Diagnostic tools are reported as summarized in Figure 1 in a chronological order (1) Preoperative; and (2) Intraoperative, from the least to the most invasive.
In order to understand why there is still no consensus concerning CBDS diagnosis and so many examinations have been and are continuing to be proposed, the following statements should be considered: The prevalence of gallbladder stones in the general population is up to 20%[2]; of these patients, up to 20% have synchronous CBDS[5]; CBDS are asymptomatic in up to half of these latter cases[7]. These data mean that up to 2% of the general population may have unknown CBDS during their life-span; CBDS may cause potentially life-threatening complications, such as acute cholangitis or acute pancreatitis, and therefore should be diagnosed and treated[29,34,37-39]; Accuracy, invasiveness, potential therapeutic use and costs of the most common imaging techniques used to identify CBDS increase together in a parallel way: they are minimal for transabdominal US and maximum for ERC, where the counterpart of intrinsic therapeutic implications (endoscopic sphincterotomy) are non-negligible morbidity/mortality[14,26]; These latter considerations contraindicate the systematic, pre-cholecystectomy use of imaging techniques other than transabdominal US as first-line imaging, and ERC as second-line examination, unless a clear indication is given by jaundice, cholangitis or high risk of synchronous CBDS[29,38] (Table 1).
| Biliary lithiasis affects 10% to 20% of general population and is associated with CBDS in up to 20% of cases |
| Clinical symptoms, liver/pancreas serology and transabdominal ultrasounds may define the “risk of carrying CBDS”, and identify: |
| “Low risk” patients, to be directly referred to laparoscopic cholecystectomy |
| “Intermediate risk” patients, needing intraoperative cholangiography, endoscopic ultrasounds or magnetic resonance cholangiography before laparoscopic cholecystectomy |
| “High risk” patients requiring endoscopic retrograde cholangiography |
| CBDS may be managed by endoscopic sphincterotomy or surgery (laparoscopic or open). This latter has seemingly slightly better results, counterbalanced by invasiveness (open surgery) or the need of specific instrumentation and advanced laparoscopic skills (laparoscopic surgery). Lithotripsy may help endoscopic CBDS retrieval or may be performed extra-corporeally in selected, unfit patients |
| CBDS management will be more and more multidisciplinary and tailored not only on a specific patient but also on the available resources of a specific environment to have the best possible management |
Here we propose a review of the most widely diffused techniques used to ascertain the presence of CBDS.
Symptoms: Characteristically, the symptom complex of choledocholithiasis consists of right upper abdominal colicky pain, radiating to the right shoulder with intermittent jaundice accompanied by pale stools and dark urine[45], whereas cutaneous itching is rarely present[46]. In contrast with patients with neoplastic obstruction of the CBD or ampulla of Vater, the gallbladder is usually not distended (Courvoisier’s law)[45]. Scholastically, Charcot’s triad[47] (jaundice associated to biliary colic pain and sepsis - hyperpyrexia and chills), indicates acute cholangitis; as choledocholithiasis is the most frequent aetiology of such a clinical picture, it should prompt immediate diagnostic confirmation and CBD drainage[38]. Acute pancreatitis, showing with transversal abdominal pain potentially irradiated to the back and associated to an increase of serum level of amylase/lipase, in the presence of gallstones, is a priori considered as being of biliary origin. Hepatic abscess(es) may also be a rarer infectious complication of CBDS whereas chronic CBD obstruction may also cause biliary cirrhosis[38]. All the reported clinical pictures should lead us to focus on the right upper quadrant in order to identify biliary lithiasis as possible aetiology.
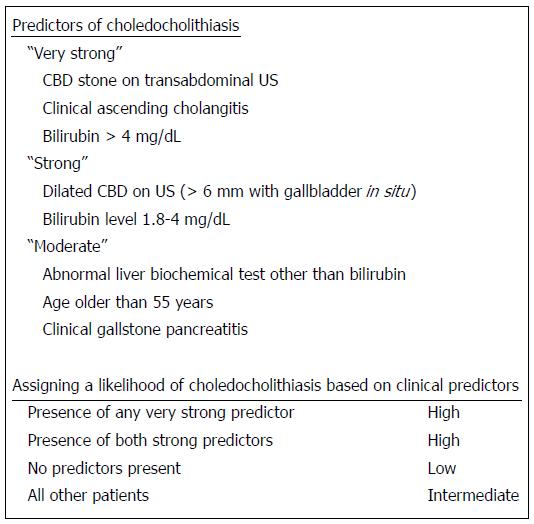
Serology: Traditionally, an alteration of the so-called cholestasis indexes (direct bilirubin, gamma-glutamil-transpherase, alkaline phosphatase)[44,48] was considered as potentially due to CBDS. Indeed, also liver cytolysis indexes were also found to be associated to unknown CBDS[3,44,49]. Nowadays, although total bilirubin is considered the main laboratory index related to the risk of synchronous CBDS (“very strong predictor”)[29] and its ensuing management, all liver biochemical tests other than bilirubin deserve a careful evaluation (“moderate predictors”)[29].
Transabdominal US: Transabdominal US represents the first line, non-expensive, non-invasive imaging examination available in virtually any environment[29,50,51], performed for any sign/symptom/condition possibly referring to liver disease. The small distance of the gallbladder from the abdominal wall and the absence of interposed gas make transabdominal US the ideal examination to study gallbladder morphology and to ascertain the presence of gallstones, where sensitivity of US is 96%[52].
Unfortunately, US accuracy in detecting CBDS drops to less than 50%[52,53], since CBDS often do not show acoustic shadowing or are located in the distal part of the CBD, where they are often obscured by gas[53]. In those cases, CBDS diagnosis often relies on indirect signs of CBD obstruction, such as CBD dilation. The definition of CBD dilation is also a matter of discussion, as suggested “normal limits” vary widely, ranging from 5 to 11 mm[7,54,55], partly because CBD diameter may increase with age and after cholecystectomy. The accuracy of US in visualizing the gallbladder and its content may allow us to identify another indirect sign of increased CBDS risk: the number and size of gallbladder stones[51,56]. As multiple, small-sized gallstones are more likely to migrate into the CBD[43,57], this US finding should be considered during CBD assessment.
Scoring systems and algorithms: The above mentioned “first-line” diagnostic criteria have been variously associated to identify patients at high risk of carrying CBDS[3,43,44,48,49,58,59]. Although they mostly showed the statistical significance of clinical, laboratory and US findings, nevertheless, the extreme variability of proposed models and the doubtful results of some studies[60] have limited the use of scoring systems and algorithms.
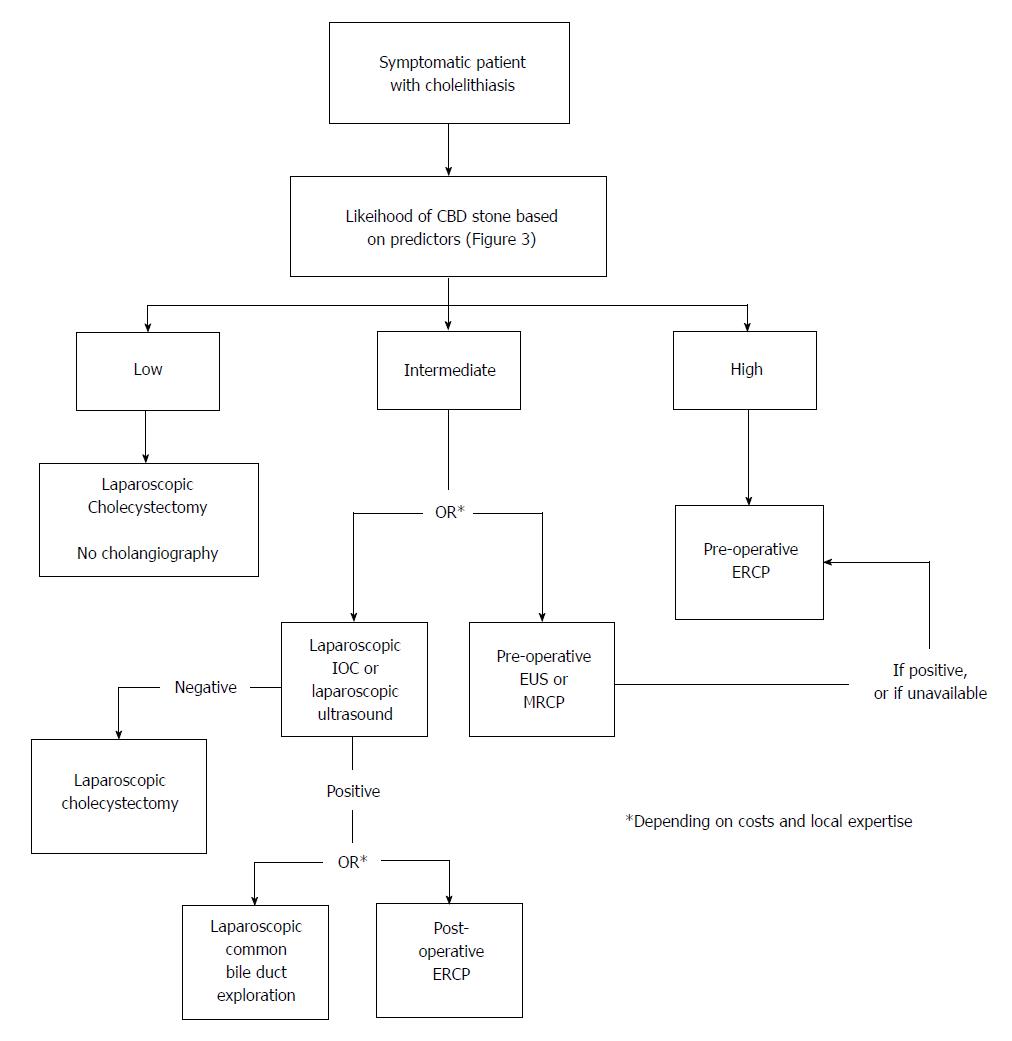
Although none of those scoring systems have ever really entered clinical practice on a large scale, they[41,42,48] have somewhat paved the way to recent guidelines[29], which have identified three classes of risk of CBDS based on symptoms, liver serology and transabdominal US. The risk of carrying CBDS is defined as low where no other examination is needed, intermediate where preoperative EUS/MRC or laparoscopic intraoperative colangiography (IOC)/US is requested, and high where patients should be directly referred to preoperative ERC (and possibly ES). The proposed algorithm based on the predictors listed in Figure 3 and is reported in Figure 4.
CT scan: CT scan is a “second line examination” for many abdominal diseases/conditions, partly owing to patients’ exposure to X-rays and higher costs compared to US. Traditionally considered more accurate than trasparietal US in identifying CBDS[61] but still inferior to MRC[62], recent advances, such as the “helical” CT scan[29,63] and CT cholangiography[46,62], have increased its accuracy. CT-cholangiography, performed after the administration of iodinated contrast agent excreted into the bile, has shown a sensitivity (88%-92% vs 88%-96%) and specificity (75%-92% vs 75%-100%) comparable to MRC[46,62]. New generation 64-detector CT at the portal venous phase is reported to have a 72%-78% sensitivity and a 96% specificity in identifying CBDS[63]. Very recently, 64-sliced CT was reported not to detect CBDS in 17% of cases, stone size < 5 mm, color black/brown and patient’s age being related to non-detectability at multivariate analysis[64]. Although the intrinsic higher “invasiveness” of CT compared to MRC makes the latter preferable nowadays, the extreme availability and diffusion of CT scan may possibly lead to its rediscovery in this field.
MRC: MRC is considered nowadays to be the most accurate non-invasive (non-endoscopic) procedure for the detection of CBDS, with 85%-92% sensitivity and 93%-97% specificity in large series[12,65,66]. A recent meta-analysis showed that the aggregated sensitivities of EUS and MRC were 93% and 85% respectively, whereas their specificities were 96% and 93% respectively, with no significant differences[12]. Regardless of overall effectiveness, it should be pointed out that MRC is a non-invasive technique, which may allow for providing a higher spatial resolution than EUS, but is probably less sensitive than EUS for detecting CBD stones smaller than 6 mm[66]. Other drawbacks of MRC are its suboptimal availability in non-tertiary care centers, and non-therapeutic purpose of the technique, which involves a further procedure to eventually treat CBDS. The impossibility of its use in specific situations (morbid obesity or claustrophobic patients, the presence of metal foreign body/device) is also a specific issue of this technique.
All endoscopic procedures share the pros and cons of peroral endoscopy. Although endoscopically reaching the second duodenum is widely considered to be an easy task for average endoscopists in average patients, some conditions can make this manoeuvre a difficult one. In some cases, the papilla major is difficult to identify and to cannulate, resulting in a challenging situation for the endoscopist (and dangerous for the patient), as it is for example when it is placed in a duodenal diverticulum[67]. Previous surgical procedures on the stomach, such as Roux-en-Y gastric by-pass[68] or gastrectomy with duodenal stump closure and Billroth II reconstruction[69], are another frequent cause of ERC failure and complications[70]. In particular, endoscopically reaching the second duodenum is difficult after a Roux-en-Y reconstruction, but also after an “Ω”-shaped anastomosis. In those cases, both CBDS diagnosis and management have to be carried out surgically (open surgery or laparoscopy).
ERC: After having been widely used for CBDS diagnosis in the late eighties/nineties, with a 75%-93% sensitivity and 100% specificity[14,71,72], ERC is nowadays being progressively abandoned as a diagnostic tool for patients with moderate to intermediate risk of carrying CBDS, as most cholangiograms result as being normal[73] and ERC is not cost-effective[74]. Compared to EUS and MRC, accuracy of ERC is suboptimal, being reduced in the case of CBD dilation and small CBDS[14]. Moreover, ERC not only involves X-ray exposure and the intrinsic invasiveness of endoscopy, but it also has non-negligible procedure-related morbidity/mortality[26,75-77], with a 2%-11% acute pancreatitis rate[13,14,78]. Moreover, once ERC is performed, endoscopic sphincterotomy is frequently associated regardless of CBDS presence, both for the risk of a post-ERC ascending cholangitis and for the possible false negatives of ERC itself. Such a practice of performing endoscopic sphincterotomy systematically after ERC is obviously a good argument against a supposed lesser invasiveness of diagnostic ERC compared with “operative” ERC. Thus, while ERC remains indicated for patients with a high risk of presenting synchronous CBDS, MRC, EUS or IOC are nowadays preferred for intermediate risk cases[29].
EUS and EUS-guided ERC: EUS is increasingly performed worldwide as a diagnostic tool, often as the first step of a potential double-technique procedure (EUS and ERC/ES)[13,14]. On the basis of the scientific evidence of no statistically significant difference in sensitivity (93% vs 85%) and specificity (96% vs 93%) between EUS and MRC[12], ASGE guidelines[29] have proposed MRC or EUS in patients at intermediate risk of CBDS. Nevertheless, the two techniques have particular pros and cons. Although EUS optimizes the low impact of US associated with the potential therapeutic option of ERC (namely ES), it involves endoscopy under sedation/anesthesia, thus being intrinsically more invasive than MRC. Compared to ERC, EUS has shown the same specificity and higher sensitivity (91% vs 75%), mostly due to cases with CBDS smaller than 4 mm or dilated CBD, where ERC presented some false positives[14]. Moreover, EUS has very limited (2%-8%), low degree morbidity, with a virtually 0 post-procedure acute pancreatitis[13,14,79]; thus, it may enable us to avoid ERC-related morbidity/mortality whenever unnecessary, as it is in 2/3 of cases[80], without increasing the risk of further endoscopic procedures in patients with an intermediate risk of carrying CBDS[13,14]. The (moderately) invasive nature of EUS, and the need for an endoscopic theatre, including instrumentation, personnel and expertise, make this option optimal whenever the risk of having CBDS is high enough to allow patients to potentially take advantage of contextual EUS/ERC/ES. In the latter case, EUS and ERC done in a single session proved to be safe, with no increase in sedation- or procedure-related complications, nor need for further endoscopic procedures[14,81], whereas postponing treatment for symptomatic CBD stones exposes the patient to biliary complications, especially cholangitis[12]. Ideally, future guidelines should take into account the specific pros and cons of MRC and EUS, thus defining subgroups of patients as being candidates for one procedure or the other.
ERC-associated intra-, extra-ductal US and cholangioscopy: During the 2000s, high-frequency (12.30 MHz) intraductal US was proposed for a further increase of ERC accuracy[72,78]. As the procedure is wire-guided, the cannulation rate of papilla major is reported to be virtually 100%[72] and sensitivity 97%-100%[72,78]. In particular, intraductal US is reported to have a 100% sensitivity in identifying non-radio-opaque stones, which may pass undiagnosed by traditional imaging[78]. Intraductal US is also reported to identify 100% of CBDS undetected at ERC[82] and 24%-40% of post-ERC/ES residual stones[82,83], thus allowing for definitive CBD clearance, by saline solution irrigation[83], and reduced CBDS recurrence[83].
Recently, extraductal endoscopic ultrasonography has been reported to have the same sensitivity as traditional EUS (90% vs 92%); since it can be performed with a traditional duodenoscope using miniprobes, it presents the advantage that, if therapeutic intervention becomes necessary, there is no need to change the scope[84].
Endoscopic cholangioscopy has also been reported to potentially increase the accuracy of ERC by detecting undiagnosed residual CBDS in 23% of ERC-negative cases[85]. State-of-the-art, ultra-slim choledochoscopes allow for magnification of intraductal imaging, differential diagnosis and stone management by basket/balloon retrieval or lithotripsy[85,86]. Although promising, these latter technologies still have a limited role in CBDS management, partly because of the need for costly instrumentation and adequate training of teams, and the uncertain clinical relevance of undiagnosed residual CBDS after ES.
All the intraoperative techniques aimed at identifying CBDS share advantages and drawbacks: on one hand, they avoid any preoperative procedure, thus potentially reducing hospitalizations, hospital stay and costs; on the other hand, they inevitably reduce the therapeutic options whenever CBDS are finally identified at surgery, since any preoperative procedure is obviously not possible anymore. The remaining options include laparoscopic CBD clearance[22,75], synchronous ERC[87,88] or postoperative ERC[31,75]. The first two options involve specific, dedicated instrumentation and surgical/endoscopic skills, and the third one has the main drawback that, whenever ERC fails (previous gastrectomy,..), a third procedure is finally needed to treat CBDS[32]. It should be noted that this latter procedure, in most environments, is an “old-fashioned” CBD exploration by laparotomy (and duodenotomy or biliodigestive anastomosis), which de facto cancels any effort to treat CBDS mini-invasively. Similarly, intraoperative techniques present the common issue of what to do whenever they are not possible or not conclusive, putting the surgeon in a challenging situation to decide whether to explore the CBD (laparoscopically or by open approach).
First reported in general surgery in the 1930s[89] and introduced in the laparoscopy armamentarium in the early nineties[18], IOC is still under debate regarding its cost-effectiveness when performed systematically or in a selected population[27,30]. IOC substantially relies on low cost laparoscopic instruments and the availability of a mobile roentgenography, and does not require advanced laparoscopic skills. Considering that it is reported to have a 59%-100% sensitivity and a 93%-100% specificity for CBDS[19], its cost-effectiveness is a strong argument in favour of its systematic use. In contrast, a recent systematic review of IOC did not show any benefit of performing IOC routinely[90], somewhat supporting ASGE Guidelines[29] indicating IOC for patients at intermediate risk of carrying CBDS, to eventually treat laparoscopically[22,27] or to refer for postoperative ERC[30]. Moreover, IOC involves the cannulation of the cystic duct to inject the CBD, which may become a difficult task in the case of intense inflammation/scarring (acute or chronic cholecystitis...), or anatomy variations, such as a short cystic duct or serrated Heister’s valves. As well as failure, such difficulties may favour specific complications of this technique, such as CBD perforation and biliary leakage, increased rates of post-surgical procedures and overall complications[91]. If we add that, whenever IOC shows CBDS, patients eventually referred to postoperative ERC and ES may possibly be exposed to the risk of a third surgical procedure for CBD clearance if ERC is unsuccessful, the greatest advantage of IOC is seemingly within a totally laparoscopic management of CBDS.
Laparoscopic US (LUS)[28,92], whose rationale is that the effectiveness of US is maximal when the probe is placed nearer to the CBD, represents the most recent tool to diagnose CBDS intraoperatively. The latter technique is obviously less invasive than IOC, although specific instrumentation and ultrasonographic skills are needed. In expert hands, LUS is reported to have a sensitivity of 92%-95%, and a specificity of 99%-100% for detecting CBDS[28,92,93]. In specialized environments, LUS has become the primary routine imaging method for evaluating the bile duct during laparoscopic cholecystectomy, thus relegating IOC to being performed in only 4%-23% of cases[28,92], whenever LUS imaging is inadequate, stronger clinical indicators of choledocholithiasis are present, or biliary anatomy remains uncertain[92]. Moreover, LUS success rate is higher than that of IOC (95%-99% vs 92%-97%)[19,92,93] and operative times are shorter (5-8 min vs 15-16 min)[19,93]. Furthermore, LUS does not subject the patient to the risk of bile duct injury, and it may be performed for pregnant patients because it involves no ionizing radiation[92]. Finally, the only equipment required for the study is the ultrasound machine and probe, making routine LUS significantly cheaper (less 46%) than routine IOC[94]. The main limitations of LUS are the learning curve associated with its initial usage[92,94] and the inability of LUS to identify bile duct anatomy in some cases, where surgeons should preferably have a low threshold for performing IOC[92]. For all these reasons, LUS has not yet become popular worldwide and, even if it finally does, IOC will probably not be abandoned, as it provides some additional information in difficult cases.
CBDS management consists of CBD clearance and may be accomplished by surgery (traditional and laparoscopic), endoscopy and lithotripsy[87,95-97]. Since, in most cases, CBDS are due to gallstone migration from the gallbladder which is still in situ, there is also a formal indication for cholecystectomy. Such a frequent eventuality may be dealt with in several ways, as a one-step or two-step-procedure, variously associating the above reported techniques and laparoscopic cholecystectomy.
A recent extensive literature review[98] of 16 published randomized trials comparing the results of cholecysto-choledochal management by open surgery, laparoscopy and various endoscopic-laparoscopic protocols, did not show any significant differences concerning overall mortality and morbidity, ranging from 0%-3% and 13%-20%, respectively. Nevertheless, the cumulative rate of retained stones was significantly lower after open surgery (6%) than after two-step protocols (including laparoscopic cholecystectomy and ERC + endoscopic sphincterotomy regardless of the sequence of procedures - 16%). Laparoscopic treatment was also associated to a lower rate of retained CBDS than post-cholecystectomy endoscopic management (ERC + ES) (9% vs 25%). Moreover, laparoscopic management resulted as being associated to shorter hospital stay and reduced in-hospital charges than sequential two-step management in some studies[96,99,100].
Although the analysis of recent literature seems to be in favour of a large-scale diffusion of one-stage laparoscopic management of cholecystocholedochal lithiasis, as witnessed by the encouragement to surgeons to train in laparoscopic CBD clearance by the British Society of Gastroenterology guidelines[38], nevertheless, in common practice, only 20%-21% of American surgeons regularly perform laparoscopic CBD exploration[100,101], and 75% consider preoperative ERC as the preferred approach[101]. The reasons for such a limited diffusion of laparoscopic CBD exploration are the necessary learning curve for a not-so-frequent procedure, the non-reproducibility of referral centers’ results in elective patients[40], long operative times and lack of equipment[101].
ERC + ES: First introduced in 1974[102], ERC followed by endoscopic sphincterotomy has become the most widely used method for imaging and treating CBD stones[103,104]. The technique consists of the endoscopic identification of the papilla major (Vater papilla), its cannulation in order to perform ERC and endoscopic sphincterotomy followed by CBDS extraction by Dormia Basket or balloon. Endoscopic sphincterotomy success rate is reported to exceed 90%[22,87,88,105,106]. Although regarded widely as a safe procedure, large series have recently shown 5%-9.8% morbidity and 0.3% to 2.3% mortality[26,75-77,106,107], mostly due to postoperative acute pancreatitis, bleeding and perforation; the latter, reported in 0.3%-1% of cases, carries a mortality rate of 16%-18%[77,106,108,109].
Timing for ERC is also a matter of debate, as ERC may be performed before, after, or even during cholecystectomy, according to the so-called “rendez-vous technique”.
Preoperative ERC[44,87,88,96] presents the drawback of needing a second surgical procedure to treat gallstones (cholecystectomy), but has the great advantage of allowing for a “strategy update” before surgery: if endoscopic sphincterotomy is successful, cholecystectomy will complete the mini-invasive management of cholecysto-choledochal lithiasis; if it is not, the “second step” will be a surgical procedure aimed at managing both gallstones and CBDS, which, in most environments, will be performed by laparotomy. The other major issue of this approach is patient selection to undergo ERC, since the systematic use of ERC is no longer acceptable in patients who are candidates for cholecystectomy (see above).
Performing ERC after cholecystectomy [22,31,75,110] is also a “two step management” of cholecysto-choledochal lithiasis, and has the great advantage of performing ERC in virtually only those cases really needing CBD clearance, thus reducing to a minimum any possible ERC-related complications and costs. Unfortunately, the main drawback of postoperative ERC is the need for a third surgical procedure whenever postoperative ERC fails.
The rendez-vous technique[87,88,111,112] avoids some of the critical issues of other techniques, since it involves CBDS diagnosis and the synchronous management of both gallstones and CBDS during the same procedure (“one step”), although it needs the systematic availability of dedicated instrumentation and a second team to perform intraoperative ERC whenever IOC/laparoscopic US shows CBDS.
Endoscopic papillary balloon dilatation: Endoscopic papillary balloon dilatation (EPBD) was first introduced with the purpose of extracting CBDS minimizing the damage to the sphincter of Oddi[113]. Several studies have shown that EPBD alone or in combination with small sphincterotomy and lithotripsy can be used for the management of difficult biliary stones[114-118]. The rationale of performing a minimal sphincterotomy before proceeding with EPBD is that it can provide a larger opening and prevent perforation and bleeding, thus making this technique especially attractive in patients who are at risk of bleeding or in those with altered anatomy in whom a full sphincterotomy cannot be achieved. After minimal sphincterotomy, a guidewire is inserted into the bile duct and a balloon catheter is guided over the wire. The balloon is inflated until it reaches a diameter of 15-20 mm. Endoscopic papillary dilation is performed slowly (approximately 1 min of balloon dilation time)[119]. The results of a Japanese multicentric trial with a mean follow up of 6.7 years demonstrated that there is a lower risk of stone recurrence following EPBD when compared with ES[120]. Nevertheless, despite its premises of lower invasiveness, a recent meta-analysis[121] including 15 randomized trials comparing endoscopic sphincterotomy and EPBD showed this latter to be associated to a lower rate of stone removal (RR= 0.90), a more frequent need for mechanical lithotripsy (RR = 1.34), and a higher risk of pancreatitis (RR = 1.96). Somewhat confirming its not so low invasiveness, more recently EPBD has been associated with higher morbidity and severe complications than sphincterotomy, including acute pancreatitis and perforation[115]. The rate of these complications can be reduced by strict patient selection, avoidance of forced procedures, optimal dilation duration and immediate conversion to an alternative procedure if any difficulty is encountered during EPBD[115]. Conversely, EPBD is associated with reduced risk of bleeding and early/long term infections, thus making this option especially indicated in older patients, those who are at risk of infection and those who are affected by coagulopathy[122].
Open surgery: Until the late 1980s, gallstones were treated by open cholecystectomy and CBDS were managed by open CBD exploration and clearance, which was performed by duodenotomy and sphincterotomy or bilio-enteric anastomosis[45,123].
Although open surgery is regarded nowadays as the last resource or obsolete therapy of CBDS, recent literature seems to show its superiority to ERC in achieving CBDS clearance[96,98], without increasing morbidity/mortality (20% vs 19% and 1% vs 3% for open surgery and ERC, respectively)[98]. An emerging issue regarding CBDS management and biliary surgery in general is that, while the treatment of gallstones is mostly performed mini-invasively by laparoscopy and/or endoscopy, open biliary surgery is performed increasingly less outside centers specialized in hepato-bilio-pancreatic surgery. Such a trend towards the “super-specialization” of surgeons, in accordance with centralization policies theoretically aimed to improve the quality of surgery and to reduce its costs, raises new issues regarding the most appropriate management of those patients, whose number is small but not negligible, presenting with complex cases or needing conversion/revision by open approach, with potentially disastrous consequences. The answer we will give to such a dilemma will definitely have an impact on the education of next generation’s general surgeons.
Laparoscopy: Since 1991[21], CBD exploration may be performed laparoscopically. After Calot’s triangle dissection, laparoscopic IOC and/or US are used in order to identify CBDS. Whenever CBDS are found, clearance is usually attempted by “water flush” by means of an irrigator. The latter procedure may be performed through the cystic duct, if it is adequately large, or through vertical choledochotomy. If this latter manoeuvre fails, choledochotomy may also allow for the introduction of a choledochoscope and CBDS retrieval by Dormia basket.
Laparoscopic CBD management, in expert hands, is reported to be at least as effective as ERC in clearing the CBD[22,75,98]. After clearance, in most cases choledochotomy is sutured without the need for any drainage (T-tube-Kehr -drain), whereas the latter may be required if the CBD is inflamed[32,124-126]. Finally, although the operating time is definitely longer than that needed to carry out a simple laparoscopic cholecystectomy, both gallbladder and CBDS are treated during the same intervention, thus avoiding a second hospitalization and procedure[22,96,100].
Conversely, laparoscopic exploration has particular drawbacks, which limit its diffusion outside specialized environments. CBD exploration/clearance needs costly instruments and adequate surgical skills. The feasibility of laparoscopic CBD exploration depends on several variables, including tissue status (inflammation, adhesions...), patient anatomy (length/size/insertion of cystic duct, size of CBD) and number/size/location of CBDS. Recently, our group proposed a “laparoscopy first” attitude in managing CBDS, involving a systematic laparoscopic exploration before deciding whether to proceed with CBD exploration or just cholecystectomy, in the latter case postponing CBDS management to postoperative ERC[32].
Some small series/case reports showed the feasibility of choledocho-jejunal anastomosis by laparoscopy for CBDS[127,128]. Nevertheless, the difficulty of the procedure, requiring very experienced surgeons, and the long operating time (300-358 min)[127,128] make this technique a late option of CBDS management in very specialized environments.
Emerging mini-invasive surgical techniques: Recent advances in minimally invasive surgery, including single incision laparoscopy (SILS), natural orifice translumenal endoscopic surgery (NOTES), and robotics, to some extent show the possible future of biliary lithiasis management, although lesser dexterity on the part of surgeons, the need for dedicated devices/advanced skills and the intrinsically technical difficulty of CBD exploration limit the diffusion of these approaches to cholecystectomy at the moment.
Although SILS cholecystectomy has been increasingly reported[129-133], interestingly, very little information is reported regarding patient selection and CBD status. IOC has not been attempted systematically (5%-93%)[129-133], probably partly because of the difficult triangulation through the single access. When attempted, IOC had a success rate of 88%-93%[129,132,133]. Moreover, when IOC finally showed CBDS, only two of the relevant papers reported the accomplishment of laparoscopic CBD clearance[129,133]; of these, only Yeo[133] reports a single port CBD clearance (1 of 5 cases with CBDS), whereas Hawasli[129] “converted” the procedure to traditional laparoscopy. SILS is presently reserved for specially selected, non-obese patients undergoing cholecystectomy[133].
NOTES has also been used for cholecystectomy, in 92%-97% of cases with a transvaginal access and in 3%-8% of cases with a transgastric one[134,135]. Interestingly, neither CBD preoperative work up nor IOC are reported in most articles[134-136], whereas others considered the need for an intraoperative diagnosis as being an exclusion criteria for NOTES[137,138]. In the only series reporting the IOC technique, significantly only 33 out of 83 of eligible patients (40%) underwent transvaginal cholecystectomy[138], 13 transvaginal IOC and 3 laparoscopic CBD clearance requiring one additional 12-mm port[138]. NOTES cholecystectomy is performed in non-complicated cases in non-obese patients, who are almost exclusively women, since it is mostly performed transvaginally.
First proposed for cholecystectomy in 1994[139], robotics may theoretically offer both the advantages of mini-invasive approach and the dexterity of open surgery, which should be useful in particular during demanding procedures such as CBD exploration. Nevertheless, few papers report any manoeuvre/procedure to diagnose/ treat CBDS. First described in 2004[140], small series of robotic laparoscopic exploration of CBD have recently been reported[141,142], showing a longer mean operating time (220 min vs 169 min) and a shorter median hospital stay (4 d vs 11 d) compared to open surgery[142]. These procedures involve the placing of five or more trocars. This technique may currently be considered at best an option for CBDS refractory to ERC[142] in order to avoid open surgery or percutaneous drainage in very specialized environments.
Single port robotics[143,144] intuitively present all the advantages of SILS and robotics. Unfortunately, switching from cholecystectomy to IOC (and potentially to CBD exploration) is not such an easy task to accomplish, owing to the difficult triangulation and the need to change instruments[144]. Single port robotic IOC involves another incision[143] and longer operating times[143,144], whereas CBD exploration has not yet been described by single port robotics.
Although papers reporting CBDS management by robotic choledocho-jejunostomy are extremely sporadic[145,146], the increasing evidence of an improved dexterity of the robotic hepatobiliary surgeon[147] seems to indicate a possible future management of complex cases.
First introduced in 1982[148], lithotripsy represents the theoretically ideal management of CBDS, as it may enable the clearing of the CBD without any interruption of the CBD wall or sphincterotomy. Although lithotripsy may technically fragmentize gallbladder stones too, it cannot be considered a radical treatment of synchronous cholecysto-choledochal lithiasis, as gallstone aetiology is considered as being due to lithogenic bile in the gallbladder (and therefore gallstones are destined to recur after lithotripsy). Moreover, since fragmentized gallstones are smaller, they may be supposed to have a higher risk of migration into the CBD, with choledocho-lithiasis recurrence. However, lithotripsy presents the advantage of being a one time (or few times) management of CBDS. For all these considerations, lithotripsy does not allow for avoiding cholecystectomy, thus becoming a very attractive option whenever cholecystectomy has already been performed or is not indicated. Reported to be performed in several ways (mechanical, electrohydraulic, laser, and extracorporeal shock wave)[23-25,149], lithotripsy also presents the drawback of needing dedicated instrumentation and skilled personnel, which are not always available, thus limiting its diffusion worldwide.
Endoscopic mechanical lithotripsy: endoscopic mechanical lithotripsy is usually performed after endoscopic sphincterotomy for CBDS that cannot be removed through a Dormia basket or balloon catheter. Introduced through the same scope utilized for ERC, lithotriptors consist of a large hard-wire basket with an additional metal spiral sheath, which is advanced over the basket into the CBD. When in position, the metal basket is opened to capture the stones and pulled back to the external hard-duct of the lithotriptor and lithotripsy is performed. The so-called “out of the scope” technique, where the endoscope is removed to introduce the Soehendra lithotriptor and crush the stones blindly[150,151], has been progressively replaced by the “through the scope” technique, where the lithotriptor is inserted in the endoscope itself and the whole procedure is performed under vision, reaching a CBD clearance in 80%-90% of cases[149,151-153].
Conditions associated to failure of lithotripsy are CBDS size exceeding 3 cm[154], since stones could not be captured, and stone impaction in the CBD[155]. Basket impaction or rupture of the basket traction wire are complications unique to mechanical lithotripsy[24,156]. Some patients cannot tolerate the prolonged lithotripsy operating time, thus, the procedure has to be conducted in several sessions[154,155], and general anaesthesia is recommended in selected cases[157]. According to several authors, a stent should be placed for bile drainage if the CBD stones could not be removed in the first session of mechanical lithotripsy[158-160].
Endoscopic electrohydraulic lithotripsy: Endoscopic electrohydraulic lithotripsy, using a “baby-mother” scope system[161,162], may also be used for difficult CBDS. The mother scope has a large operating channel to accommodate the baby scope carrying the 4.5 Fr calibre probe with an electrohydraulic shock wave generator set at an output of 2000 V, generating high-frequency hydraulic pressure waves. Because these shock waves can also destroy normal tissues, it is important that the probe is placed close to the stone. The CBDS removal rate ranges from 74% to 98%[161,162]. However, this procedure needs costly and fragile endoscopes[97] as well as the excellent coordination of two very experienced endoscopists. The latter problem has been potentially solved by the introduction of the so-called SpyGlass system[163,164], where the same endoscopist controls both duodenoscope and cholangioscope (SpyScope). After ES, the SpyScope is introduced through the therapeutic duodenoscope into the biliary tree, where electrohydraulic lithotripsy can be used via a 3-Fr electrohydraulic lithotripsy probe.
Endoscopic laser lithotripsy: Since the mid-nineties, laser-induced shock wave lithotripsy has been used in shattering huge stones under fluoroscopic visualization, which was reqested, because of the risk of heat-induced biliary perforation[165]. Nowadays, single-operator steerable cholangioscopy (SpyGlass) allows the safer use of laser lithotripsy under direct vision[166,167]. A 93%-97% removal rate of the bile duct stones has been reported, with mild complications in 4%-13% of cases[167-169]. Among the several types of laser lithotriptors developed, the holmium laser (holmium:yttrium aluminum garnet or Ho:YAG) is the newest one, but its use is still limited, also owing to the need for costly equipment[119]. Recent laser lithotriptors combine the advantages of dye and solid-state lasers at a reasonably low price[166], thus potentially allowing for a future progressive diffusion of laser lithotripsy worldwide[166,167].
Following the encouraging results of laser lithotripsy by endoscopy, laser lithotripsy has recently been proposed during laparoscopy[170], open surgery[171], or percutaneously[172].
Extracorporeal shock-wave lithotripsy: External shock wave using extracorporeal shock wave lithotripsy (ESWL) is also used for difficult CBDS under ultrasound control or fluoroscopic guidance, after nasobiliary tube placement for contrast instillation [97,173]. In the case of first generation lithotriptors, the patient needed to be immersed in a water bath, whereas modern lithotriptors employ water-filled compressible bags. General anaesthesia is usually needed as the discomfort produced may not be adequately controlled by conscious sedation. The critical determining factors for success of ESWL are stone size/structure[174] and CBD diameter[175]. ESWL has particular contraindications, such as portal thrombosis and varices of the umbilical plexus, and may be associated with adverse events such as transient biliary colic, subcutaneous ecchymosis, cardiac arrhythmia, self-limited haemobilia, cholangitis, ileus, pancreatitis[176,177], and, more rarely, biliary obstruction, bowel perforation, lung injury and splenic rupture[174]. Multiple ESWL sessions may be required. The recurrence rate of CBD stones during a 1 to 2 year follow up is around 14%[178]. In a randomized trial comparing fluoroscopy-guided ESWL and laser lithotripsy, the latter is preferable for a successful stone free rate (97% vs 73%)[173]. Therefore, ESWL is at present not considered as being the first line treatment of difficult bile duct stones[97].
Acute cholecystitis: Whether acute cholecystitis is associated with CBDS is a matter of debate, as some authors have found CBDS in 9.1%-16.5% of patients presenting with acute cholecystitis (vs 6.6%-7.7% of those with uncomplicated lithiasis[179,180]), while others did not[181]; we ourselves found acute cholecystitis to be related to the absence of CBDS, thus entering in our score system[44].
Diagnosis of CBDS may be difficult in the presence of acute cholecystitis, where various alterations in liver serology and hyperpyrexia may be observed, thus possibly ‘‘hiding’’ CBDS. Moreover, since the effectiveness of US and CT is suboptimal in diagnosing CBDS, they can pass undiagnosed. Recently, the systematic use of MRC has been proposed for the diagnosis of acute cholecystitis, coexistent choledocholithiasis, and possible complications such as gangrene and perforation in patients with inconclusive clinical, laboratory, and sonographic findings[182,183].
Since acute cholecystitis may include a wide variety of clinical pictures, ranging from asymptomatic cases presenting merely the doubling of the gallbladder wall at US, to those with abdominal pain/tenderness suitable for conservative treatment, or with life-threatening conditions needing emergency surgery, the management of synchronous acute cholecystitis and CBDS should be tailored considering gallbladder status and the patient’s general conditions. In general, the presence of acute cholecystitis may increase the difficulty of dissecting Calot’s triangle by laparoscopy, as well as of cannulating the cystic duct to perform IOC or proceed with CBD laparoscopic exploration. Therefore, CBDS associated with acute cholecystitis are generally considered an indication for ERC[184], as confirmed by a recent survey of 859,747 patients who underwent emergency and/or urgent laparoscopic cholecystectomy and where IOC was performed in only 29% of cases and CBD exploration in no more than 1%[185]. Nevertheless, the laparoscopic management of acute cholecystitis and CBDS is feasible, with an acceptable 12% conversion rate and 29% morbidity[186]. As we have already mentioned[32], a laparoscopy-first attitude, intraoperatively evaluating the real feasibility of laparoscopic CBD exploration, may allow for CBD clearance in roughly 2/3 of cases, thus reducing the number of postoperative ERCs to a minimum, without excessively long/risky procedures and inopportune conversions.
Acute cholangitis: Biliary decompression is considered as being the primary treatment of gallstone-related acute cholangitis. As acute cholangitis may have various clinical presentations, elective biliary decompression may be planned by endoscopy or laparoscopy in mild to moderate disease, whereas emergency endoscopic sphincterotomy has to be performed in the case of severe sepsis not responding to antibiotic therapy[29,38]. Any surgical treatment in these patients is associated with higher mortality than for endoscopy and therefore should be avoided[187,188]. Given the potentially life-threatening condition, significantly, even if ERC does not show any CBDS, there is a formal indication for endoscopic sphincterotomy followed by balloon or basket trawl of the CBD[38].
Acute pancreatitis: The management of biliary acute pancreatitis has evolved over the last 30 years. Following the results of randomized studies[189,190], the United Kingdom guidelines for the management of acute pancreatitis advocated urgent therapeutic ERC in every patient with acute pancreatitis by suspected gallstone etiology[191]. With the advent of EUS and MRC[192], this management policy has been modified, according to the principle that every effort should be made to identify biliary obstruction before resorting to ERC[193]. Lastly, the American Gastroenterological Association Institute guidelines on acute pancreatitis recommend that early ERC is not indicated in patients with predicted severe pancreatitis without concomitant cholangitis or high suspicion of CBDS[194].
The aging population of Western countries together with the increasingly less invasive nature of CBDS management is rekindling the debate concerning the most appropriate treatment not only after the age of 65 (elderly) but also after the age of 80 (very elderly), where procedure-related complications may become severe or fatal.
Although the small size and retrospective nature of the studies do not confer a clear evidence, no clear benefit of any technique has been shown over another in this class of patients. ERC and endoscopic sphincterotomy are proposed for CBDS retrieval in the very elderly[157,195-197]. Because elderly patients often receive anticoagulant/antiplatelet agents for underlying heart and cerebrovascular diseases, the risk of bleeding after endoscopic sphincterotomy is higher in these patients[77,198] and the ASGE guideline has recommended discontinuing anticoagulant agents before ES[199]. Moreover, patients who are old[200] and/or have haemostasis problems (such as cirrhosis)[201] have been recommended for management by endoscopic balloon dilation instead of ERC, with similar results to those of younger patients (apart from a higher number of endoscopic sessions)[200]. In very old patients and those with serious co-morbidities, where other endoscopic or surgical procedures may confer unacceptably high risks, endoscopic biliary stenting is a useful alternative[202].
Similarly to sequential treatment (ERC followed by delayed laparoscopic cholecystectomy)[203], the rendez-vous technique has shown longer operating times and hospital stays in octogenarians compared to younger patients[112], whereas the two approaches did not show significant differences from each other among the octogenarian population[112]. In general, although results of mini-invasive CBDS management in the very elderly patients are seemingly worse than in younger patients, they mostly consist of longer operating times and hospital stays; since clinical impact and morbidity are seemingly lower than those of traditional surgery, age should not contraindicate per se CBDS management.
Biliary stent placement is a possible alternative in elderly patients[158,204], but is likely to put the patients at risk for cholangitis as a result of stent clogging. To avoid this adverse event, a periodic (e.g., every 3 mo) exchange of biliary stents is needed, which makes this option far less attractive whenever any other technique is feasible.
An emerging dilemma is whether to proceed with cholecystectomy after successful CBD clearance in patients of 80 years old or older, where laparoscopic cholecystectomy may be presumed to be associated to increased morbidity/mortality. Indeed, a review[203] reporting the results of laparoscopic cholecystectomy in the very elderly showed a cumulative mortality rate of 2% in octogenarians, but this value dropped to virtually 0 if patients presenting with acute cholecystitis and/or concomitant severe disease were excluded, to some extent suggesting not postponing elective laparoscopic cholecystectomy in fit octogenarians.
The wide variety of treatment options for CBDS available nowadays makes it impossible to draw up any algorithm/protocol including any possible challenging situations for endoscopists and surgeons. Any systematic approach to CBDS by a single technique, as has been proposed for decades by authors having a great expertise in only one field (endoscopy, laparoscopy, lithotripsy), is slowly but progressively giving way to a more patient-tailored approach, where the pros and cons of different approaches are taken into account[31,33]. Obviously, the increasing availability of instruments and skills in the same environment will allow for a diffusion of such a multidisciplinary approach to CBDS. Moreover, as already pointed out, it is nowadays suggested that asymptomatic CBDS should be managed more conservatively, as some will pass through the papilla within a few weeks of cholecystectomy[31,35]. Thus, following this more multidisciplinary and cautious policy, an unsuccessful laparoscopic CBD exploration, for example, is increasingly often considered an indication not for conversion to open surgery and transduodenal CBD clearance or biliodigestive anastomosis, but rather for a postoperative conservative or mini-invasive approach by ERC[32], based on the assumption that a difficult laparoscopic CBD clearance does not necessarily mean a difficult endoscopic management.
Postcholecystectomy CBDS may be due to residual CBDS misdiagnosed at surgery, as happens after up to 2% of cholecystectomies performed without cholangiogram[205], or recurrent CBDS. The latter are due to intrahepatic or intraCBD lithiasis and are therefore not treated in this review. Other than the already described clinical pictures, residual CBDS are the most frequent cause of a “postcholecystectomy syndrome”, including symptoms originally attributed to gallstones, such as abdominal pain, nausea, vomiting, dyspepsia, and persistent biliary colic and liver serology alterations[206].
Diagnosis of CBDS after cholecystectomy may be difficult, as the absence of a gallbladder may lead to the exclusion of a biliary origin of aspecific symptoms/laboratory test alterations, thus causing a delay in diagnosis. Moreover, transabdominal US is less accurate in postcholecystectomy patients, since the CBD diameter may be larger because of cholecystectomy or old age of patients[55,207,208], where the upper limit for a “normal” CBD diameter is raised to 7.6-10 mm[207-209]. Since the total number of patients undergoing second-line examinations for residual CBDS and finally presenting this feature is 33%-43%[208,210], systematic ERC should result as being unnecessary in more than half of the patients and is therefore seemingly not indicated as a second-line examination. Among less invasive imaging techniques, although MRC and EUS have shown similar results in this setting[208,210], the need for ERC in 1/3 of patients or more should prompt us to favour EUS, possibly followed by ERC and endoscopic sphincterotomy during the same session, for a theoretical one-step-management of CBDS.
The absence of gallbladder and the consequent possibility to avoid surgery and general anaesthesia make endoscopy the most attractive option for several authors[211,212]. For the same reason, lithotripsy may intuitively be useful in this setting[213], whereas open surgery is the last option after the failure of mini-invasive approaches[214]. Although laparoscopic CBD clearance is also possible in such a context in expert hands[215], such a procedure is likely to result as being more difficult owing to the sequelae of cholecystectomy (adhesions, scarring), thus requiring advanced laparoscopic skills.
CBDS diagnosis and management can nowadays rely on various imaging techniques and mini-invasive treatments, including endoscopy and laparoscopy. Concerning diagnosis, IOC, EUS and MRC have similar results and very low morbidity and may be used indiscriminately. There is no consensus regarding the best management of CBDS, where surgery has seemingly slightly better results and a lower pancreatitis rate, which are counterbalanced by the need for specific instrumentation and advanced laparoscopic skills. In this regard, significantly, the British Society of Gastroenterologists encourage surgeons to train in laparoscopic CBD exploration[38] while, conversely, laparoscopic surgeons mostly prefer ERC to treat CBDS[101]. According to recent guidelines, the management of CBDS will be increasingly tailored not only to a specific patient but also to the available resources of a specific environment in order to have the best possible management. The worldwide diffusion of mini-invasive management (by laparoscopy/endoscopy) of biliary disease goes in the opposite direction of the recent trend of the “centralization” of open biliary surgery, which is being performed increasingly less outside specialized centers. Such considerations raise new issues regarding the most appropriate management of complex cases needing conversion/exploration by an open approach, as the new generation of laparoscopic surgeons may not be able to deal with their own complications.
P- Reviewer: Rausei S, Zhu JF S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632-639. [PubMed] |
| 2. | Aerts R, Penninckx F. The burden of gallstone disease in Europe. Aliment Pharmacol Ther. 2003;18 Suppl 3:49-53. [PubMed] |
| 3. | Menezes N, Marson LP, debeaux AC, Muir IM, Auld CD. Prospective analysis of a scoring system to predict choledocholithiasis. Br J Surg. 2000;87:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Videhult P, Sandblom G, Rasmussen IC. How reliable is intraoperative cholangiography as a method for detecting common bile duct stones? : A prospective population-based study on 1171 patients. Surg Endosc. 2009;23:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Borzellino G, Rodella L, Saladino E, Catalano F, Politi L, Minicozzi A, Cordiano C. Treatment for retained [corrected] common bile duct stones during laparoscopic cholecystectomy: the rendezvous technique. Arch Surg. 2010;145:1145-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484-487. [PubMed] |
| 7. | Sarli L, Costi R, Gobbi S, Sansebastiano G, Roncoroni L. Asymptomatic bile duct stones: selection criteria for intravenous cholangiography and/or endoscopic retrograde cholangiography prior to laparoscopic cholecystectomy. Eur J Gastroenterol Hepatol. 2000;12:1175-1180. [PubMed] |
| 8. | Cohn EM, Freiman HD. The diagnosis of common duct stones by intravenous cholangiography compared with common duct exploration. Am J Gastroenterol. 1961;35:513-517. [PubMed] |
| 9. | Mather JH, Williams WR. Cholecystography: the results of 125 consecutive cases examined by the oral administration of the salt. Br Med J. 1927;1:614-615. [PubMed] |
| 10. | Sarli L, Pietra N, Franzé A, Colla G, Costi R, Gobbi S, Trivelli M. Routine intravenous cholangiography, selective ERCP, and endoscopic treatment of bile duct stones before laparoscopic cholecystectomy. Gastrointest Endosc. 1999;50:200-208. [PubMed] |
| 11. | Hammarström LE, Holmin T, Stridbeck H, Ihse I. Routine preoperative infusion cholangiography versus intraoperative cholangiography at elective cholecystectomy: a prospective study in 995 patients. J Am Coll Surg. 1996;182:408-416. [PubMed] |
| 12. | Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc. 2006;64:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Polkowski M, Regula J, Tilszer A, Butruk E. Endoscopic ultrasound versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: a randomized trial comparing two management strategies. Endoscopy. 2007;39:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Karakan T, Cindoruk M, Alagozlu H, Ergun M, Dumlu S, Unal S. EUS versus endoscopic retrograde cholangiography for patients with intermediate probability of bile duct stones: a prospective randomized trial. Gastrointest Endosc. 2009;69:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 15. | Peters JH, Ellison EC, Innes JT, Liss JL, Nichols KE, Lomano JM, Roby SR, Front ME, Carey LC. Safety and efficacy of laparoscopic cholecystectomy. A prospective analysis of 100 initial patients. Ann Surg. 1991;213:3-12. [PubMed] |
| 16. | Soper NJ, Stockmann PT, Dunnegan DL, Ashley SW. Laparoscopic cholecystectomy. The new ‘gold standard’? Arch Surg. 1992;127:917-921; discussion 921-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 300] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Kiviluoto T, Sirén J, Luukkonen P, Kivilaakso E. Randomised trial of laparoscopic versus open cholecystectomy for acute and gangrenous cholecystitis. Lancet. 1998;351:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 254] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Phillips E, Daykhovsky L, Carroll B, Gershman A, Grundfest WS. Laparoscopic cholecystectomy: instrumentation and technique. J Laparoendosc Surg. 1990;1:3-15. [PubMed] |
| 19. | Machi J, Tateishi T, Oishi AJ, Furumoto NL, Oishi RH, Uchida S, Sigel B. Laparoscopic ultrasonography versus operative cholangiography during laparoscopic cholecystectomy: review of the literature and a comparison with open intraoperative ultrasonography. J Am Coll Surg. 1999;188:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Jacobs M, Verdeja JC, Goldstein HS. Laparoscopic choledocholithotomy. J Laparoendosc Surg. 1991;1:79-82. [PubMed] |
| 21. | Petelin JB. Laparoscopic approach to common duct pathology. Surg Laparosc Endosc. 1991;1:33-41. [PubMed] |
| 22. | Nathanson LK, O’Rourke NA, Martin IJ, Fielding GA, Cowen AE, Roberts RK, Kendall BJ, Kerlin P, Devereux BM. Postoperative ERCP versus laparoscopic choledochotomy for clearance of selected bile duct calculi: a randomized trial. Ann Surg. 2005;242:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Siegel JH, Ben-Zvi JS, Pullano WE. Endoscopic electrohydraulic lithotripsy. Gastrointest Endosc. 1990;36:134-136. [PubMed] |
| 24. | Lux G, Ell C, Hochberger J, Müller D, Demling L. The first successful endoscopic retrograde laser lithotripsy of common bile duct stones in man using a pulsed neodymium-YAG laser. Endoscopy. 1986;18:144-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 67] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Ponchon T, Martin X, Barkun A, Mestas JL, Chavaillon A, Boustière C. Extracorporeal lithotripsy of bile duct stones using ultrasonography for stone localization. Gastroenterology. 1990;98:726-732. [PubMed] |
| 26. | Ong TZ, Khor JL, Selamat DS, Yeoh KG, Ho KY. Complications of endoscopic retrograde cholangiography in the post-MRCP era: a tertiary center experience. World J Gastroenterol. 2005;11:5209-5212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 27. | Wu SC, Chen FC, Lo CJ. Selective intraoperative cholangiography and single-stage management of common bile duct stone in laparoscopic cholecystectomy. World J Surg. 2005;29:1402-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Machi J, Oishi AJ, Tajiri T, Murayama KM, Furumoto NL, Oishi RH. Routine laparoscopic ultrasound can significantly reduce the need for selective intraoperative cholangiography during cholecystectomy. Surg Endosc. 2007;21:270-274. [PubMed] |
| 29. | ASGE Standards of Practice Committee, Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, Ikenberry SO, Jain R, Khan K, Krinsky ML, Strohmeyer L, Dominitz JA. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 30. | Byrne MF, McLoughlin MT, Mitchell RM, Gerke H, Kim K, Pappas TN, Branch MS, Jowell PS, Baillie J. For patients with predicted low risk for choledocholithiasis undergoing laparoscopic cholecystectomy, selective intraoperative cholangiography and postoperative endoscopic retrograde cholangiopancreatography is an effective strategy to limit unnecessary procedures. Surg Endosc. 2009;23:1933-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Ammori BJ, Birbas K, Davides D, Vezakis A, Larvin M, McMahon MJ. Routine vs “on demand” postoperative ERCP for small bile duct calculi detected at intraoperative cholangiography. Clinical evaluation and cost analysis. Surg Endosc. 2000;14:1123-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Costi R, Mazzeo A, Tartamella F, Manceau C, Vacher B, Valverde A. Cholecystocholedocholithiasis: a case-control study comparing the short- and long-term outcomes for a “laparoscopy-first” attitude with the outcome for sequential treatment (systematic endoscopic sphincterotomy followed by laparoscopic cholecystectomy). Surg Endosc. 2010;24:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 33. | Urbach DR, Khajanchee YS, Jobe BA, Standage BA, Hansen PD, Swanstrom LL. Cost-effective management of common bile duct stones: a decision analysis of the use of endoscopic retrograde cholangiopancreatography (ERCP), intraoperative cholangiography, and laparoscopic bile duct exploration. Surg Endosc. 2001;15:4-13. [PubMed] |
| 34. | Scientific Committee of the European Association for Endoscopic Surgery. Diagnosis and treatment of common bile duct stones (CBDS). Results of a consensus development conference. Surg Endosc. 1998;12:856-864. [PubMed] |
| 35. | Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg. 2004;239:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 319] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | Pring CM, Skelding-Millar L, Goodall RJ. Expectant treatment or cholecystectomy after endoscopic retrograde cholangiopancreatography for choledocholithiasis in patients over 80 years old? Surg Endosc. 2005;19:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Treckmann J, Sauerland S, Frilling A, Paul A. Common Bile Duct Stones - Update 2006. EAES guidelines for endoscopic surgery. Berlin Heidelberg: Springer 2006; 329-333. |
| 38. | Williams EJ, Green J, Beckingham I, Parks R, Martin D, Lombard M. Guidelines on the management of common bile duct stones (CBDS). Gut. 2008;57:1004-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 39. | Maple JT, Ikenberry SO, Anderson MA, Appalaneni V, Decker GA, Early D, Evans JA, Fanelli RD, Fisher D, Fisher L. The role of endoscopy in the management of choledocholithiasis. Gastrointest Endosc. 2011;74:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 40. | De Palma GD. Minimally invasive treatment of cholecysto-choledocal lithiasis: The point of view of the surgical endoscopist. World J Gastrointest Surg. 2013;5:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Prat F, Meduri B, Ducot B, Chiche R, Salimbeni-Bartolini R, Pelletier G. Prediction of common bile duct stones by noninvasive tests. Ann Surg. 1999;229:362-368. [PubMed] |
| 42. | Tse F, Barkun JS, Barkun AN. The elective evaluation of patients with suspected choledocholithiasis undergoing laparoscopic cholecystectomy. Gastrointest Endosc. 2004;60:437-448. [PubMed] |
| 43. | Huguier M, Bornet P, Charpak Y, Houry S, Chastang C. Selective contraindications based on multivariate analysis for operative cholangiography in biliary lithiasis. Surg Gynecol Obstet. 1991;172:470-474. [PubMed] |
| 44. | Sarli L, Costi R, Gobbi S, Iusco D, Sgobba G, Roncoroni L. Scoring system to predict asymptomatic choledocholithiasis before laparoscopic cholecystectomy. A matched case-control study. Surg Endosc. 2003;17:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Schwartz SI. Gallbladder and extrahepatic biliary system. Principles of Surgery. Second Edition. New York: McGraw-Hill 1974; 1221-1254. |
| 46. | Kondo S, Isayama H, Akahane M, Toda N, Sasahira N, Nakai Y, Yamamoto N, Hirano K, Komatsu Y, Tada M. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol. 2005;54:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Charcot JM. Leçons sur les maladies du foie, des voies biliaires et des reins. Paris: Faculté de Médecine de Paris 1877; . |
| 48. | Barkun AN, Barkun JS, Fried GM, Ghitulescu G, Steinmetz O, Pham C, Meakins JL, Goresky CA. Useful predictors of bile duct stones in patients undergoing laparoscopic cholecystectomy. McGill Gallstone Treatment Group. Ann Surg. 1994;220:32-39. [PubMed] |
| 49. | Houdart R, Perniceni T, Darne B, Salmeron M, Simon JF. Predicting common bile duct lithiasis: determination and prospective validation of a model predicting low risk. Am J Surg. 1995;170:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Topal B, Van de Moortel M, Fieuws S, Vanbeckevoort D, Van Steenbergen W, Aerts R, Penninckx F. The value of magnetic resonance cholangiopancreatography in predicting common bile duct stones in patients with gallstone disease. Br J Surg. 2003;90:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Costi R, Sarli L, Caruso G, Iusco D, Gobbi S, Violi V, Roncoroni L. Preoperative ultrasonographic assessment of the number and size of gallbladder stones: is it a useful predictor of asymptomatic choledochal lithiasis? J Ultrasound Med. 2002;21:971-976. [PubMed] |
| 52. | Stott MA, Farrands PA, Guyer PB, Dewbury KC, Browning JJ, Sutton R. Ultrasound of the common bile duct in patients undergoing cholecystectomy. J Clin Ultrasound. 1991;19:73-76. [PubMed] |
| 53. | Einstein DM, Lapin SA, Ralls PW, Halls JM. The insensitivity of sonography in the detection of choledocholithiasis. AJR Am J Roentgenol. 1984;142:725-728. [PubMed] |
| 54. | Majeed AW, Ross B, Johnson AG, Reed MW. Common duct diameter as an independent predictor of choledocholithiasis: is it useful? Clin Radiol. 1999;54:170-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Lacaine F, Corlette MB, Bismuth H. Preoperative evaluation of the risk of common bile duct stones. Arch Surg. 1980;115:1114-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Wilson TG, Hall JC, Watts JM. Is operative cholangiography always necessary? Br J Surg. 1986;73:637-640. [PubMed] |
| 57. | Taylor TV, Armstrong CP, Rimmer S, Lucas SB, Jeacock J, Gunn AA. Prediction of choledocholithiasis using a pocket microcomputer. Br J Surg. 1988;75:138-140. [PubMed] |
| 58. | Abboud PA, Malet PF, Berlin JA, Staroscik R, Cabana MD, Clarke JR, Shea JA, Schwartz JS, Williams SV. Predictors of common bile duct stones prior to cholecystectomy: a meta-analysis. Gastrointest Endosc. 1996;44:450-455. [PubMed] |
| 59. | Montariol T, Rey C, Charlier A, Marre P, Khabtani H, Hay JM, Fingerhut A, Lacaine F. Preoperative evaluation of the probability of common bile duct stones. French Association for Surgical Research. J Am Coll Surg. 1995;180:293-296. [PubMed] |
| 60. | Topal B, Fieuws S, Tomczyk K, Aerts R, Van Steenbergen W, Verslype C, Penninckx F. Clinical models are inaccurate in predicting bile duct stones in situ for patients with gallbladder. Surg Endosc. 2009;23:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Mitchell SE, Clark RA. A comparison of computed tomography and sonography in choledocholithiasis. AJR Am J Roentgenol. 1984;142:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Soto JA, Alvarez O, Múnera F, Velez SM, Valencia J, Ramírez N. Diagnosing bile duct stones: comparison of unenhanced helical CT, oral contrast-enhanced CT cholangiography, and MR cholangiography. AJR Am J Roentgenol. 2000;175:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Anderson SW, Rho E, Soto JA. Detection of biliary duct narrowing and choledocholithiasis: accuracy of portal venous phase multidetector CT. Radiology. 2008;247:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Kim CW, Chang JH, Lim YS, Kim TH, Lee IS, Han SW. Common bile duct stones on multidetector computed tomography: attenuation patterns and detectability. World J Gastroenterol. 2013;19:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Ainsworth AP, Rafaelsen SR, Wamberg PA, Durup J, Pless TK, Mortensen MB. Is there a difference in diagnostic accuracy and clinical impact between endoscopic ultrasonography and magnetic resonance cholangiopancreatography? Endoscopy. 2003;35:1029-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 67. | Iida F, Kusama J. Surgical evaluation of endoscopic retrograde cholangiography for biliary tract diseases. Jpn J Surg. 1982;12:257-261. [PubMed] |
| 68. | Patel JA, Patel NA, Shinde T, Uchal M, Dhawan MK, Kulkarni A, Colella JJ. Endoscopic retrograde cholangiopancreatography after laparoscopic Roux-en-Y gastric bypass: a case series and review of the literature. Am Surg. 2008;74:689-693; discussion 693-694. [PubMed] |
| 69. | Balik E, Eren T, Keskin M, Ziyade S, Bulut T, Buyukuncu Y, Yamaner S. Parameters That May Be Used for Predicting Failure during Endoscopic Retrograde Cholangiopancreatography. J Oncol. 2013;2013:201681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Faylona JM, Qadir A, Chan AC, Lau JY, Chung SC. Small-bowel perforations related to endoscopic retrograde cholangiopancreatography (ERCP) in patients with Billroth II gastrectomy. Endoscopy. 1999;31:546-549. [PubMed] |
| 71. | Prat F, Amouyal G, Amouyal P, Pelletier G, Fritsch J, Choury AD, Buffet C, Etienne JP. Prospective controlled study of endoscopic ultrasonography and endoscopic retrograde cholangiography in patients with suspected common-bileduct lithiasis. Lancet. 1996;347:75-79. [PubMed] |
| 72. | Tseng LJ, Jao YT, Mo LR, Lin RC. Over-the-wire US catheter probe as an adjunct to ERCP in the detection of choledocholithiasis. Gastrointest Endosc. 2001;54:720-723. [PubMed] |
| 73. | Macintyre IM, Goulbourne IA, Gollock JM, Grieve DC. Operative cholangiography: a study of observer variation. J R Coll Surg Edinb. 1988;33:65-67. [PubMed] |
| 74. | Swanstrom LL, Marcus DR, Kenyon T. Laparoscopic treatment of known choledocholithiasis. Surg Endosc. 1996;10:526-528. [PubMed] |
| 75. | Rhodes M, Sussman L, Cohen L, Lewis MP. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet. 1998;351:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 288] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 76. | Heinerman PM, Boeckl O, Pimpl W. Selective ERCP and preoperative stone removal in bile duct surgery. Ann Surg. 1989;209:267-272. [PubMed] |
| 77. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1716] [Cited by in RCA: 1689] [Article Influence: 58.2] [Reference Citation Analysis (2)] |
| 78. | Lu J, Guo CY, Xu XF, Wang XP, Wan R. Efficacy of intraductal ultrasonography in the diagnosis of non-opaque choledocholith. World J Gastroenterol. 2012;18:275-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Bournet B, Migueres I, Delacroix M, Vigouroux D, Bornet JL, Escourrou J, Buscail L. Early morbidity of endoscopic ultrasound: 13 years’ experience at a referral center. Endoscopy. 2006;38:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 80. | Petrov MS, Savides TJ. Systematic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Benjaminov F, Stein A, Lichtman G, Pomeranz I, Konikoff FM. Consecutive versus separate sessions of endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) for symptomatic choledocholithiasis. Surg Endosc. 2013;27:2117-2121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Ang TL, Teo EK, Fock KM, Lyn Tan JY. Are there roles for intraductal US and saline solution irrigation in ensuring complete clearance of common bile duct stones? Gastrointest Endosc. 2009;69:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | Tsuchiya S, Tsuyuguchi T, Sakai Y, Sugiyama H, Miyagawa K, Fukuda Y, Ando T, Saisho H, Yokosuka O. Clinical utility of intraductal US to decrease early recurrence rate of common bile duct stones after endoscopic papillotomy. J Gastroenterol Hepatol. 2008;23:1590-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Wehrmann T, Martchenko K, Riphaus A. Catheter probe extraductal ultrasonography vs. conventional endoscopic ultrasonography for detection of bile duct stones. Endoscopy. 2009;41:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Huang SW, Lin CH, Lee MS, Tsou YK, Sung KF. Residual common bile duct stones on direct peroral cholangioscopy using ultraslim endoscope. World J Gastroenterol. 2013;19:4966-4972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Parsi MA, Jang S, Sanaka M, Stevens T, Vargo JJ. Diagnostic and therapeutic cholangiopancreatoscopy: performance of a new digital cholangioscope. Gastrointest Endosc. 2014;79:936-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Morino M, Baracchi F, Miglietta C, Furlan N, Ragona R, Garbarini A. Preoperative endoscopic sphincterotomy versus laparoendoscopic rendezvous in patients with gallbladder and bile duct stones. Ann Surg. 2006;244:889-893; discussion 893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 88. | Tzovaras G, Baloyiannis I, Zachari E, Symeonidis D, Zacharoulis D, Kapsoritakis A, Paroutoglou G, Potamianos S. Laparoendoscopic rendezvous versus preoperative ERCP and laparoscopic cholecystectomy for the management of cholecysto-choledocholithiasis: interim analysis of a controlled randomized trial. Ann Surg. 2012;255:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Hicken NF, Best RR, Hunt HB. Cholangiography: Visualization of the gallbladder and bile ducts during and after operation. Ann Surg. 1936;103:210-229. [PubMed] |
| 90. | Ford JA, Soop M, Du J, Loveday BP, Rodgers M. Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 2012;99:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 91. | Ragulin-Coyne E, Witkowski ER, Chau Z, Ng SC, Santry HP, Callery MP, Shah SA, Tseng JF. Is routine intraoperative cholangiogram necessary in the twenty-first century? A national view. J Gastrointest Surg. 2013;17:434-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Perry KA, Myers JA, Deziel DJ. Laparoscopic ultrasound as the primary method for bile duct imaging during cholecystectomy. Surg Endosc. 2008;22:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Halpin VJ, Dunnegan D, Soper NJ. Laparoscopic intracorporeal ultrasound versus fluoroscopic intraoperative cholangiography: after the learning curve. Surg Endosc. 2002;16:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Falcone RA, Fegelman EJ, Nussbaum MS, Brown DL, Bebbe TM, Merhar GL, Johannigman JA, Luchette FA, Davis K, Hurst JM. A prospective comparison of laparoscopic ultrasound vs intraoperative cholangiogram during laparoscopic cholecystectomy. Surg Endosc. 1999;13:784-788. [PubMed] |
| 95. | Martin DJ, Vernon DR, Toouli J. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev. 2006;CD003327. |
| 96. | Clayton ES, Connor S, Alexakis N, Leandros E. Meta-analysis of endoscopy and surgery versus surgery alone for common bile duct stones with the gallbladder in situ. Br J Surg. 2006;93:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Chang WH, Chu CH, Wang TE, Chen MJ, Lin CC. Outcome of simple use of mechanical lithotripsy of difficult common bile duct stones. World J Gastroenterol. 2005;11:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 98. | Dasari BV, Tan CJ, Gurusamy KS, Martin DJ, Kirk G, McKie L, Diamond T, Taylor MA. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev. 2013;12:CD003327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Rábago LR, Vicente C, Soler F, Delgado M, Moral I, Guerra I, Castro JL, Quintanilla E, Romeo J, Llorente R. Two-stage treatment with preoperative endoscopic retrograde cholangiopancreatography (ERCP) compared with single-stage treatment with intraoperative ERCP for patients with symptomatic cholelithiasis with possible choledocholithiasis. Endoscopy. 2006;38:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Poulose BK, Arbogast PG, Holzman MD. National analysis of in-hospital resource utilization in choledocholithiasis management using propensity scores. Surg Endosc. 2006;20:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 101. | Bingener J, Schwesinger WH. Management of common bile duct stones in a rural area of the United States: results of a survey. Surg Endosc. 2006;20:577-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Demling L, Koch H, Classen M, Belohlavek D, Schaffner O, Schwamberger K, Stolte M. [Endoscopic papillotomy and removal of gall-stones: animal experiments and first clinical results (author’s transl)]. Dtsch Med Wochenschr. 1974;99:2255-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 103. | Barwood NT, Valinsky LJ, Hobbs MS, Fletcher DR, Knuiman MW, Ridout SC. Changing methods of imaging the common bile duct in the laparoscopic cholecystectomy era in Western Australia: implications for surgical practice. Ann Surg. 2002;235:41-50. [PubMed] |
| 104. | Ghazi A, McSherry CK. Endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 1984;199:21-27. [PubMed] |
| 105. | Bergman JJ, Rauws EA, Fockens P, van Berkel AM, Bossuyt PM, Tijssen JG, Tytgat GN, Huibregtse K. Randomised trial of endoscopic balloon dilation versus endoscopic sphincterotomy for removal of bileduct stones. Lancet. 1997;349:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [PubMed] |
| 107. | Dunham F, Bourgeois N, Gelin M, Jeanmart J, Toussaint J, Cremer M. Retroperitoneal perforations following endoscopic sphincterotomy; clinical course and management. Endoscopy. 1982;14:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 613] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 109. | Elder JB. Surgical treatment of duodenal ulcer. Postgrad Med J. 1988;64 Suppl 1:54-59. [PubMed] |
| 110. | Erickson RA, Carlson B. The role of endoscopic retrograde cholangiopancreatography in patients with laparoscopic cholecystectomies. Gastroenterology. 1995;109:252-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 111. | Zang JF, Zhang C, Gao JY. Endoscopic retrograde cholangiopancreatography and laparoscopic cholecystectomy during the same session: feasibility and safety. World J Gastroenterol. 2013;19:6093-6097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Di Mauro D, Faraci R, Mariani L, Cudazzo E, Costi R. Rendezvous technique for cholecystocholedochal lithiasis in octogenarians: is it as effective as in younger patients, or should endoscopic sphincterotomy followed by laparoscopic cholecystectomy be preferred? J Laparoendosc Adv Surg Tech A. 2014;24:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 113. | Staritz M, Ewe K, Meyer zum Büschenfelde KH. Endoscopic papillary dilatation, a possible alternative to endoscopic papillotomy. Lancet. 1982;1:1306-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 114. | Ersoz G, Tekesin O, Ozutemiz AO, Gunsar F. Biliary sphincterotomy plus dilation with a large balloon for bile duct stones that are difficult to extract. Gastrointest Endosc. 2003;57:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 115. | Disario JA, Freeman ML, Bjorkman DJ, Macmathuna P, Petersen BT, Jaffe PE, Morales TG, Hixson LJ, Sherman S, Lehman GA. Endoscopic balloon dilation compared with sphincterotomy for extraction of bile duct stones. Gastroenterology. 2004;127:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 116. | Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Endoscopic sphincterotomy combined with large balloon dilation can reduce the procedure time and fluoroscopy time for removal of large bile duct stones. Am J Gastroenterol. 2009;104:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 117. | Stefanidis G, Viazis N, Pleskow D, Manolakopoulos S, Theocharis L, Christodoulou C, Kotsikoros N, Giannousis J, Sgouros S, Rodias M. Large balloon dilation vs. mechanical lithotripsy for the management of large bile duct stones: a prospective randomized study. Am J Gastroenterol. 2011;106:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 118. | Attasaranya S, Cheon YK, Vittal H, Howell DA, Wakelin DE, Cunningham JT, Ajmere N, Ste Marie RW, Bhattacharya K, Gupta K. Large-diameter biliary orifice balloon dilation to aid in endoscopic bile duct stone removal: a multicenter series. Gastrointest Endosc. 2008;67:1046-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 119. | Shim CS. How Should Biliary Stones be Managed? Gut Liver. 2010;4:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 120. | Yasuda I, Fujita N, Maguchi H, Hasebe O, Igarashi Y, Murakami A, Mukai H, Fujii T, Yamao K, Maeshiro K. Long-term outcomes after endoscopic sphincterotomy versus endoscopic papillary balloon dilation for bile duct stones. Gastrointest Endosc. 2010;72:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 121. | Feng Y, Zhu H, Chen X, Xu S, Cheng W, Ni J, Shi R. Comparison of endoscopic papillary large balloon dilation and endoscopic sphincterotomy for retrieval of choledocholithiasis: a meta-analysis of randomized controlled trials. J Gastroenterol. 2012;47:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 122. | Weinberg BM, Shindy W, Lo S. Endoscopic balloon sphincter dilation (sphincteroplasty) versus sphincterotomy for common bile duct stones. Cochrane Database Syst Rev. 2006;CD004890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 123. | Patel L, Patel JC. Chirurgie de la litiase biliaire et de ses conséquences lésionelles. Tome XII, fascicule 2: Voies biliaires extra-hépatiques – Pancreas. Nouveau traité de technique chirurgicale. Paris: Masson 1969; 81-155. |
| 124. | Kanamaru T, Sakata K, Nakamura Y, Yamamoto M, Ueno N, Takeyama Y. Laparoscopic choledochotomy in management of choledocholithiasis. Surg Laparosc Endosc Percutan Tech. 2007;17:262-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 125. | Karaliotas C, Sgourakis G, Goumas C, Papaioannou N, Lilis C, Leandros E. Laparoscopic common bile duct exploration after failed endoscopic stone extraction. Surg Endosc. 2008;22:1826-1831. [PubMed] |
| 126. | Jameel M, Darmas B, Baker AL. Trend towards primary closure following laparoscopic exploration of the common bile duct. Ann R Coll Surg Engl. 2008;90:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 127. | O’Rourke RW, Lee NN, Cheng J, Swanstrom LL, Hansen PD. Laparoscopic biliary reconstruction. Am J Surg. 2004;187:621-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Han HS, Yi NJ. Laparoscopic Roux-en-Y choledochojejunostomy for benign biliary disease. Surg Laparosc Endosc Percutan Tech. 2004;14:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Hawasli A, Kandeel A, Meguid A. Single-incision laparoscopic cholecystectomy (SILC): a refined technique. Am J Surg. 2010;199:289-293; discussion 293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Kravetz AJ, Iddings D, Basson MD, Kia MA. The learning curve with single-port cholecystectomy. JSLS. 2009;13:332-336. [PubMed] |
| 131. | Bresadola F, Pasqualucci A, Donini A, Chiarandini P, Anania G, Terrosu G, Sistu MA, Pasetto A. Elective transumbilical compared with standard laparoscopic cholecystectomy. Eur J Surg. 1999;165:29-34. [PubMed] |
| 132. | Rawlings A, Hodgett SE, Matthews BD, Strasberg SM, Quasebarth M, Brunt LM. Single-incision laparoscopic cholecystectomy: initial experience with critical view of safety dissection and routine intraoperative cholangiography. J Am Coll Surg. 2010;211:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 133. | Yeo D, Mackay S, Martin D. Single-incision laparoscopic cholecystectomy with routine intraoperative cholangiography and common bile duct exploration via the umbilical port. Surg Endosc. 2012;26:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Zorron R, Palanivelu C, Galvão Neto MP, Ramos A, Salinas G, Burghardt J, DeCarli L, Henrique Sousa L, Forgione A, Pugliese R. International multicenter trial on clinical natural orifice surgery--NOTES IMTN study: preliminary results of 362 patients. Surg Innov. 2010;17:142-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 135. | Arezzo A, Zornig C, Mofid H, Fuchs KH, Breithaupt W, Noguera J, Kaehler G, Magdeburg R, Perretta S, Dallemagne B. The EURO-NOTES clinical registry for natural orifice transluminal endoscopic surgery: a 2-year activity report. Surg Endosc. 2013;27:3073-3084. [PubMed] |
| 136. | Zornig C, Siemssen L, Emmermann A, Alm M, von Waldenfels HA, Felixmüller C, Mofid H. NOTES cholecystectomy: matched-pair analysis comparing the transvaginal hybrid and conventional laparoscopic techniques in a series of 216 patients. Surg Endosc. 2011;25:1822-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 137. | Linke GR, Tarantino I, Hoetzel R, Warschkow R, Lange J, Lachat R, Zerz A. Transvaginal rigid-hybrid NOTES cholecystectomy: evaluation in routine clinical practice. Endoscopy. 2010;42:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 138. | Ogredici O, Linke GR, Lamm S, Rosenthal R, Zerz A, Steinemann DC. Routine cholangiography during rigid-hybrid transvaginal natural orifice transluminal endoscopic cholecystectomy. Surg Endosc. 2014;28:910-917. [PubMed] |
| 139. | Gagner M, Begin E, Hurteau R, Pomp A. Robotic interactive laparoscopic cholecystectomy. Lancet. 1994;343:596-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 140. | Roeyen G, Chapelle T, Ysebaert D. Robot-assisted choledochotomy: feasibility. Surg Endosc. 2004;18:165-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 141. | Ji WB, Zhao ZM, Dong JH, Wang HG, Lu F, Lu HW. One-stage robotic-assisted laparoscopic cholecystectomy and common bile duct exploration with primary closure in 5 patients. Surg Laparosc Endosc Percutan Tech. 2011;21:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Alkhamesi NA, Davies WT, Pinto RF, Schlachta CM. Robot-assisted common bile duct exploration as an option for complex choledocholithiasis. Surg Endosc. 2013;27:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 143. | Kroh M, El-Hayek K, Rosenblatt S, Chand B, Escobar P, Kaouk J, Chalikonda S. First human surgery with a novel single-port robotic system: cholecystectomy using the da Vinci Single-Site platform. Surg Endosc. 2011;25:3566-3573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 144. | Morel P, Hagen ME, Bucher P, Buchs NC, Pugin F. Robotic single-port cholecystectomy using a new platform: initial clinical experience. J Gastrointest Surg. 2011;15:2182-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 145. | Lai EC, Tang CN, Yang GP, Li MK. Approach to manage the complications of choledochoduodenostomy: robot-assisted laparoscopic Roux-en-Y hepaticojejunostomy. Surg Laparosc Endosc Percutan Tech. 2011;21:e228-e231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 146. | Chan OC, Tang CN, Lai EC, Yang GP, Li MK. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci. 2011;18:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 147. | Jayaraman S, Quan D, Al-Ghamdi I, El-Deen F, Schlachta CM. Does robotic assistance improve efficiency in performing complex minimally invasive surgical procedures? Surg Endosc. 2010;24:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 148. | Riemann JF, Seuberth K, Demling L. Clinical application of a new mechanical lithotripter for smashing common bile duct stones. Endoscopy. 1982;14:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 149. | Van Dam J, Sivak MV. Mechanical lithotripsy of large common bile duct stones. Cleve Clin J Med. 1993;60:38-42. [PubMed] |
| 150. | Binmoeller KF, Brückner M, Thonke F, Soehendra N. Treatment of difficult bile duct stones using mechanical, electrohydraulic and extracorporeal shock wave lithotripsy. Endoscopy. 1993;25:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 170] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 151. | Chung SC, Leung JW, Leong HT, Li AK. Mechanical lithotripsy of large common bile duct stones using a basket. Br J Surg. 1991;78:1448-1450. [PubMed] |
| 152. | Hintze RE, Adler A, Veltzke W. Outcome of mechanical lithotripsy of bile duct stones in an unselected series of 704 patients. Hepatogastroenterology. 1996;43:473-476. [PubMed] |
| 153. | Leung JW, Neuhaus H, Chopita N. Mechanical lithotripsy in the common bile duct. Endoscopy. 2001;33:800-804. [PubMed] |
| 154. | Cipolletta L, Costamagna G, Bianco MA, Rotondano G, Piscopo R, Mutignani M, Marmo R. Endoscopic mechanical lithotripsy of difficult common bile duct stones. Br J Surg. 1997;84:1407-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 155. | Garg PK, Tandon RK, Ahuja V, Makharia GK, Batra Y. Predictors of unsuccessful mechanical lithotripsy and endoscopic clearance of large bile duct stones. Gastrointest Endosc. 2004;59:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 156. | Moon JH, Cha SW, Ryu CB, Kim YS, Hong SJ, Cheon YK, Cho YD, Kim YS, Lee JS, Lee MS. Endoscopic treatment of retained bile-duct stones by using a balloon catheter for electrohydraulic lithotripsy without cholangioscopy. Gastrointest Endosc. 2004;60:562-566. [PubMed] |
| 157. | Sugiyama M, Atomi Y. Endoscopic sphincterotomy for bile duct stones in patients 90 years of age and older. Gastrointest Endosc. 2000;52:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 158. | Bergman JJ, Rauws EA, Tijssen JG, Tytgat GN, Huibregtse K. Biliary endoprostheses in elderly patients with endoscopically irretrievable common bile duct stones: report on 117 patients. Gastrointest Endosc. 1995;42:195-201. [PubMed] |
| 159. | Maxton DG, Tweedle DE, Martin DF. Retained common bile duct stones after endoscopic sphincterotomy: temporary and longterm treatment with biliary stenting. Gut. 1995;36:446-449. [PubMed] |
| 160. | Chopra KB, Peters RA, O’Toole PA, Williams SG, Gimson AE, Lombard MG, Westaby D. Randomised study of endoscopic biliary endoprosthesis versus duct clearance for bileduct stones in high-risk patients. Lancet. 1996;348:791-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 161. | Farrell JJ, Bounds BC, Al-Shalabi S, Jacobson BC, Brugge WR, Schapiro RH, Kelsey PB. Single-operator duodenoscope-assisted cholangioscopy is an effective alternative in the management of choledocholithiasis not removed by conventional methods, including mechanical lithotripsy. Endoscopy. 2005;37:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 162. | Arya N, Nelles SE, Haber GB, Kim YI, Kortan PK. Electrohydraulic lithotripsy in 111 patients: a safe and effective therapy for difficult bile duct stones. Am J Gastroenterol. 2004;99:2330-2334. [PubMed] |
| 163. | Reavis KM, Melvin WS. Advanced endoscopic technologies. Surg Endosc. 2008;22:1533-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | Draganov PV, Lin T, Chauhan S, Wagh MS, Hou W, Forsmark CE. Prospective evaluation of the clinical utility of ERCP-guided cholangiopancreatoscopy with a new direct visualization system. Gastrointest Endosc. 2011;73:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 165. | Neuhaus H, Hoffmann W, Gottlieb K, Classen M. Endoscopic lithotripsy of bile duct stones using a new laser with automatic stone recognition. Gastrointest Endosc. 1994;40:708-715. [PubMed] |
| 166. | Hochberger J, Tex S, Maiss J, Hahn EG. Management of difficult common bile duct stones. Gastrointest Endosc Clin N Am. 2003;13:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 167. | Patel SN, Rosenkranz L, Hooks B, Tarnasky PR, Raijman I, Fishman DS, Sauer BG, Kahaleh M. Holmium-yttrium aluminum garnet laser lithotripsy in the treatment of biliary calculi using single-operator cholangioscopy: a multicenter experience (with video). Gastrointest Endosc. 2014;79:344-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 168. | Neuhaus H, Zillinger C, Born P, Ott R, Allescher H, Rösch T, Classen M. Randomized study of intracorporeal laser lithotripsy versus extracorporeal shock-wave lithotripsy for difficult bile duct stones. Gastrointest Endosc. 1998;47:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 169. | Maydeo A, Kwek BE, Bhandari S, Bapat M, Dhir V. Single-operator cholangioscopy-guided laser lithotripsy in patients with difficult biliary and pancreatic ductal stones (with videos). Gastrointest Endosc. 2011;74:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 170. | Varban O, Assimos D, Passman C, Westcott C. Video. Laparoscopic common bile duct exploration and holmium laser lithotripsy: a novel approach to the management of common bile duct stones. Surg Endosc. 2010;24:1759-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 171. | Bark K, Gamblin TC, Zuckerman R, Geller DA. Operative choledochoscopic laser lithotripsy for impacted intrahepatic gallstones: a novel surgical approach. Surg Endosc. 2009;23:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 172. | Rimon U, Kleinmann N, Bensaid P, Golan G, Garniek A, Khaitovich B, Winkler H. Percutaneous transhepatic endoscopic holmium laser lithotripsy for intrahepatic and choledochal biliary stones. Cardiovasc Intervent Radiol. 2011;34:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 173. | DiSario J, Chuttani R, Croffie J, Liu J, Mishkin D, Shah R, Somogyi L, Tierney W, Song LM, Petersen BT. Biliary and pancreatic lithotripsy devices. Gastrointest Endosc. 2007;65:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 174. | Tandan M, Reddy DN, Santosh D, Reddy V, Koppuju V, Lakhtakia S, Gupta R, Ramchandani M, Rao GV. Extracorporeal shock wave lithotripsy of large difficult common bile duct stones: efficacy and analysis of factors that favor stone fragmentation. J Gastroenterol Hepatol. 2009;24:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 175. | Amplatz S, Piazzi L, Felder M, Comberlato M, Benvenuti S, Zancanella L, Di Fede F, de’Guelmi A, Bertozzo A, Farris P. Extracorporeal shock wave lithotripsy for clearance of refractory bile duct stones. Dig Liver Dis. 2007;39:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 176. | McHenry L, Lehman G. Difficult bile duct stones. Curr Treat Options Gastroenterol. 2006;9:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 177. | Trikudanathan G, Navaneethan U, Parsi MA. Endoscopic management of difficult common bile duct stones. World J Gastroenterol. 2013;19:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 178. | Conigliaro R, Camellini L, Zuliani CG, Sassatelli R, Mortilla MG, Bertoni G, Formisano D, Bedogni G. Clearance of irretrievable bile duct and pancreatic duct stones by extracorporeal shockwave lithotripsy, using a transportable device: effectiveness and medium-term results. J Clin Gastroenterol. 2006;40:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 179. | Peng WK, Sheikh Z, Nixon SJ, Paterson-Brown S. Role of laparoscopic cholecystectomy in the early management of acute gallbladder disease. Br J Surg. 2005;92:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 180. | Nebiker CA, Baierlein SA, Beck S, von Flüe M, Ackermann C, Peterli R. Is routine MR cholangiopancreatography (MRCP) justified prior to cholecystectomy? Langenbecks Arch Surg. 2009;394:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 181. | Videhult P, Sandblom G, Rudberg C, Rasmussen IC. Are liver function tests, pancreatitis and cholecystitis predictors of common bile duct stones? Results of a prospective, population-based, cohort study of 1171 patients undergoing cholecystectomy. HPB (Oxford). 2011;13:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 182. | Tonolini M, Ravelli A, Villa C, Bianco R. Urgent MRI with MR cholangiopancreatography (MRCP) of acute cholecystitis and related complications: diagnostic role and spectrum of imaging findings. Emerg Radiol. 2012;19:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 183. | Wong HP, Chiu YL, Shiu BH, Ho LC. Preoperative MRCP to detect choledocholithiasis in acute calculous cholecystitis. J Hepatobiliary Pancreat Sci. 2012;19:458-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 184. | Rogers SJ, Cello JP, Horn JK, Siperstein AE, Schecter WP, Campbell AR, Mackersie RC, Rodas A, Kreuwel HT, Harris HW. Prospective randomized trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Arch Surg. 2010;145:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 185. | Csikesz N, Ricciardi R, Tseng JF, Shah SA. Current status of surgical management of acute cholecystitis in the United States. World J Surg. 2008;32:2230-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 186. | Chiarugi M, Galatioto C, Decanini L, Puglisi A, Lippolis P, Bagnato C, Panicucci S, Pelosini M, Iacconi P, Seccia M. Laparoscopic transcystic exploration for single-stage management of common duct stones and acute cholecystitis. Surg Endosc. 2012;26:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 187. | Jain MK, Jain R. Acute bacterial cholangitis. Curr Treat Options Gastroenterol. 2006;9:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 188. | Hui CK, Lai KC, Wong WM, Yuen MF, Lam SK, Lai CL. A randomised controlled trial of endoscopic sphincterotomy in acute cholangitis without common bile duct stones. Gut. 2002;51:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 189. | Neoptolemos JP, Carr-Locke DL, London NJ, Bailey IA, James D, Fossard DP. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 563] [Reference Citation Analysis (1)] |
| 190. | Fan ST, Lai EC, Mok FP, Lo CM, Zheng SS, Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993;328:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 439] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 191. | Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut. 2005;54 Suppl 3:iii1-iii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 192. | Kapetanos DJ. ERCP in acute biliary pancreatitis. World J Gastrointest Endosc. 2010;2:25-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 193. | Garrow D, Miller S, Sinha D, Conway J, Hoffman BJ, Hawes RH, Romagnuolo J. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 194. | Forsmark CE, Baillie J. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 505] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 195. | Deenitchin GP, Konomi H, Kimura H, Ogawa Y, Naritomi G, Chijiiwa K, Tanaka M, Ikeda S. Reappraisal of safety of endoscopic sphincterotomy for common bile duct stones in the elderly. Am J Surg. 1995;170:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 196. | Mitchell RM, O’Connor F, Dickey W. Endoscopic retrograde cholangiopancreatography is safe and effective in patients 90 years of age and older. J Clin Gastroenterol. 2003;36:72-74. [PubMed] |
| 197. | Katsinelos P, Paroutoglou G, Kountouras J, Zavos C, Beltsis A, Tzovaras G. Efficacy and safety of therapeutic ERCP in patients 90 years of age and older. Gastrointest Endosc. 2006;63:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 198. | Park DH, Kim MH, Lee SK, Lee SS, Choi JS, Song MH, Seo DW, Min YI. Endoscopic sphincterotomy vs. endoscopic papillary balloon dilation for choledocholithiasis in patients with liver cirrhosis and coagulopathy. Gastrointest Endosc. 2004;60:180-185. [PubMed] |
| 199. | Zuckerman MJ, Hirota WK, Adler DG, Davila RE, Jacobson BC, Leighton JA, Qureshi WA, Rajan E, Hambrick RD, Fanelli RD. ASGE guideline: the management of low-molecular-weight heparin and nonaspirin antiplatelet agents for endoscopic procedures. Gastrointest Endosc. 2005;61:189-194. [PubMed] |
| 200. | Ito Y, Tsujino T, Togawa O, Yamamoto N, Isayama H, Nakata R, Kawabe T, Omata M. Endoscopic papillary balloon dilation for the management of bile duct stones in patients 85 years of age and older. Gastrointest Endosc. 2008;68:477-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 201. | Kawabe T, Komatsu Y, Tada M, Toda N, Ohashi M, Shiratori Y, Omata M. Endoscopic papillary balloon dilation in cirrhotic patients: removal of common bile duct stones without sphincterotomy. Endoscopy. 1996;28:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 202. | Lee DK, Jahng JH. Alternative methods in the endoscopic management of difficult common bile duct stones. Dig Endosc. 2010;22 Suppl 1:S79-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 203. | Costi R, DiMauro D, Mazzeo A, Boselli AS, Contini S, Violi V, Roncoroni L, Sarli L. Routine laparoscopic cholecystectomy after endoscopic sphincterotomy for choledocholithiasis in octogenarians: is it worth the risk? Surg Endosc. 2007;21:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 204. | De Palma GD, Catanzano C. Stenting or surgery for treatment of irretrievable common bile duct calculi in elderly patients? Am J Surg. 1999;178:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 205. | Ahrendt SA, Pitt HA. Biliary tract. Sabiston textbook of surgery: the biological basis of modern surgical practice. Seventeenth Edition. Philadelphia: Elsevier 2004; 1597-1639. |
| 206. | Glasgow RE, Mulvihill SJ. Treatment of gallstone disease. Sleisenger and Fordtran’s gastrointestinal and liver diseases. Eight Edition. Philadelphia: Saunders 2006; 1419-1437. |
| 207. | Senturk S, Miroglu TC, Bilici A, Gumus H, Tekin RC, Ekici F, Tekbas G. Diameters of the common bile duct in adults and postcholecystectomy patients: a study with 64-slice CT. Eur J Radiol. 2012;81:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 208. | Terhaar OA, Abbas S, Thornton FJ, Duke D, O’Kelly P, Abdullah K, Varghese JC, Lee MJ. Imaging patients with “post-cholecystectomy syndrome”: an algorithmic approach. Clin Radiol. 2005;60:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 209. | Benjaminov F, Leichtman G, Naftali T, Half EE, Konikoff FM. Effects of age and cholecystectomy on common bile duct diameter as measured by endoscopic ultrasonography. Surg Endosc. 2013;27:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 210. | Canto MI, Chak A, Stellato T, Sivak MV. Endoscopic ultrasonography versus cholangiography for the diagnosis of choledocholithiasis. Gastrointest Endosc. 1998;47:439-448. [PubMed] |
| 211. | Tsujino T, Kawabe T, Isayama H, Yashima Y, Yagioka H, Kogure H, Sasaki T, Arizumi T, Togawa O, Ito Y. Management of late biliary complications in patients with gallbladder stones in situ after endoscopic papillary balloon dilation. Eur J Gastroenterol Hepatol. 2009;21:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 212. | Sugiyama M, Suzuki Y, Abe N, Masaki T, Mori T, Atomi Y. Endoscopic retreatment of recurrent choledocholithiasis after sphincterotomy. Gut. 2004;53:1856-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 213. | Adamek HE, Kudis V, Jakobs R, Buttmann A, Adamek MU, Riemann JF. Impact of gallbladder status on the outcome in patients with retained bile duct stones treated with extracorporeal shockwave lithotripsy. Endoscopy. 2002;34:624-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 214. | Matsushima K, Soybel DI. Operative management of recurrent choledocholithiasis. J Gastrointest Surg. 2012;16:2312-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 215. | Li LB, Cai XJ, Mou YP, Wei Q. Reoperation of biliary tract by laparoscopy: experiences with 39 cases. World J Gastroenterol. 2008;14:3081-3084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |