Published online Sep 21, 2014. doi: 10.3748/wjg.v20.i35.12462
Revised: March 25, 2014
Accepted: May 19, 2014
Published online: September 21, 2014
Chronic active hepatitis (CAH) is acknowledged as an imperative risk factor for the development of liver injury and hepatocellular carcinoma. The histological end points of CAH are chronic inflammation, fibrosis and cirrhosis which are coupled with increased DNA synthesis in cirrhotic vs healthy normal livers. The potential mechanism involved in CAH includes a combination of processes leading to liver cell necrosis, inflammation and cytokine production and liver scaring (fibrosis). The severity of liver damage is regulated by Hepatitis B virus genotypes and viral components. The viral and cellular factors that contribute to liver injury are discussed in this article. Liver injury caused by the viral infection affects many cellular processes such as cell signaling, apoptosis, transcription, DNA repair which in turn induce radical effects on cell survival, growth, transformation and maintenance. The consequence of such perturbations is resulted in the alteration of bile secretion, gluconeogenesis, glycolysis, detoxification and metabolism of carbohydrates, proteins, fat and balance of nutrients. The identification and elucidation of the molecular pathways perturbed by the viral proteins are important in order to design effective strategy to minimize and/or restore the hepatocytes injury.
Core tip: There are over 400 million people infected with hepatitis B virus worldwide and chronic active hepatitis is recognized as an important risk factor for liver injury and hepatocellular carcinoma. Cirrhosis is the histological end point of this chronic inflammatory and fibrotic process and is coupled with increased DNA synthesis in cirrhotic as compared to normal livers. The potential mechanism(s) involved in chronic active hepatitis include a combination of processed leading to liver cell necrosis, inflammation and cytokine synthesis and fibrosis. The severity of liver damage is regulated by Hepatitis B virus genotypes and viral components. The viral and cellular factors that contribute to liver injury are discussed in this article. Liver injury caused by the viral infection affects many cellular process such as cell signaling, apoptosis, transcription, DNA repair which in turn induce radical effects on cell survival, growth, transformation and maintenance. The consequence of such perturbations is dictated in bile secretion, gluconeogenesis, glycolysis, detoxification and metabolism of carbohydrates, proteins, and fat and balance of nutrients. The identification and elucidation of the molecular pathways perturbed by the viral protein(s) are important in order to design effective strategy to minimize and/or restore the hepatocyte injury.
- Citation: Suhail M, Abdel-Hafiz H, Ali A, Fatima K, Damanhouri GA, Azhar E, Chaudhary AG, Qadri I. Potential mechanisms of hepatitis B virus induced liver injury. World J Gastroenterol 2014; 20(35): 12462-12472
- URL: https://www.wjgnet.com/1007-9327/full/v20/i35/12462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i35.12462
Human hepatitis B virus (HBV) is one of the foremost causes of fulminant, acute and chronic hepatitis[1-5]. HBV is a partially double stranded DNA virus and is a member of the Hepadnaviridae virus family. HBV replication is not directly cytopathic per se but increases the risk of hepatocellular carcinoma (HCC) and liver injury. The viral genome contains a small (3.2 kb), covalently closed circular DNA (cccDNA) that is transcribed to generate four known transcripts of 3.5 kb, 2.4 kb, 2.1 kb, and 0.7 kb size[5]. These transcripts encode to generate Polymerase, HBcAg, HBeAg, and HBsAg and HBx that have defined role in HBV life cycle and liver injury[6,7]. The HBV infection that leads to persistence is present approximately 5% in adults and 95% in neonates[8] while in 90%-95% of adulthood cases the HBV is rapidly cleared during the acute phase of infection. Irrespective to this, HBV infection leads to chronic infection in 90% of perinatal or early childhood cases[9-14]. That leads to the development of liver cirrhosis and finally toward HCC at some stage in the 20-30 years of asymptomatic infection and often ¼th of the patients perishes due to complications of liver injury.
A number of studies are being done on several animal models for HBV infection to address these issues. In transgenic animal model studies, different HBV DNA inserts were tested to demonstrate the importance of liver cell regeneration after necrosis. In the natural course of infection of woodchucks and ground squirrels by the woodchuck hepatitis virus (WHV) and ground squirrel hepatitis virus, respectively, HCC development occur in the absence of cirrhosis[15-17]. In another study, the transgenic mice was shown to develop HCC and liver cell regeneration by triggering the immune response to HBV[18]. Furthermore, extensive oxidative DNA damage has been detected in HBV positive transgenic mice explaining the increased sensitivity to chemical carcinogens[18]. These animal models offer an attractive choice to study the liver injury caused by HBV. In the section below, we discuss the mechanisms of liver injury by which different genotypes and viral gene products (C, S, HBx) induce a subtle and sustained effect on the host cellular pathways during this prolonged period of acute infection (Table 1).
| HBV genotypes | Geographical distribution | Risk of developing diseases | Ref. |
| A | North America, Europe, Africa | HCC | [21,22] |
| B | Southeast Asia, Southern China, Alaska, Canada, and Greenland | HCC and Cirrhosis at a younger age | [19,23] |
| C | East Asia, Northern China, Korea, Taiwan | Cirrhosis and HCC | [19,24] |
| D | Africa, Europe, India, Russia and North America | Liver fibrosis and HCC | [22,25] |
| E | West Africa | PC and BCP mutations but disease outcome unknown | [26] |
| F | Central America, South America | Fulminate hepatitis associated with HDV coinfection | [13] |
| G | France, Germany, United States | Coinfection with genotype A; increased association with acute hepatitis, liver fibrosis, and HCC (Hepatocellular carcinoma) in Vietnam | [27,28] |
| H | Central America (Mexico and Nicaragua) | Most closely connected to genotype F but disease outcome is not known | [13,29] |
| I | Laos, Vietnam | Not studied | [30] |
| J | Japan, Ryukyu | Not studied | [30] |
There are at least 10 different genotypes of HBV named (A-J), which are based on > 8% genetic diversity. These are further grouped into many sub-genotypes which are different by 4%-8%[11,19]. The contribution of HBV genetic variability in the genesis of liver cirrhosis is still under investigation. A number of HBV genotypes have been identified based on specific geographical areas and liver diseases (Table 1). Several studies have documented that the genotypes B and C are foremost risk factors for HBV-associated HCC[20]. However the changes in the promoter core region and HBx-encoding sequence are overlapping in the patients serum samples. Few mutations are present in various HBV genotypes. Finally, mutations in one sequence of open reading frames (ORF) may affect the protein expression pattern of HBV genome. HBV genotype A is prevalent in North America while B and C genotypes are geographically localized to Asia (specially China)[19]. Central America populations generally have HBV genotype H and disease correlation outcome is not known with this genotype. The geographical distribution pattern of these genotypes and associated liver disease is presented in Table 1.
Different mechanisms and cellular processes that are regulated by virus and viral proteins to govern the outcome of liver injury are presented. These changes lead to cell proliferation, tissue invasion, metastasis, angiogenesis and resistance to growth inhibition, restructuring of energy metabolism, and resistance to programmed cell death (apoptosis)[31].
In several models of HBV infection, it has been observed that the liver injuries in chronic infection are considered to be associated with the activity of HBV specific T cells. However, few reports suggest that certain chemokine-mediated neutrophils infiltration, nature killer cells and lymphocytes also played role in HBV-related liver damage[32]. The virus elimination may also be hindered due to imbalances in cytokine production. Viral infection usually causes inflammatory reaction characterized by release of cytokines and chemokines which may lead to cancer development[33]. It has been observed that molecular and cellular changes of host gene expression are being supported by the virus replication that protect virally infected hepatocytes from immune-mediated destruction and facilitate tumorigenesis. The oxidative stress induced by Inflammation incurs Kupffer cells to promote stellate cells activation via NF-κB and AP1[34]. The persistent activation of these genes leads to cirrhosis, fibrosis and severe liver damage[1,34] leading to the development of HCC. It has been reported that cirrhosis increases the risk for HCC about 300% in the patients with chronic infection[35-38].
In liver inflammatory lesions, lymphocytes which are virus-specific can be detectable spontaneously but they are not capable to remove active virus infection[39]. HBV can cause chronic inflammation that leads to the development of cirrhosis and HCC[40]. It has also been observed that HBsAg could inhibit the production of interferon (IFN) in chronic HBV infection[41]. On the other hand, interferon has been used in the treatment of chronic hepatitis B. A recent study indicate that IFN prevented or delayed the progress of HCC and liver cirrhosis in chronic HBV individuals[42].
Dendritic cells are an important linkage between innate and adaptive immunity which is gravely affected with HBV infection[43]. The immune responses are either obstructed or altered by HBV and the acute infection outcome is affected by virus titer and CD4+CD25+FOX3P+ regulatory T (Treg) cells. They all play a vital role on immune tolerance within the liver. In the HBV transgenic mice, virus replication is inhibited by Toll like receptors (TLR) signaling, activating the innate immunity[44]. TLRs are essential for HBV recognition and this process is initiated by activation of critical downstream inflammatory signaling cascades such as nuclear factor-κB, INF regulatory factor, mitogen activated protein kinases, and proinflammatory cytokines[44,45]. When studied the TLRs induced signaling cascade in peripheral blood mononuclear cells during chronic HBV infestation vs healthy controls, the expression of interferon regulatory factor 7, interleukin-1 receptor-associated kinase 4, and TNF receptor-associated factor 3 was not affected. The expression level of these genes decreased significantly with elevated level of IL-12 and IL-6 leading to impairment of the immune response against HBV infection which was observed in all the chronically infected HBV patients[46]. However, HBV altered TLR signaling[47] is not efficient in the virus removal. It has been observed in chimpanzees that the acute HBV infection could not generate innate immunity with the initial stage of infection[40]. Another major signaling molecule is NF-κB which activates the expression of hepatoprotective and pro-inflammatory genes[48].
In viral infected cells, the Treg cells are known to be induced by HBx via increased transforming growth factor-β1 production[49]. In the course of HBV infection, the immune response for HBV-specific CD8+ T cells plays a significant role to ward off the course of liver injury. The hypo responsiveness of these cells present in chronic infection is likely related to high antigen level in these patients[50]. The pro-apoptotic and anti-apoptotic states of HBV proteins have been well documented depending on each experimental system. HBV promote inappropriate cell growth upon infection to quiescent hepatocytes, which then triggers growth arrest or apoptosis. However, researchers have observed that the HBx protein inhibits apoptosis of hepatic progenitor cells by activating WNT/β-catenin signaling pathway[51,52]. This resistance to apoptosis in HBV infected hepatocytes enhances persistence and is a characteristic of tumor cells.
It has been reported that increased pro-oxidants and decreased antioxidant levels affect the enzyme activities which are associated with severe liver injury and hepatocarcinogenesis. The elevated levels of reactive oxygen species (ROS) may cause damage of host DNA, activation of MAP kinase signaling pathways and inactivation and degradation of mitogen activated protein kinase (MAPK) phosphatases which control cell death or survival and subsequent proliferation[53]. Using a trimmed part of the larger surface (S) of protein, the increased ROS and activated cyclin A expression and c-Raf-1/Erk2 signaling cascade has been shown. These activities were further linked with the hepatocyte proliferation[54]. Furthermore, HBV infected transgenic mice also show sign of oxidative stress[55]. It has been shown that the upregulation of forkhead box O4 (FOXO4) induced by HBx contributes to the cellular resistance to oxidative stress-induced death[56]. HBx association with mitochondria is also known to trigger oxidative stress resulting in apoptosis[57,58]. Similarly, HBx increase ROS level by promoting cytosolic calcium signaling and by activating the PYK2 and SRC kinase. This further decreases the level of mitochondrial permeability transition which causes the production of ROS[59].
Accumulation of HBV proteins in the endoplasmic reticulum (ER) activates ER stress which then leads to stimulation of DNA damage and increased oxidative stress, and pre-disposed virally infected hepatocytes to transformation[60,61]. Under the provisionally mild ER stress, unfolded protein response (UPR) attenuates the protein synthesis followed by increased protein degradation[61,62]. If the ER stress severe or prolonged, UPR activates hepatocytes apoptosis through unknown and poorly defined mechanisms[61]. It is important to note that UPR contributes to elevated intracellular ROS production through up-regulating the oxidative flop machinery[63,64]. This further form disulfide bonds by oxidizing thiol groups which plays important role in the supply of intracellular ROS[65]. The UPR-mediated up regulation of ROS was reported in association with ER stress-triggered cell apoptosis[63]. Furthermore, recent evidence suggests that HBV infection induces ER stress due to large amount of viral and cellular misfolded proteins synthesized in infected cells[62].
However, when ER stress is persistent, the autophagy is activated to degrade the unfolded proteins as well as maintains the cell viability. During autophagy ER integrity is restored to maintain the viral replication[66]. Therefore, autophagy promotes the hepatocyte survival[67] and in turn helps to maintain the viral persistence which is serious risk factors for liver cancer[68]. Taken together these observations support the idea that HBV proteins harbor oncogenic effects, mediated by the induction of ROS and ER stress factors. Thus, it is pertinent to control the liver injury caused by induced ROS and ER stress by effective antioxidants and ER stress relief strategies.
In chronically HBV infected hepatocyte, the cirrhotic nodule formation is accompanied by the low level of vasculature in the liver. This occurs in localized regions with the development of a hypoxic environment which in turn can enhance cell migration through the enhanced activity of matrix metalloproteinases[69]. Hypoxia is a major determining factor in cancer development, because it contributes to inflammation, apoptosis and liver regeneration inhibition. The cellular adaptive response to hypoxia triggers the upregulated expression of hypoxia-inducible factor 1-alpha (HIF1α), which is known to promote angiogenesis by up regulating vascular endothelial growth factor (VEGF). During HBV infection and protein synthesis, HBx is known to stabilizes HIF1α through direct binding and also stimulates the transcription of HIF1α[70,71], thus promotes angiogenesis[72]. HBx protein also enhances angiogenesis by activated expression of pro-angiogenic growth factor angiopoietin 2, via the activation of MAPK pathway[73]. In many diseases, the role of angiogenesis has been highlighted by using the kinase inhibitor sorafenib[74], which specifically target the VEGF receptor (VEGFR). Since both the PI3K-AKT signaling cascade and EGFR are activated by HBx[75], the pathways of HIF and VEGFR activation emphasize the importance in the pathogenesis of HBV induced HCC.
In HCC tumor samples, the viral DNA is frequently found to be integrated into host genome[76]. The HBV viral genome integration is also shown to occur in patients affected with chronic hepatitis. In some cases, viral DNA integration is also reported to occur during the early stage of HBV infection. Full length viral genome integration are not common but partial deletions of genome and more complex rearrangements are frequently detected[77]. It has been shown that viral integration affects the host genome through insertional mutagenesis, viral transcriptional upregulation, and induction of genomic instability[78]. The integration of DNA shows some common insertion or activation of proto-oncogenes of the myc family, primarily the N-myc2. It was also studied that N-myc2 locus could be expressed by WHV integration in win and b3n locus[79]. It is mentioned in some cases that the regeneration of infected hepatocytes may have occurred due to integration of HBx and occasionally with a truncated S and pre-S regions.
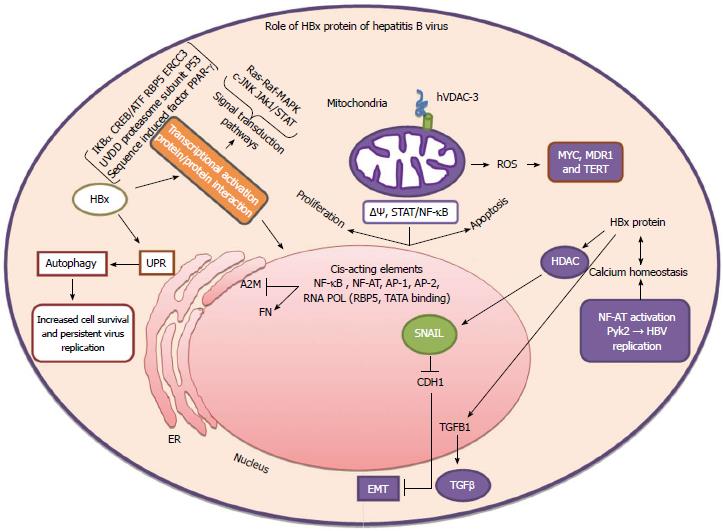
HBx is a 17 kDa multifunctional regulatory protein, necessary for viral cccDNA transcription and/or replication[80,81] and also major player in HBV induced oncogenicity. HBx exhibits pleiotropic effects involving intracellular calcium, stress signaling transcriptional activation, ROS, apoptosis and cell division[2,82,83]. HBx protein does not bind directly to double-stranded DNA on viral or cellular promoters but has been shown to bind to short stretches of ssDNA in vitro[84]. It acts on various cellular promoters through the protein-protein interactions and alters the cytoplasmic signaling pathways. However, HBx can also activate stellate cells as a paracrine factor[85,86]. HBx is known to transactivate many cellular promoters and enhancers containing binding site for activator protein-1 (AP-1), -2 (AP-2), nuclear factor-kappa-B (NF-κB), activated transcription factor, CCAAT-enhancer-binding protein and cAMP response element binding protein. Other cellular target of HBx includes the RNA polymerase binding protein, epidermal growth factor receptor, nuclear factor of activated T-cells, transforming growth factor β, IL-8, cytosolic signal transduction pathways as Ras/Raf mitogen-activated protein kinase, tumor necrosis factor (TNF), Src kinases, cjun N-terminal kinase, protein kinase and Jak1/STAT[82,83]. Direct binding of HBx has also been reported with p53, DNA repair protein DDB1, TFIIH which in turn affects DNA repair functions and may allow the amassing of genetic mutations[87,88]. The peptidyl-prolyl cis/trans isomerase Pin1 functions are enhanced by direct HBx binding and subsequent transactivation of target genes to increase cellular proliferation[89]. The complex nature of this protein contributes to activation of overlapping pathways that have effects on cell viability and cell proliferation in HBV infected hepatocytes.
During the low level expression of HBx in both acute and chronic hepatitis the humoral and cellular immune response are activated. The HBV replication is also modulated due to the interactions of HBx with proteasome complex and Src tyrosine kinase activation[90]. It also blocks TNFα and FAS-mediated apoptosis pathway by activation of NF-κB[91]. This aspect signifies that infected hepatocytes survive immune-mediated damage and the uninfected hepatocytes undergo broad cell death in CLD[92]. HBx interactions with DNA repair genes DDB1[93,94], ERCC2 and ERCC3 subunit of TFIIH[87] suggest that a compromised DNA repair process and accumulation of mutations in key house keeping genes may favor the fine balance leading to liver cancer.
HBx is known to negatively regulate proteosome function thus have a role in degradation of viral and cellular proteins[95,96]. The mitochondrial functions are regulated by HBx either due to its channel-forming activity or indirectly through the interactions with endogenous channels[97,98]. Several studies have shown that HBx interacts with mitochondrial heat shock proteins -60 (hsp60) and -70 (hsp70) and the voltage dependent anion channel (VDAC) isoform VDAC3[99,100]. Additionally Wnt/beta-catenin signaling is also up-regulated by HBx via activation of the cytoplasmic beta-catenin[101,102] (Figure 1). Moreover, additional damage to the hepatocytes can occur due to many factors initiated by HBx such as (1) multipolar spindle formation; (2) chromosome segregation; and (3) by inducing aberrant centrosome duplication. These actions are hypothesized due to nuclear transport receptor Crm1 sequestration by HBx in the cytoplasm[103]. The induction of lagging chromosomes by binding to BubR1 and increased expression of matrix metalloproteinase are alternative mechanisms[104]. The later is also accountable of cellular migration[105]. All these biochemical properties of HBx and its influence on above described cellular process are detrimental to the healthy hepatocytes and may eventually lead to serious liver stress and injury.
The HBV encoded intracellular HBcAg or nucleocapsid protein (p21) is approximately 21 kDa and is composed of 183 residues. The protein includes a C-terminal arginine-rich DNA binding domain required for viral genome packaging. Studies have postulated that preC-C, which encodes both HBeAg and HBcAg can play a significant part in the establishment of viral persistence[106]. It is reported that HBeAg attenuates the host immune response and adversely influence the innate and adaptive immune responses during viral infection[107,108]. The precore region shares 149 common residues with the core, but contains a unique amino-terminal hydrophobic cysteine rich ten-mer region. Overall there is strong homology between pre core and core relevant to specific serological, structural and immunological distinctiveness. Recent studies have revealed pleiotropic functions of HBc that affect host cellular processes, including HBc induced expression profile change of an innate immune protein, toll like receptor 2[109,110]. These evidences demonstrate that HBc can affect the physiological functions of innate immune system which are relevant to the health of hepatocytes.
The surface proteins of HBV consist of at least three known structurally related proteins referred to as pre-S1, pre-S2, and S. These proteins are translated from two mRNA transcripts (1) Pre-S1 Mrna; and (2) Pre-S2/S mRNA, from which the L (Pre-S1) and M/S (Pre-S2 + S/S) proteins are translated, respectively. Several studies have reported that HBsAg titers directly correlate with the amount HBV DNA in serum and intrahepatic cccDNA levels. However this level varies in different liver disease phases[111-113]. However, during HBeAg-negative state, the HBsAg production and secretion appears to be incoherent from that of virion production[113,114]. In HBeAg-negative patients, serum HBsAg titer, serum HBV DNA level and liver cccDNA level are all reduced relative to HBeAg-positive chronic patients where HBsAg titers are noted to be preserved relative to serum cccDNA[113-115]. The rationale for such surveillance is indistinct but may be correlated due to integration of HBV S regions in the genome. A preferential control of the replicative pathway over HBsAg transcription/secretion, may occur in cases where virion production is inhibited[115]. The Pre S2 protein also activates MAPK, which is a key signaling molecule involved in hepatocyte proliferation[116]. Moreover, PreS2 protein can also accumulates in the endoplasmic reticulum (ER) of infected hepatocytes leading to ER stress, a process definitely implicated in liver injury[117]. All these mechanisms are consistent with the hypothesis that S can induce liver carcinogenesis.
Finally, it is also noteworthy that HBsAg may also modify detoxification pathways implicating in cytochrome P450. A modulated P450 may enhance the metabolism of carcinogens with adverse effects on the liver. There seems to a strong clinical significance of quantitative changes in HBsAg during the acute and chronic phase of HBV infection[118,119]. The amount of HBsAg has been found to be closely related to HBV replication within hepatocytes[113] and this could contribute to the immuno pathogenesis during persistent infection.
While HBV polymerase (Pol) is involved in viral replication, it has been shown that it can block cellular processes such as IRF signaling. This indicates that HBV Pol can counteracts the host innate immune defense response[120]. Additionally, HBV Pol is known to inhibit TANK-binding kinase 1 (TBK1)/IkB kinase-e (IKKe) and the effector kinases of IRF signaling. The TBK1/IKKe activity is inhibited by HBV Pol by disrupting the interaction between IKKe and DDX3 DEAD box RNA helicase. This unforeseen role of HBV Pol may provide some details why HBV evades innate immune response in the early phase. A therapeutic implication of this work should involved strategy to interfere with the HBV Pol-DDX3 interaction might lead to the resolution of enduring persistent infection of HBV[120]. While nucleoside and nucleotide analogues may inhibit the HBV-DNA de-novo minus and/or plus strand synthesis by interfering with the reverse transcriptase[121] it may have serious consequence on the survival and health of hepatocytes.
The overall cellular pathways that seem to be regulated by HBV to induce liver injury are very complex. However the slow changes in the liver gene expression are significant for the virus to maintain a safe haven for viral life cycle, replication and productive infection. The chronic hepatitis is characterized by augmented regenerative cell proliferation, a process in which cells are more susceptible to gene mutations. This is further supported by the experiments that increased DNA synthesis is inadequate to induce liver carcinogenesis unless genetic alteration, induced by various factors and cellular processes are gradually accumulated. A compromised DNA repair pathways, activation of signal transduction, modulated epigenetic control of liver genes and transactivation of key hepatic genes disturb the fine balance within the liver. Inflammatory cytokines exacerbate the effect further and changes observed after 2-3 decades of effect and injury are ultimately translated into more serious hepatic injury and dysfunctions. Thus, it is important to understand all those biochemical and molecular pathways that are disturbed by viral gene product or HBV DNA integration, in order to restore the healthy outlook of the most vital organ of the body.
We wish to thank the King Fahd Medical Research Center and Center of Genomic Medicine for financial support and technical help in the preparation of this manuscript. We thank Dr. Zainab Younis and Ms Nadia Rashid of IQ Institute of Infection and Immunity for helpful discussion and editing.
P- Reviewer: Bellanti F, Conti B, Silva LD, Penkova-Radicheva MP S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1700] [Cited by in F6Publishing: 1651] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 2. | Ganem D. Virology. The X files--one step closer to closure. Science. 2001;294:2299-2300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1838] [Cited by in F6Publishing: 1721] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 4. | Chen DS. From hepatitis to hepatoma: lessons from type B viral hepatitis. Science. 1993;262:369-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 266] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985;317:489-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 934] [Cited by in F6Publishing: 919] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 6. | Summers J, Mason WS. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1108] [Cited by in F6Publishing: 1087] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 7. | Mason WS, Aldrich C, Summers J, Taylor JM. Asymmetric replication of duck hepatitis B virus DNA in liver cells: Free minus-strand DNA. Proc Natl Acad Sci USA. 1982;79:3997-4001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 170] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 595] [Cited by in F6Publishing: 582] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 9. | Kane MA. Global status of hepatitis B immunisation. Lancet. 1996;348:696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 117] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1734] [Cited by in F6Publishing: 1695] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 11. | Kim BK, Revill PA, Ahn SH. HBV genotypes: relevance to natural history, pathogenesis and treatment of chronic hepatitis B. Antivir Ther. 2011;16:1169-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Lai CL, Yuen MF. The natural history of chronic hepatitis B. J Viral Hepat. 2007;14 Suppl 1:6-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int. 2009;3:334-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 307] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Summers J, Smolec JM, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533-4537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 446] [Cited by in F6Publishing: 483] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Marion PL, Van Davelaar MJ, Knight SS, Salazar FH, Garcia G, Popper H, Robinson WS. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci USA. 1986;83:4543-4546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Tennant BC, Toshkov IA, Peek SF, Jacob JR, Menne S, Hornbuckle WE, Schinazi RD, Korba BE, Cote PJ, Gerin JL. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology. 2004;127:S283-S293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Brechot C, Kremsdorf D, Soussan P, Pineau P, Dejean A, Paterlini-Brechot P, Tiollais P. Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC): molecular mechanisms and novel paradigms. Pathol Biol (Paris). 2010;58:278-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 684] [Cited by in F6Publishing: 668] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 20. | Liu H, Ye L, Wang QW, Yan QX, Yu JM. Effect of a conserved peptide derived from Kunitz domain of hepatitis B virus x protein on the cell cycle and apoptosis of HepG2 cells via the proteasome pathway. Chin Med J (Engl). 2009;122:460-465. [PubMed] [Cited in This Article: ] |
| 21. | Kew MC. Hepatocellular carcinoma in African Blacks: Recent progress in etiology and pathogenesis. World J Hepatol. 2010;2:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Kuo A, Gish R. Chronic hepatitis B infection. Clin Liver Dis. 2012;16:347-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Gish RG. Epidemiologic and virologic characteristics of hepatitis B virus genotype B having the recombination with genotype C. Gastroenterology. 2003;124:925-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 366] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 25. | Zacharakis GH, Koskinas J, Kotsiou S, Papoutselis M, Tzara F, Vafeiadis N, Archimandritis AJ, Papoutselis K. Natural history of chronic HBV infection: a cohort study with up to 12 years follow-up in North Greece (part of the Interreg I-II/EC-project). J Med Virol. 2005;77:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Suzuki S, Sugauchi F, Orito E, Kato H, Usuda S, Siransy L, Arita I, Sakamoto Y, Yoshihara N, El-Gohary A. Distribution of hepatitis B virus (HBV) genotypes among HBV carriers in the Cote d’Ivoire: complete genome sequence and phylogenetic relatedness of HBV genotype E. J Med Virol. 2003;69:459-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Stuyver L, De Gendt S, Van Geyt C, Zoulim F, Fried M, Schinazi RF, Rossau R. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J Gen Virol. 2000;81:67-74. [PubMed] [Cited in This Article: ] |
| 28. | Lacombe K, Massari V, Girard PM, Serfaty L, Gozlan J, Pialoux G, Mialhes P, Molina JM, Lascoux-Combe C, Wendum D. Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. AIDS. 2006;20:419-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Devesa M, Pujol FH. Hepatitis B virus genetic diversity in Latin America. Virus Res. 2007;127:177-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Tanwar S, Dusheiko G. Is there any value to hepatitis B virus genotype analysis? Curr Gastroenterol Rep. 2012;14:37-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39812] [Cited by in F6Publishing: 43011] [Article Influence: 3308.5] [Reference Citation Analysis (4)] |
| 32. | Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5245] [Cited by in F6Publishing: 5425] [Article Influence: 235.9] [Reference Citation Analysis (0)] |
| 34. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1691] [Cited by in F6Publishing: 1665] [Article Influence: 83.3] [Reference Citation Analysis (2)] |
| 35. | Villa E, Grottola A, Buttafoco P, Trande P, Merighi A, Fratti N, Seium Y, Cioni G, Manenti F. Evidence for hepatitis B virus infection in patients with chronic hepatitis C with and without serological markers of hepatitis B. Dig Dis Sci. 1995;40:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Berasain C, Betés M, Panizo A, Ruiz J, Herrero JI, Civeira MP, Prieto J. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut. 2000;47:429-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Chemin I, Zoulim F, Merle P, Arkhis A, Chevallier M, Kay A, Cova L, Chevallier P, Mandrand B, Trépo C. High incidence of hepatitis B infections among chronic hepatitis cases of unknown aetiology. J Hepatol. 2001;34:447-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Borzio M, Trerè D, Borzio F, Ferrari AR, Bruno S, Roncalli M, Colloredo G, Leandro G, Oliveri F, Derenzini M. Hepatocyte proliferation rate is a powerful parameter for predicting hepatocellular carcinoma development in liver cirrhosis. Mol Pathol. 1998;51:96-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 820] [Article Influence: 29.3] [Reference Citation Analysis (1)] |
| 40. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1202] [Cited by in F6Publishing: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 41. | Akbar SM, Inaba K, Onji M. Upregulation of MHC class II antigen on dendritic cells from hepatitis B virus transgenic mice by interferon-gamma: abrogation of immune response defect to a T-cell-dependent antigen. Immunology. 1996;87:519-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat. 2009;16:265-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J, Janssen HL. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269-7272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 330] [Cited by in F6Publishing: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 45. | Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886-1900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 332] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 46. | Momeni M, Zainodini N, Bidaki R, Hassanshahi G, Daneshvar H, Khaleghinia M, Ebrahim M, Karimi-Googheri M, Askari A, Arababadi MK. Decreased expression of toll like receptor signaling molecules in chronic HBV infected patients. Hum Immunol. 2014;75:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 47. | Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 268] [Article Influence: 17.9] [Reference Citation Analysis (3)] |
| 48. | Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2509] [Cited by in F6Publishing: 2457] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 49. | Yoo YD, Ueda H, Park K, Flanders KC, Lee YI, Jay G, Kim SJ. Regulation of transforming growth factor-beta 1 expression by the hepatitis B virus (HBV) X transactivator. Role in HBV pathogenesis. J Clin Invest. 1996;97:388-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 126] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118:1835-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | Jahan S, Ashfaq UA, Khaliq S, Samreen B, Afzal N. Dual behavior of HCV Core gene in regulation of apoptosis is important in progression of HCC. Infect Genet Evol. 2012;12:236-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Ng SA, Lee C. Hepatitis B virus X gene and hepatocarcinogenesis. J Gastroenterol. 2011;46:974-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 53. | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8502] [Cited by in F6Publishing: 8384] [Article Influence: 465.8] [Reference Citation Analysis (0)] |
| 54. | Hildt E, Munz B, Saher G, Reifenberg K, Hofschneider PH. The PreS2 activator MHBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice. EMBO J. 2002;21:525-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Yang F, Yan S, He Y, Wang F, Song S, Guo Y, Zhou Q, Wang Y, Lin Z, Yang Y. Expression of hepatitis B virus proteins in transgenic mice alters lipid metabolism and induces oxidative stress in the liver. J Hepatol. 2008;48:12-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Srisuttee R, Koh SS, Park EH, Cho IR, Min HJ, Jhun BH, Yu DY, Park S, Park do Y, Lee MO. Up-regulation of Foxo4 mediated by hepatitis B virus X protein confers resistance to oxidative stress-induced cell death. Int J Mol Med. 2011;28:255-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Vandermeeren AM, Gómez CE, Patiño C, Domingo-Gil E, Guerra S, González JM, Esteban M. Subcellular forms and biochemical events triggered in human cells by HCV polyprotein expression from a viral vector. Virol J. 2008;5:102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Clippinger AJ, Bouchard MJ. Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential. J Virol. 2008;82:6798-6811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 59. | Yang B, Bouchard MJ. The hepatitis B virus X protein elevates cytosolic calcium signals by modulating mitochondrial calcium uptake. J Virol. 2012;86:313-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 60. | Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023-2032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 61. | Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4586] [Cited by in F6Publishing: 4821] [Article Influence: 283.6] [Reference Citation Analysis (0)] |
| 62. | He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 63. | Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 489] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 64. | Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1554] [Cited by in F6Publishing: 1513] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 65. | Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 763] [Cited by in F6Publishing: 773] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 66. | Li J, Liu Y, Wang Z, Liu K, Wang Y, Liu J, Ding H, Yuan Z. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J Virol. 2011;85:6319-6333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 67. | Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220-9231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1361] [Cited by in F6Publishing: 1428] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 68. | Liu TT, Fang Y, Xiong H, Chen TY, Ni ZP, Luo JF, Zhao NQ, Shen XZ. A case-control study of the relationship between hepatitis B virus DNA level and risk of hepatocellular carcinoma in Qidong, China. World J Gastroenterol. 2008;14:3059-3063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Vrancken K, Paeshuyse J, Liekens S. Angiogenic activity of hepatitis B and C viruses. Antivir Chem Chemother. 2012;22:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 70. | Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18:382-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 71. | Yoo YG, Oh SH, Park ES, Cho H, Lee N, Park H, Kim DK, Yu DY, Seong JK, Lee MO. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor-1alpha through activation of mitogen-activated protein kinase pathway. J Biol Chem. 2003;278:39076-39084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 833] [Cited by in F6Publishing: 856] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 73. | Sanz-Cameno P, Martín-Vílchez S, Lara-Pezzi E, Borque MJ, Salmerón J, Muñoz de Rueda P, Solís JA, López-Cabrera M, Moreno-Otero R. Hepatitis B virus promotes angiopoietin-2 expression in liver tissue: role of HBV x protein. Am J Pathol. 2006;169:1215-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9515] [Article Influence: 594.7] [Reference Citation Analysis (1)] |
| 75. | Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, Clergue F, Poupon R, Barbu V, Rosmorduc O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 76. | Imazeki F, Omata M, Yokosuka O, Okuda K. Integration of hepatitis B virus DNA in hepatocellular carcinoma. Cancer. 1986;58:1055-1060. [PubMed] [Cited in This Article: ] |
| 77. | Wang Y, Cui F, Lv Y, Li C, Xu X, Deng C, Wang D, Sun Y, Hu G, Lang Z. HBsAg and HBx knocked into the p21 locus causes hepatocellular carcinoma in mice. Hepatology. 2004;39:318-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 78. | Wang Y, Lau SH, Sham JS, Wu MC, Wang T, Guan XY. Characterization of HBV integrants in 14 hepatocellular carcinomas: association of truncated X gene and hepatocellular carcinogenesis. Oncogene. 2004;23:142-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Ma B. Animal models for hepatitis B virus and liver cancer. Primary liver cancer: etiological and progression factors. Boca Raton: CRC Press 1994; 211-224. [Cited in This Article: ] |
| 80. | Belloni L, Pollicino T, De Nicola F, Guerrieri F, Raffa G, Fanciulli M, Raimondo G, Levrero M. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. Proc Natl Acad Sci USA. 2009;106:19975-19979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 364] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 81. | Lucifora J, Arzberger S, Durantel D, Belloni L, Strubin M, Levrero M, Zoulim F, Hantz O, Protzer U. Hepatitis B virus X protein is essential to initiate and maintain virus replication after infection. J Hepatol. 2011;55:996-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 322] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 82. | Nguyen DH, Ludgate L, Hu J. Hepatitis B virus-cell interactions and pathogenesis. J Cell Physiol. 2008;216:289-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 84. | Qadri I, Ferrari ME, Siddiqui A. The hepatitis B virus transactivator protein, HBx, interacts with single-stranded DNA (ssDNA). Biochemical characterizations of the HBx-ssDNA interactions. J Biol Chem. 1996;271:15443-15450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Martín-Vílchez S, Sanz-Cameno P, Rodríguez-Muñoz Y, Majano PL, Molina-Jiménez F, López-Cabrera M, Moreno-Otero R, Lara-Pezzi E. The hepatitis B virus X protein induces paracrine activation of human hepatic stellate cells. Hepatology. 2008;47:1872-1883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 86. | Tralhao JG, Roudier J, Morosan S, Giannini C, Tu H, Goulenok C, Carnot F, Zavala F, Joulin V, Kremsdorf D. Paracrine in vivo inhibitory effects of hepatitis B virus X protein (HBx) on liver cell proliferation: an alternative mechanism of HBx-related pathogenesis. Proc Natl Acad Sci USA. 2002;99:6991-6996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Qadri I, Conaway JW, Conaway RC, Schaack J, Siddiqui A. Hepatitis B virus transactivator protein, HBx, associates with the components of TFIIH and stimulates the DNA helicase activity of TFIIH. Proc Natl Acad Sci USA. 1996;93:10578-10583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 88. | Bouchard MJ, Navas-Martin S. Hepatitis B and C virus hepatocarcinogenesis: lessons learned and future challenges. Cancer Lett. 2011;305:123-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 89. | Pang R, Lee TK, Poon RT, Fan ST, Wong KB, Kwong YL, Tse E. Pin1 interacts with a specific serine-proline motif of hepatitis B virus X-protein to enhance hepatocarcinogenesis. Gastroenterology. 2007;132:1088-1103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 90. | Leupin O, Bontron S, Schaeffer C, Strubin M. Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death. J Virol. 2005;79:4238-4245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 91. | Pan J, Lian Z, Wallett S, Feitelson MA. The hepatitis B x antigen effector, URG7, blocks tumour necrosis factor alpha-mediated apoptosis by activation of phosphoinositol 3-kinase and beta-catenin. J Gen Virol. 2007;88:3275-3285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Jin YM, Yun C, Park C, Wang HJ, Cho H. Expression of hepatitis B virus X protein is closely correlated with the high periportal inflammatory activity of liver diseases. J Viral Hepat. 2001;8:322-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Mathonnet G, Lachance S, Alaoui-Jamali M, Drobetsky EA. Expression of hepatitis B virus X oncoprotein inhibits transcription-coupled nucleotide excision repair in human cells. Mutat Res. 2004;554:305-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Lee AT, Ren J, Wong ET, Ban KH, Lee LA, Lee CG. The hepatitis B virus X protein sensitizes HepG2 cells to UV light-induced DNA damage. J Biol Chem. 2005;280:33525-33535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Zhang Z, Protzer U, Hu Z, Jacob J, Liang TJ. Inhibition of cellular proteasome activities enhances hepadnavirus replication in an HBX-dependent manner. J Virol. 2004;78:4566-4572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Sirma H, Weil R, Rosmorduc O, Urban S, Israël A, Kremsdorf D, Bréchot C. Cytosol is the prime compartment of hepatitis B virus X protein where it colocalizes with the proteasome. Oncogene. 1998;16:2051-2063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 97. | Henkler F, Hoare J, Waseem N, Goldin RD, McGarvey MJ, Koshy R, King IA. Intracellular localization of the hepatitis B virus HBx protein. J Gen Virol. 2001;82:871-882. [PubMed] [Cited in This Article: ] |
| 98. | Lee YI, Hwang JM, Im JH, Lee YI, Kim NS, Kim DG, Yu DY, Moon HB, Park SK. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J Biol Chem. 2004;279:15460-15471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 99. | Tanaka Y, Kanai F, Kawakami T, Tateishi K, Ijichi H, Kawabe T, Arakawa Y, Kawakami T, Nishimura T, Shirakata Y. Interaction of the hepatitis B virus X protein (HBx) with heat shock protein 60 enhances HBx-mediated apoptosis. Biochem Biophys Res Commun. 2004;318:461-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 100. | Zhang SM, Sun DC, Lou S, Bo XC, Lu Z, Qian XH, Wang SQ. HBx protein of hepatitis B virus (HBV) can form complex with mitochondrial HSP60 and HSP70. Arch Virol. 2005;150:1579-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 475] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 102. | Longato L, de la Monte S, Kuzushita N, Horimoto M, Rogers AB, Slagle BL, Wands JR. Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant alterations in the liver. Hepatology. 2009;49:1935-1943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Forgues M, Difilippantonio MJ, Linke SP, Ried T, Nagashima K, Feden J, Valerie K, Fukasawa K, Wang XW. Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol Cell Biol. 2003;23:5282-5292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 104. | Kim S, Park SY, Yong H, Famulski JK, Chae S, Lee JH, Kang CM, Saya H, Chan GK, Cho H. HBV X protein targets hBubR1, which induces dysregulation of the mitotic checkpoint. Oncogene. 2008;27:3457-3464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int J Cancer. 2007;120:1208-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Ou JH. Molecular biology of hepatitis B virus e antigen. J Gastroenterol Hepatol. 1997;12:S178-S187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 107. | Chen MT, Billaud JN, Sällberg M, Guidotti LG, Chisari FV, Jones J, Hughes J, Milich DR. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci USA. 2004;101:14913-14918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 108. | Milich DR, Chen MK, Hughes JL, Jones JE. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J Immunol. 1998;160:2013-2021. [PubMed] [Cited in This Article: ] |
| 109. | Kwon JA, Rho HM. Transcriptional repression of the human p53 gene by hepatitis B viral core protein (HBc) in human liver cells. Biol Chem. 2003;384:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 110. | Razanskas R, Sasnauskas K. Interaction of hepatitis B virus core protein with human GIPC1. Arch Virol. 2010;155:247-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | Nguyen T, Thompson AJ, Bowden S, Croagh C, Bell S, Desmond PV, Levy M, Locarnini SA. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 112. | Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, Flisiak R, Bock CT, Manns MP, Wedemeyer H. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 113. | Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, Slavin J, Bowden S, Gane EJ, Abbott W. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933-1944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 114. | Lin LY, Wong VW, Zhou HJ, Chan HY, Gui HL, Guo SM, Wang H, Huang L, Bao SS, Xie Q. Relationship between serum hepatitis B virus DNA and surface antigen with covalently closed circular DNA in HBeAg-negative patients. J Med Virol. 2010;82:1494-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 115. | Volz T, Lutgehetmann M, Wachtler P, Jacob A, Quaas A, Murray JM, Dandri M, Petersen J. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology. 2007;133:843-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 116. | Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384-46392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 117. | Kadowaki H, Nishitoh H, Ichijo H. Survival and apoptosis signals in ER stress: the role of protein kinases. J Chem Neuroanat. 2004;28:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 118. | Gramenzi A, Loggi E, Micco L, Cursaro C, Fiorino S, Galli S, Gitto S, Galli C, Furlini G, Bernardi M. Serum hepatitis B surface antigen monitoring in long-term lamivudine-treated hepatitis B virus patients. J Viral Hepat. 2011;18:e468-e474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, Tillmann HL, Kao JH, Jia JD, Wedemeyer H. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol. 2011;55:1121-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 245] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 120. | Wang H, Ryu WS. Hepatitis B virus polymerase blocks pattern recognition receptor signaling via interaction with DDX3: implications for immune evasion. PLoS Pathog. 2010;6:e1000986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 160] [Article Influence: 11.4] [Reference Citation Analysis (2)] |
| 121. | Urban S, Schulze A, Dandri M, Petersen J. The replication cycle of hepatitis B virus. J Hepatol. 2010;52:282-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |