Published online Sep 7, 2014. doi: 10.3748/wjg.v20.i33.11904
Revised: March 28, 2014
Accepted: April 21, 2014
Published online: September 7, 2014
Processing time: 222 Days and 6.1 Hours
In October 2009, a 71-year-old female was diagnosed with a cystic tumor in the tail of the pancreas with an irregular dilatation of the main pancreatic duct in the body and tail of the pancreas. A distal pancreatectomy with splenectomy, and partial resection of the duodenum, jejunum and transverse colon was performed. In March 2011, a follow-up computed tomography scan showed a low density mass at the head of the remnant pancreas. We diagnosed it as a recurrence of the tumor and performed a total pancreatectomy for the remnant pancreas. In the histological evaluation of the resected specimen of the distal pancreas, the neoplastic cells formed an acinar and papillary structure that extended into the main pancreatic duct. Mucin5AC, α1-antitrypsin (α-AT) and carcinoembryonic antigen (CEA) were detected in the tumor cells by immunohistochemistry. In the resected head of the pancreas, the tumor was composed of both acinar and ductal elements with a mottled pattern. The proportions of each element were approximately 40% and 60%, respectively. Strongly positive α-AT cells were detected in the acinar element. Some tumor cells were also CEA positive. However, the staining for synaptophysin and chromogranin A was negative in the tumor cells. Ultimately, we diagnosed the tumor as a recurrence of mixed acinar-ductal carcinoma in the remnant pancreas. In conclusion, we report here a rare case of repeated pancreatic resection for multicentric lesions of mixed acinar-ductal carcinoma of the pancreas.
Core tip: We report a rare case of multicentric mixed acinar-ductal carcinoma lesions of the pancreas. The patient lived for 39 mo after the first operation without a second recurrence.
- Citation: Shonaka T, Inagaki M, Akabane H, Yanagida N, Shomura H, Yanagawa N, Oikawa K, Nakano S. Total pancreatectomy for metachronous mixed acinar-ductal carcinoma in a remnant pancreas. World J Gastroenterol 2014; 20(33): 11904-11909
- URL: https://www.wjgnet.com/1007-9327/full/v20/i33/11904.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i33.11904
Although several cases of mixed acinar-endocrine carcinoma of the pancreas, which is composed of both acinar and endocrine tumor cells, have been reported[1], there have only been a few cases of resected mixed acinar-ductal carcinoma worldwide. According to the World Health Organization (WHO) classification, mixed acinar-ductal carcinoma is considered to be a sub-class of acinar cell neoplasms[2]. Mixed acinar-ductal carcinoma is defined in cases where greater than 25% of the tumor exhibits acinar and ductal elements in the pathological findings. Mixed acinar-ductal carcinoma usually exhibits an aggressive behavior, and it has a poor prognosis[3].
Herein we report a case of repeat pancreatectomy for multicentric lesions diagnosed as mixed acinar-ductal carcinoma, discuss the clinicopathological features, and present a review of the literature.
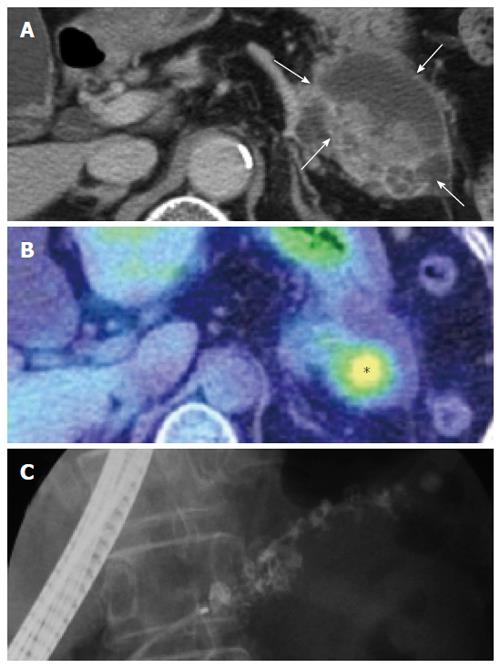
A 71-year-old female presented with epigastric pain in October 2009. Abdominal computed tomography (CT) showed a cystic lesion with a solid component in the tail of the pancreas (Figure 1A) and positron emission tomography-CT (PET-CT) showed intense fluorodeoxy glucose (FDG) uptake at the solid lesion (Figure 1B). Endoscopic retrograde cholangiopancreatography (ERCP) revealed irregular dilatation of the main pancreatic duct in the body and tail of the pancreas (Figure 1C). We diagnosed the patient with intraductal papillary mucinous neoplasms. During the operation, we found that the pancreas tumor was adhered to the duodenum, jejunum and transverse colon. We did not want to peel away the tumor, so we performed a distal pancreatectomy with splenectomy and partial resections of the duodenum, jejunum and transverse colon.
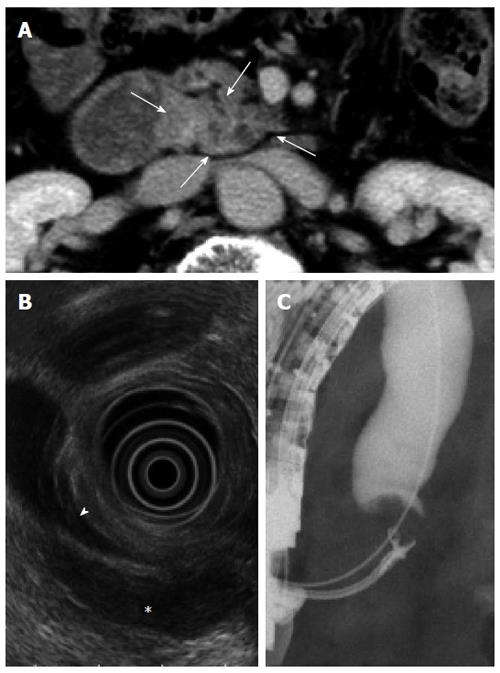
In March 2011 (15 mo after the first operation), the patient had did not exhibit any symptoms, but a follow-up CT examination showed a low density area at the uniciate process of the pancreas (Figure 2A). FDG/PET-CT revealed intense FDG uptake in the same region (data not shown). An endoscopic ultrasound examination showed a hypo-echoic lesion in the uniciate process of the pancreas that was approximately 3 cm in diameter, and this tumor compressed the inferior common bile duct (Figure 2B). ERCP revealed the presence of inferior common bile duct stenosis, and adenocarcinoma was suggested by a biopsy of the tumor lesion (Figure 2C). Based on these findings, we suspected a recurrence of mixed-acinar ductal carcinoma. Total resection of the remnant pancreas was performed. The patient was discharged successful on postoperative day 32. She remained alive 21 mo after the second operation and had no recurrence.
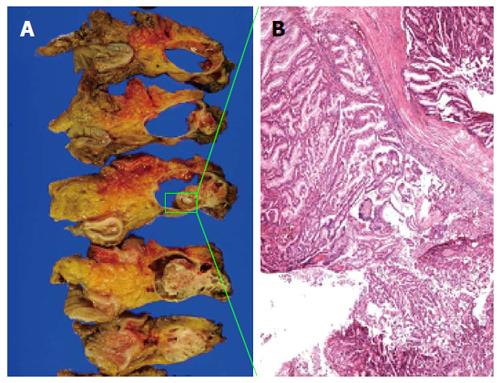
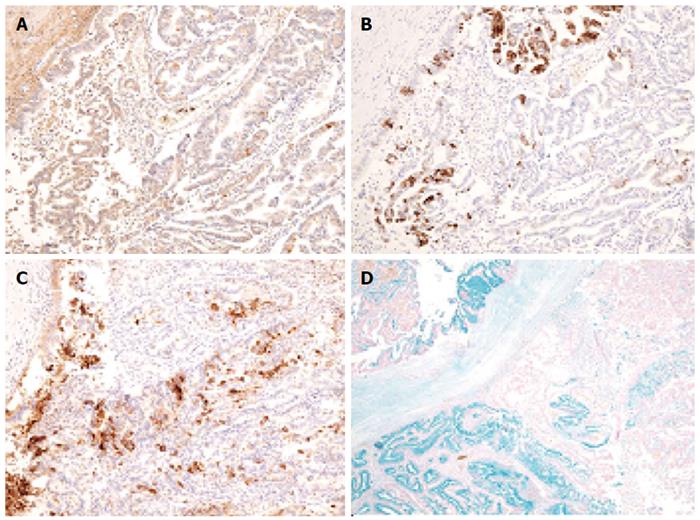
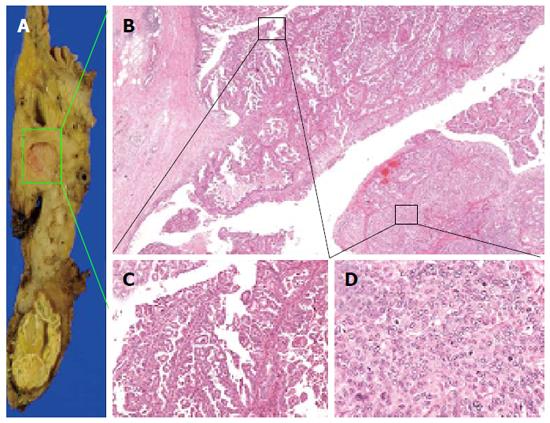
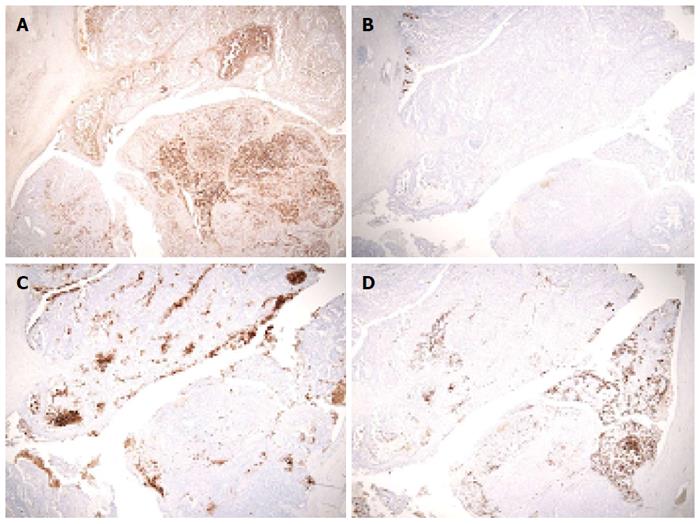
During the distal pancreatectomy, a cystic lesion that was 7.2 cm in diameter with a partial solid component was noted in the specimen (Figure 3A). Hematoxylin and eosin (HE) staining showed both papillary and acinar-like structures in the solid component that extended into the main and the branch pancreatic duct (Figure 3B). In the former region, cube-shaped or high columnar cells exhibiting with mucin production formed the papillary structure resembling intraductal papillary neoplasms. Acinar-like structures were also observed in certain areas. No malignant cells were detected in the proximal stump of the pancreas. The tumor was localized to the pancreas without invasion into the duodenum, jejunum or, colon. We used an EnVision™ FLEX system for immunostaining. We assessed the ductal and acinar elements using immunohistochemistry. The results showed, positive staining for α1-antitrypsin (α-AT) and carcinoembryonic antigen (CEA). The gastrointestinal element was negative for mucin 2 (MUC2). Mucin 5AC (MUC5AC) was minimally detected in the tumor cells; however the we thought intraductal papillary mucinous neoplasm was negative (Figure 4). The islet element was negative for synaptophysin and chromogranin A (data not shown). Based on these histological findings, we diagnosed the tumor as a mixed acinar-ductal carcinoma. Extended papillary growth outside the cystic lesion was also observed. A total of 8 lymph nodes were extracted, but no metastasis of the resected lymph nodes was detected. The pathological stage was IIIb (T3N0M0) based on the 7th edition criteria of the Union for International Center Control[4].
The specimen taken during the second operation revealed another tumor approximately 3 cm in diameter tumor in the head of the pancreas (Figure 5A). Acinar-like structures and papillary growths were observed, with a mottled pattern were observed(Figure 5B). Some regions of the tumor had a papillary growth pattern (Figure 5C).
Some of the tumor cells with mild atypia showed poor mucus production with eosinophilic cytoplasm. The tumor exhibited a papillary proliferation pattern, with delicate fibervascular stroma and acinar-like structure pattern in some regions (Figure 5D). The tumor exhibited an almost solid growth, and the glandular lumen was unclear. We detected abundant mitotic figures in the tumor. In the microscopic findings, approximately 40% of the tumor had an acinar-like structure, and 60% of it exhibited a papillary growth pattern. There was intense α-AT staining in the acinar-like part of the tumor. MUC5AC was not detected. Mild positive staining for mucin1 and CEA was observed (Figure 6). The staining for synaptophysin A and chromogranin were negative (data not shown). The tumor showed both an acinar-like and papillary growth pattern in the main pancreatic duct. As a result, we diagnosed the tumor as a mixed acinar-ductal carcinoma in the remnant pancreas. A total of 7 lymph nodes were extracted, but metastasis was not detected.
Combined acinar and ductal phenotype carcinoma of the pancreas is very rare[5-7]. The WHO classification categorizes mixed acinar-ductal pancreatic carcinoma as a sub-class of acinar cell neoplasms[2]. Mixed carcinomas of the pancreas have distinctive histological features suggesting more than one line of differentiation. Mixed acinar-ductal carcinomas are defined as those in which at least 25 percent of the neoplastic cells show an acinar and ductal line of differentiation[2,8].
Stelow et al[3] reported a study of 11 cases of mixed acinar-ductal carcinoma. In their report, all cases showed significant evidence of acinar and ductal differentiation that were, estimated to include at least 25% of the neoplastic cells. In the present case, acinar differentiation was shown to involve 40% of the tumor, with the remaining portion exhibiting ductal differentiation during the second operation. All but one of the carcinomas showed predominantly acinar differentiation, based on routine histological and immunohistochemical analyses[3]. The 18 previously reported cases of mixed-acinar ductal carcinoma in the English and Japanese literatures are summarized in Table 1[3,9-11]. In five cases, the tumors were present in the tail of the pancreas, while in the remaining cases, the tumors were located in the head of the pancreas (the tumor diameters ranged from 35-72 mm). With regard to treatment, only one case received radiotherapy, and the other cases underwent surgical resection. There were no reports of a recurrence of the tumor or additional resection of the remnant pancreas.
| Case | Age | Sex | Symptoms at presentation | Size | Location | Treatment | Follow-up | Prognosis | Reference |
| 1 | 74 | M | Painless jaundice | 31 | Head | RTx, CTx | 20 | Alive | [3] |
| 2 | 75 | M | Weight loss and diarrhea | 25 | Head | RTx, CTx | 39 | Dead | [3] |
| 3 | 73 | M | Not available | 20 | Tail | RTx, CTx | 52 | Dead | [3] |
| 4 | 74 | M | Weight loss and diarrhea | 40 | Head | RTx, CTx | 51 | Dead | [3] |
| 5 | 70 | M | Pain | 40 | Head | RTx, Rdx | 38 | Dead | [3] |
| 6 | 77 | F | Weight loss | 30 | Head | Rdx | 9 | Dead | [3] |
| 7 | 77 | M | Weight loss and pain | 37 | Head | RTx | 0.5 | Dead1 | [3] |
| 8 | 52 | M | Pain | 55 | Head | RTx, CTx | 12 | Dead | [3] |
| 9 | 76 | M | Painless jaundice | 35 | Head | RTx, CTx | 8 | Dead | [3] |
| 10 | 79 | M | Painless jaundice | 34 | Head | RTx, CTx | 11 | Alive | [3] |
| 11 | 69 | M | Painless jaundice | 54 | Head | RTx, CTx | 36 | Alive | [3] |
| 12 | 71 | M | Not mentioned | 30 | Head | - | 12 | Dead | [10] |
| 13 | 51 | F | Not mentioned | - | Tail | - | 3 | Dead | [10] |
| 14 | 51 | F | Not mentioned | 30 | Tail | - | 4 | Dead | [10] |
| 15 | 85 | M | Not mentioned | - | Tail | - | 6 | Dead | [10] |
| 16 (J) | 63 | M | Abdominal pain | 65 | Head | RTx | 39 | Alive | [11] |
| 17 (J) | 63 | M | Worsening of diabetes | 35 | Head | RTx | 8 | Dead | [12] |
| 18 | 71 | F | Epigastric discomfort | 72 | Tail | RTx | 36 | Alive2 | |
| 35 | Head | RTx |
Kobayashi et al[12] reported an intraductal growth of tumor cells into the main pancreatic duct with acinar endocrine carcinomas. For this reason, it is possible that mixed acinar and ductal phenotype carcinoma is biologically more closely related to acinar cell carcinoma than to pancreatic duct carcinoma. Five of the previously reported cases had a “mucinous acinar cell carcinoma” pattern that, which is characterized by the production of mucin in the acinar cell carcinoma. Six cases were reported to exhibit a “combined acinar ductal” pattern which finds mottled component of acinar cell carcinoma and ductal carcinoma. Our case was a “mucinous acinar cell carcinoma” at the first operation and a “combined acinar ductal carcinoma” at the second operation[3]. Mucin production was observed in all cases, with a high positive rate of immunostaining for MUC1 and CEA that, was useful in the diagnosis. This was also the case consistent with our patient. The clinical course was considered to be aggressive, and seven of the previous patients died at a mean of 29 mo after the operation[3]. However, our patinet remained alive without recurrence 39 mo after the first operation.
Our patient underwent repeated pancreatectomy due to a metachronous mixed acinar-ductal carcinoma. A metachrounous tumor in the remnant pancreas of mixed acinar-ductal carcinoma has not previously been reported, although repeated pancreatectomy for some cases of pancreatic duct carcinoma, intraductal papillary, mucinous tumors and endocrine tumors has[13-18]. There was only one previous case of a recurrence of acinar carcinoma[14].
In conclusion, we reported a rare case of mixed acinar-ductal carcinoma. The patient developed metachrounous tumors in the remnant pancreas and underwent a repeated resection. She remained alive 39 mo after the first operation without a second recurrence.
We thank Tomoko Mitsuhashi (Department of Surgical Pathology, Hokkaido University Hospital) for her expertise regarding this case.
P- Reviewer: Eysselein VE, Iacono C, Liu SH S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Ohike N, Jürgensen A, Pipeleers-Marichal M, Klöppel G. Mixed ductal-endocrine carcinomas of the pancreas and ductal adenocarcinomas with scattered endocrine cells: characterization of the endocrine cells. Virchows Arch. 2003;442:258-265. [PubMed] |
| 2. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 9th ed. Lyon: IARC/WHO 2010; 279-337. |
| 3. | Stelow EB, Shaco-Levy R, Bao F, Garcia J, Klimstra DS. Pancreatic acinar cell carcinomas with prominent ductal differentiation: Mixed acinar ductal carcinoma and mixed acinar endocrine ductal carcinoma. Am J Surg Pathol. 2010;34:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Sobin L, Gospodarowic M, Wittekind C. TNM Classification of Malignant tumours. 7th ed. New York: Wiley-Blackwell 2009; . |
| 5. | Schron DS, Mendelsohn G. Pancreatic carcinoma with duct, endocrine, and acinar differentiation. A histologic, immunocytochemical, and ultrastructural study. Cancer. 1984;54:1766-1770. [PubMed] |
| 6. | Okada Y, Mori H, Tsutsumi A. Duct-acinar-islet cell tumor of the pancreas. Pathol Int. 1995;45:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Tanakaya K, Teramoto N, Konaga E, Takeuchi H, Yasui Y, Takeda A, Yunoki Y, Murakami I. Mixed duct-acinar-islet cell tumor of the pancreas: report of a case. Surg Today. 2001;31:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas afip Atlas of Tumor Pathology. 4th Series Fascicle. Washington DC: American Registry of Pathology 2007; 211-218. |
| 9. | Webb JN. Acinar cell neoplasms of the exocrine pancreas. J Clin Pathol. 1977;30:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Inaba N, Kasahara K, Kashii A, Kanazawa K, Yamaguchi T, Saito K, Kamisawa T. [Mixed ductal and acinar cell cancer of the pancreas head; report of a case]. Nihon Geka Gakkai Zasshi. 1987;88:773-778. [PubMed] |
| 11. | Sakai M, Takeda S, Ishikawa T, Kanazumi N, Inoue S, Kaneko T, Nakao A, Nagasaka T. Mixed duct-acinar cell carcinoma of the pancreas: Report of a case. Jpn J Gastroenterol Surg. 2005;38:1821-1827. |
| 12. | Kobayashi S, Asakura T, Ohike N, Enomoto T, Sakurai J, Koizumi S, Watanabe T, Nakano H, Otsubo T. Mixed acinar-endocrine carcinoma of the pancreas with intraductal growth into the main pancreatic duct: Report of a case. Surg Today. 2010;40:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Inagaki M, Aoyagi T, Nomura A, Yokoo H, Akabane M, Kawata A, Nakano S, Chiba A, Fujii T, Sakurai H. Surgical approach to tumor recurrence and metastatic lesions of the liver in a patient with malignant endocrine tumors of the pancreas: case report. J Hepatobiliary Pancreat Surg. 2003;10:325-328. [PubMed] |
| 14. | Miura F, Takada T, Amano H, Yoshida M, Isaka T, Toyota N, Wada K, Takagi K, Kato K. Repeated pancreatectomy after pancreatoduodenectomy. J Gastrointest Surg. 2007;11:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, Friess H, Büchler MW. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Inagaki M, Obara M, Kino S, Goto J, Suzuki S, Ishizaki A, Tanno S, Kohgo Y, Tokusashi Y, Miyokawa N. Pylorus-preserving total pancreatectomy for an intraductal papillary-mucinous neoplasm of the pancreas. J Hepatobiliary Pancreat Surg. 2007;14:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Zacharias T, Oussoultzoglou E, Jaeck D, Pessaux P, Bachellier P. Surgery for recurrence of periampullary malignancies. J Gastrointest Surg. 2009;13:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Ogino T, Ueda J, Sato N, Takahata S, Mizumoto K, Nakamura M, Oda Y, Tanaka M. Repeated Pancreatectomy for Recurrent Pancreatic Carcinoma after Pylorus-Preserving Pancreatoduodenectomy: Report of Two Patients. Case Rep Gastroenterol. 2010;4:429-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |