Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10637
Revised: January 26, 2014
Accepted: April 30, 2014
Published online: August 14, 2014
Processing time: 224 Days and 0.4 Hours
Splenic hamartoma (SH) is a rare benign tumor usually detected accidentally, which is composed of an aberrant mixture of normal splenic elements. Here, we report a case of 54-year-old man who presented with symptomatic multinodular SH and was admitted initially for thrombocytopenia and anemia. Physical examination revealed that the patients had an anemic appearance and palpable spleen, extending 10 cm below the costal margin. Preoperative ultrasound and computed tomography (CT) indicated splenomegaly with multinodular lesions. On enhanced CT scanning, during the arterial phase, the lesions demonstrated inhomogeneous enhancement, and in the portal phase the lesions were more hyperdense than the splenic parenchyma. The images were highly suggestive of a metastatic tumor. Splenectomy was performed 1 wk later. The tumor was eventually diagnosed as SH according to the morphological features and immunohistochemical detection, by which CD34 was positive in lining cells and some spindle cells, vimentin was positive in the tumor, factor-VIII-related antigen was positive multifocally in lining cells, and smooth muscle actin was positive in some spindle cells. Thrombocytopenia and anemia were cured after splenectomy.
Core tip: Splenic hamartoma (SH) is a rare benign tumor that is usually detected accidentally. This case represents one of the largest SHs reported in the literature, and we report the use of immunohistochemistry as a tool to confirm the diagnosis. Thrombocytopenia and anemia were cured after splenectomy.
- Citation: Wang RT, Xu XS, Hou HL, Qu K, Bai JG. Symptomatic multinodular splenic hamartoma preoperatively suspected as metastatic tumor: A case report. World J Gastroenterol 2014; 20(30): 10637-10641
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10637.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10637
Splenic hamartoma (SH) is a rare benign tumor composed of an aberrant mixture of normal splenic elements. It is usually a single lesion, with rare occurrence of multiple or diffuses lesions[1,2]. Most patients are asymptomatic, with no specific imaging findings, making it difficult to distinguish it from other benign and malignant splenic diseases, and thus surgery is necessary to confirm the clinical suspicion[3,4]. We here report a symptomatic multinodular SH that was suspected as tumor metastasis preoperatively.
A 54-year-old male patient was admitted to our department for weak and left upper quadrant pain. Physical examination revealed that the patient had an anemic appearance and palpable spleen, extending 10 cm below the costal margin. The spleen had a nodular surface, hard texture, and was tender. Routine blood tests showed: red blood cell (RBC) count 2.03 × 1012/L, hemoglobin 65 g/L, white blood cell (WBC) count 5.18 × 109/L and platelet count 36 × 109/L.
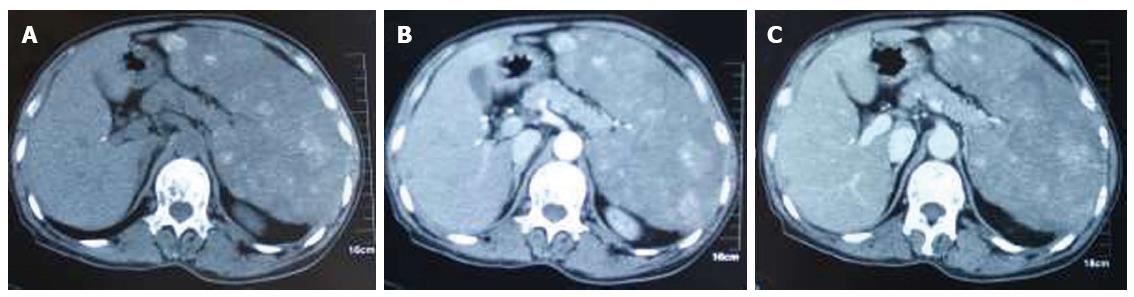
Ultrasound revealed splenomegaly, with multiple hyperechoic masses in the spleen, and some lesions were fused. The diameter of the larger lesions was about 5 cm. The borders were not clear, with irregular shape and rough capsules, which were suggestive of multiple splenic metastases. Further investigation with computed tomography (CT) was performed. Splenomegaly and multiple hyperintense lesions were also seen on CT plain scanning (Figure 1A). On enhanced CT scanning, during the arterial phase, the lesions demonstrated inhomogeneous enhancement (Figure 1B), and in the portal phase, the lesions were more hyperdense than the splenic parenchyma (Figure 1C).
Splenectomy was performed 1 wk later. On day 7 after surgery, routine blood tests showed: RBC count 2.85 × 1012/L, hemoglobin 90 g/L, WBC count 9.60 × 109/L and platelet count 234 × 109/L. Finally, the patient was discharged from hospital on postoperative day 10. Blood counts returned to normal 1 mo postoperatively, and 6 mo after the operation, the patient was in general good condition with normal blood counts.
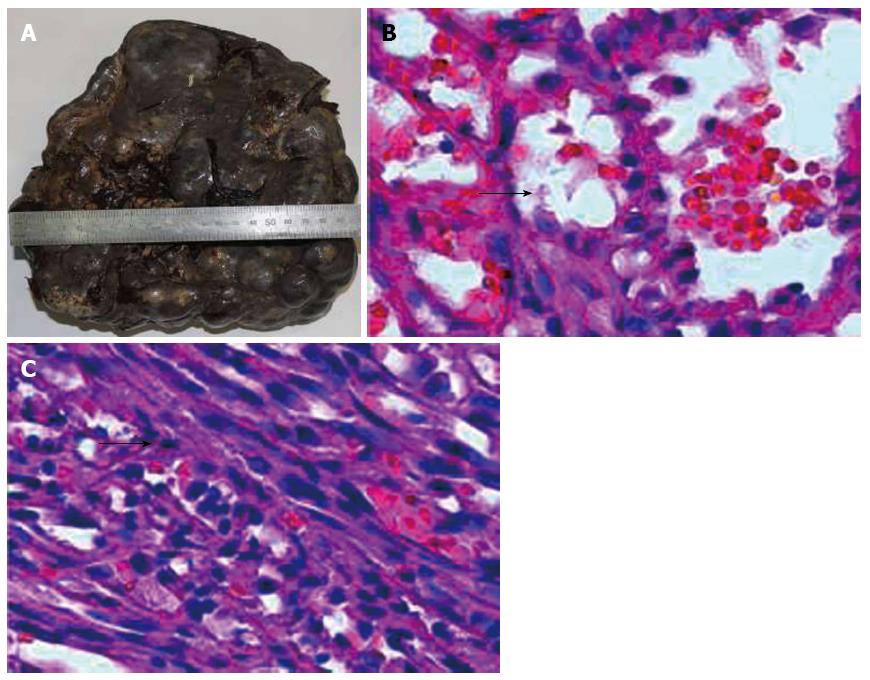
The resected specimen was 2130 g in weight and 24 cm × 19 cm × 11 cm in size. On the surface there were multiple nodules of different sizes (Figure 2A). The cut surface showed round, well-circumscribed and unencapsulated bulging nodules compressing the adjacent normal splenic parenchyma. Local fibrosis and cystic areas were also seen. The lesions were dark red mixed with yellow stripes, ranging from 1.3 to 5 cm.
Histologically, the normal spleen structure had disappeared, and was replaced by nodular lesions of various sizes, similar to the red pulp of the spleen, that merged imperceptibly with the surrounding splenic parenchyma. The nodules were mainly composed of variably dilated, unorganized vascular channels, which were lined by endothelial cells (Figure 2B). These vascular channels contained RBCs, mature granulocytes, lymphoid cells, and tissue cells. Spindle cells could also be seen in local vascular channels, which were arranged in fascicules, with unclear boundaries (Figure 2C). These cells were of regular shape and size, with short spindle-shaped nuclei, fine chromatin, small eosinophilic nucleoli, lightly stained cytoplasm, and light eosin staining. Hyaline degeneration and focal calcification were seen in the area of the spindle cells. A small amount of atrophic white pulp residue was observed between the lesions.
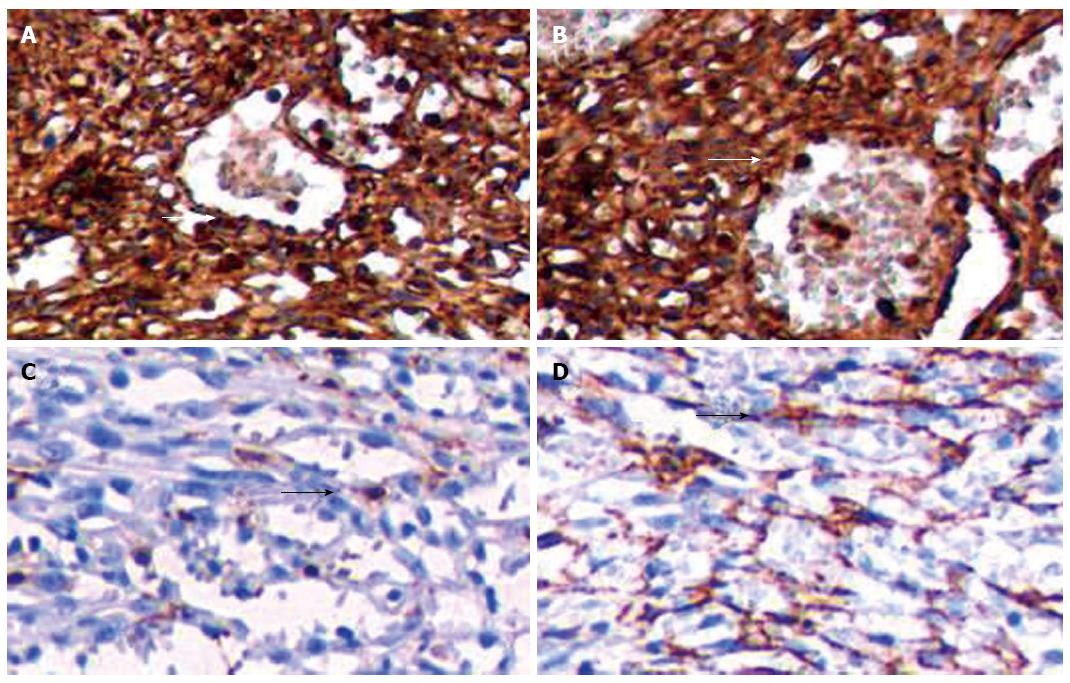
Immunohistochemical staining indicated that CD34 was positive in lining cells and some spindle cells (Figure 3A); vimentin was positive in the tumor (Figure 3B); factor-VIII-related antigen was multifocally positive in lining cells (Figure 3C); and smooth muscle actin (SMA) was positive in some spindle cells (Figure 3D). Combined with the features of morphology and immunohistochemistry, it was eventually diagnosed as SH.
SH was first described in 1861 by Rokitansky. It is a rare benign tumor with an incidence of 3/200000 splenectomies reported by a single center and 0.024%-0.13% in autopsies[1,2]. SH can occur in any age group and has the same male and female occurrence[5,6]. It tends to be larger in women, probably due to hormonal influence on tumor growth[3,7], whereas our case was an adult man with splenomegaly. The majority of patients are asymptomatic, with SH being an incidental finding. A minority of patients have symptoms such as pain, palpable mass, or spontaneous splenic rupture associated with large lesions. Hypersplenism, including thrombocytopenia, anemia, pancytopenia, or malignant hematological conditions, has also been reported[6,8,9]. Our patient complained of weak and left upper quadrant pain. Routine blood tests showed severe anemia and thrombocytopenia.
Imaging findings of SH are nonspecific. On ultrasound, it is usually a hypoechoic solid mass, or occasionally isoechoic or hyperechoic. Due to ischemia or hemorrhage, cystic degeneration and calcification are sometimes demonstrated[4-6]. In our patient, ultrasound revealed multiple hyperechoic masses in the spleen, with some lesions fused.
For red pulp type, SH is usually isointense to normal parenchyma on CT plain scanning, with the only abnormality being the splenic contours. A heterogeneous enhancement in the arterial phase and on delayed images has also been reported. On magnetic resonance imaging (MRI), SH is isointense on T1-weighted images and hyperintense on T2-weighted images[10,11]. For white pulp type composed of lymphatic tissue, the imaging is similar to lymphoma, in that it is usually hypodense or isointense on CT plain scanning, shows delayed enhancement on enhanced CT scanning[10,11], and is hypodense or isointense on MRI T1-weighted images and hyperintense on T2-weighted images. For mixed type, imaging is related to the different composition. For fibrous type mainly composed of fibrous tissue, the tumor is hypodense on CT plain scanning, shows delayed enhancement on enhanced CT scanning, and is isointense on T1-weighted images and hypointense on T2-weighted images[10-12]. In our case, multiple hyperintense nodular lesions were seen in the splenic parenchyma on CT plain scanning. During the arterial phase, the lesions demonstrated heterogeneous enhancement. In the portal phase the lesions were more hyperdense than the splenic parenchyma.
SHs are usually solitary, round, well-circumscribed, unencapsulated nodules, with local fibrosis and occasional cystic areas. The size of SH ranges from a few millimeters to centimeters, with a median size of 5 cm, but lesions as large as 20 cm have seldom been reported[7,13-15]. The color of the cut surface varies because of different tissue types, and is usually grayish white, grayish yellow, grayish red, or dark brown, and often accompanied by bleeding[4-6]. SH can be divided into red pulp type (disorganized splenic sinus), white pulp type (lymphoid tissue), mixed type (red and white pulp), and fibrous type[1]. Immunohistochemically, the lining cells of the vascular channels of the hamartoma are positive for endothelial markers CD8, CD31, vimentin and factor-VIII-related antigen. Immunostaining results for the lining cells with CD34 is inconsistent and CD68 is positive in scattered stromal macrophages but negative in the lining cells of the vascular channels[3,6,13-17]. The main feature of our case was that the normal splenic structure disappeared and was replaced by hyperplastic nodules of various sizes. Spindle cells were seen in the local vascular channels, which looked like splenic red pulp. The size of these cells and their nuclei were consistent, and their morphology was regular. Immunohistochemical staining indicated that CD34 was positive in the lining cells and some spindle cells; vimentin was positive in the tumor; factor-VIII-related antigen was multifocally positive in the lining cells; and SMA was positive in some spindle cells.
SH is a rare benign tumor. It must be distinguished from other benign and malignant primary splenic lesions before surgery, including lymphoma, metastatic lesions, inflammatory myofibroblastic tumor, disseminated fungal or mycobacterial infections, sarcoidosis, and vascular tumors of the spleen, including hemangioma, littoral cell angioma, lymphangioma, hemangioendothelioma, sclerosing angiomatoid nodular transformation, and angiosarcoma[17]. However, the majority of patients with SH are asymptomatic and the imaging findings are not specific. Consequently, it is difficult to diagnose before surgery. Fine needle aspiration biopsy may be useful to establish a pathological diagnosis. However, this technique is associated with some serious complications including bleeding and abdominal seeding[18]. Hence, surgery becomes necessary. For single small lesions, especially in children, partial splenectomy should be adopted, to avoid potential risks of total splenectomy. For larger lesions, multiple lesions, or when the malignancy cannot be ruled out, total splenectomy is needed[6,17]. The prognosis of SH is good, without postoperative recurrence and metastasis. In our case, blood counts returned to normal 1 mo postoperatively. At 6 mo postoperatively, the patient is in general good condition and has normal blood counts.
A 54-year-old man presented with symptomatic multinodular splenic hamartoma (SH).
The patient was initially admitted for thrombocytopenia and anemia.
Routine blood tests showed: red blood cell count 2.03 × 1012/L, hemoglobin 65 g/L, white blood cell count 5.18 × 109/L and platelet count 36 × 109/L.
Preoperative ultrasound and computed tomography indicated splenomegaly with multinodular lesions.
Morphological features and immunohistochemistry indicated that CD34 was positive in lining cells and some spindle cells; vimentin was positive in the tumor; factor-VIII-related antigen was multifocally positive in lining cells; and smooth muscle actin was positive in some spindle cells.
Splenectomy was performed 1 wk later, after which, thrombocytopenia and anemia were cured.
There are some reports of SH with a median size of 5 cm, but lesions as large as 20 cm have seldom been reported.
SH is asymptomatic and the imaging findings are not specific. Fine needle aspiration biopsy may be useful to establish a pathological diagnosis. However, SH is associated with complications such as bleeding, thus making surgery necessary.
This article is interesting and will be of interest to the readership.
P- Reviewer: Chetty R, Nakayama Y S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Silverman ML, LiVolsi VA. Splenic hamartoma. Am J Clin Pathol. 1978;70:224-229. [PubMed] |
| 2. | Lam KY, Yip KH, Peh WC. Splenic vascular lesions: unusual features and a review of the literature. Aust N Z J Surg. 1999;69:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Lee H, Maeda K. Hamartoma of the spleen. Arch Pathol Lab Med. 2009;133:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 4. | Chou YH, Chiou HJ, Tiu CM, Chiou SY, Hsia CY, Tsay SH. Splenic hamartoma: presentation on contrast-enhanced sonography. J Clin Ultrasound. 2004;32:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Iozzo RV, Haas JE, Chard RL. Symptomatic splenic hemartoma: a report of two cases and review of the literature. Pediatrics. 1980;66:261-265. [PubMed] |
| 6. | Hayes TC, Britton HA, Mewborne EB, Troyer DA, Saldivar VA, Ratner IA. Symptomatic splenic hamartoma: case report and literature review. Pediatrics. 1998;101:E10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Jia HB, Li YP, Han DE, Liu Y, Zhang B, Wu DQ, Chen X, Jiang Y, Zheng LX, Du JR. Splenic hamartoma: case report and review of literature. Chin Med J (Engl). 2006;119:1403-1408. [PubMed] |
| 8. | Abramowsky C, Alvarado C, Wyly JB, Ricketts R. “Hamartoma” of the spleen (splenoma) in children. Pediatr Dev Pathol. 2004;7:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Wirbel RJ, Uhlig U, Futterer KM. Case report: splenic hamartoma with hematologic disorders. Am J Med Sci. 1996;311:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Warshauer DM, Hall HL. Solitary splenic lesions. Semin Ultrasound CT MR. 2006;27:370-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Yu RS, Zhang SZ, Hua JM. Imaging findings of splenic hamartoma. World J Gastroenterol. 2004;10:2613-2615. [PubMed] |
| 13. | Tsitouridis I, Michaelides M, Tsitouridis K, Davidis I, Efstratiou I. Symptomatic splenoma (hamartoma) of the spleen. A case report. Hippokratia. 2010;14:54-56. [PubMed] |
| 14. | Pisani Ceretti A, Bislenghi G, Virdis M, Maroni N, Gatti A, Opocher E. Laparoscopic splenectomy for splenic hamartoma: a case report. Case Rep Gastrointest Med. 2012;2012:435802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Garvin DF, King FM. Cysts and nonlymphomatous tumors of the spleen. Pathol Annu. 1981;16 Pt 1:61-80. [PubMed] |
| 16. | Conlon S, Royston D, Murphy P. Splenic hamartoma. Cytopathology. 2007;18:200-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Basso SM, Sulfaro S, Marzano B, Fanti G, Chiara GB, Lumachi F. Incidentally discovered asymptomatic splenic hamartoma with rapidly expansive growth: a case report. In Vivo. 2012;26:1049-1052. [PubMed] |
| 18. | Lee SH. Fine-needle aspiration cytology of splenic hamartoma. Diagn Cytopathol. 2003;28:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |