Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10585
Revised: March 3, 2014
Accepted: May 12, 2014
Published online: August 14, 2014
Processing time: 237 Days and 17.8 Hours
AIM: To study the diagnostic value of controlled attenuation parameter (CAP), evaluated by transient elastography, for liver steatosis in patients with chronic hepatitis B (CHB).
METHODS: Eighty-eight patients with CHB were enrolled in this study. All of the patients were subjected to transient elastography to determine CAP. These patients also underwent liver biopsy in the same period. Using liver biopsy as a reference, we determined receiver operating characteristic (ROC) curves for different endpoints. Areas under the ROC curves (AUCs) were used to evaluate the diagnostic importance of CAP for liver steatosis in patients with CHB.
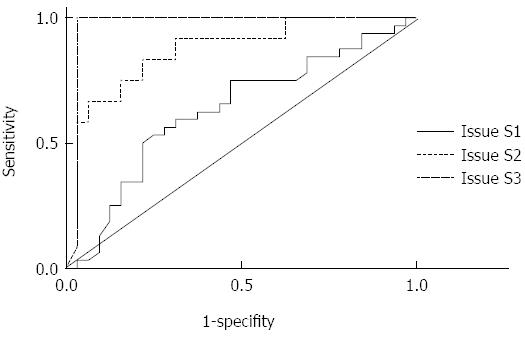
RESULTS: A positive correlation was observed between the AUCs of CAP and liver pathological stage (r = 0.582, P < 0.05). CAP was not correlated with inflammation degree and fibrosis degree (r = -0.025, P > 0.05; r = 0. 068, P > 0.05). The mean CAP value at S0 was 209.59 ± 41.25 dB/m, 223.84 ± 35.28 dB/m at S1, 274.17 ± 43.69 dB/m at S2, and 312.50 ± 25.44 dB/m at S3. CAP values among S0, S1, S2, and S3 were significantly different (F = 17.79, P < 0.01). The AUC values for CAP were 0.711 (0.592-0.870), 0.868 (0.748-0.989), and 0.974 (0.922-1.026) for S1, S2, and S3, respectively. The optimal cut-off values were 219.5, 230.0, and 283.5 dB/m.
CONCLUSION: CAP is a novel tool that can be used to assess the degree of steatosis.
Core tip: In recent years, numerous patients suffer from steatosis combined with hepatitis B virus infection. Hence, the amount of fat in the liver of these patients should be evaluated. This study aimed to investigate the diagnostic importance of controlled attenuation parameter, evaluated by transient elastography, for liver steatosis in patients with chronic hepatitis B.
- Citation: Wang CY, Lu W, Hu DS, Wang GD, Cheng XJ. Diagnostic value of controlled attenuation parameter for liver steatosis in patients with chronic hepatitis B. World J Gastroenterol 2014; 20(30): 10585-10590
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10585.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10585
Steatosis results from the accumulation of fatty droplets in the liver cells and can be a result of several causes such as alcohol consumption, viral hepatitis and metabolic dysfunction (obesity, type 2 diabetes, hyperglycemia, hypertriglyceridemia)[1]. Steatosis is a reversible and benign condition[2]. However, in many cases, steatosis can be associated with inflammation (steatohepatitis), which may result in liver fibrosis and might progress to cirrhosis, liver failure or hepatocellular carcinoma[1].
In recent years, numerous patients suffer from steatosis combined with hepatitis B virus infection. Hence, the amount of fat in the liver of these patients should be evaluated.
Liver biopsy has been regarded as the gold standard for steatosis assessment. However, liver biopsy has a potential sampling error, is an invasive and often painful procedure and can result in severe complications. Therefore, the procedure is accepted by patients with some reluctance. Furthermore, it can only be applied in selected subjects and not readily repeated for the follow-up of patients.
To evaluate hepatic steatosis degree, medical practitioners employ advanced transient elastography as an optimal technique. Transient elastography is also a non-invasive, painless, rapid, simple, and objective quantitative detection method. This technique can be used to determine controlled attenuation parameter (CAP) to evaluate the degree of hepatic steatosis. This parameter is based on the ultrasonic properties of the radiofrequency back-propagated signals acquired by the Fibroscan.
Therefore, this study applied transient elastography to determine CAP and evaluate the degree of hepatic steatosis among 88 patients treated at our hospital.
A total of 88 patients with chronic hepatitis B (CHB) treated at Tianjin Second People’s Hospital from August 2012 to December 2012 were selected as our research subjects. The diagnostic criteria were based on the Guideline of Prevention and Treatment for Chronic Hepatitis B (version 2010)[3]. Among these patients, 62 were male and 26 were female, with ages ranging from 15 years to 67 years (an average of 38.32 ± 12.99 years). The duration of positive hepatitis B surface antigen and hepatitis B virus (HBV)-DNA was at least six months. Alanine aminotransferase exacerbated or stimulated another onset of abnormality. The detected results for other hepadnaviruses were negative. At the same stage, hepatic pathological examination and CAP detection were conducted.
The detection method was based on the transient elastography user’s manual. The decision points were positioned between the seventh and eighth ribs or between the eighth and ninth ribs from the right anterior axillary line to the midaxillary line. After 10 consecutive valid detections, median was selected as the ultimate measurement result and expressed using CAP (dB/m). The success rate of ultimate detection was required to exceed 60%, and the interquartile range should be less than 1/3 of the median.
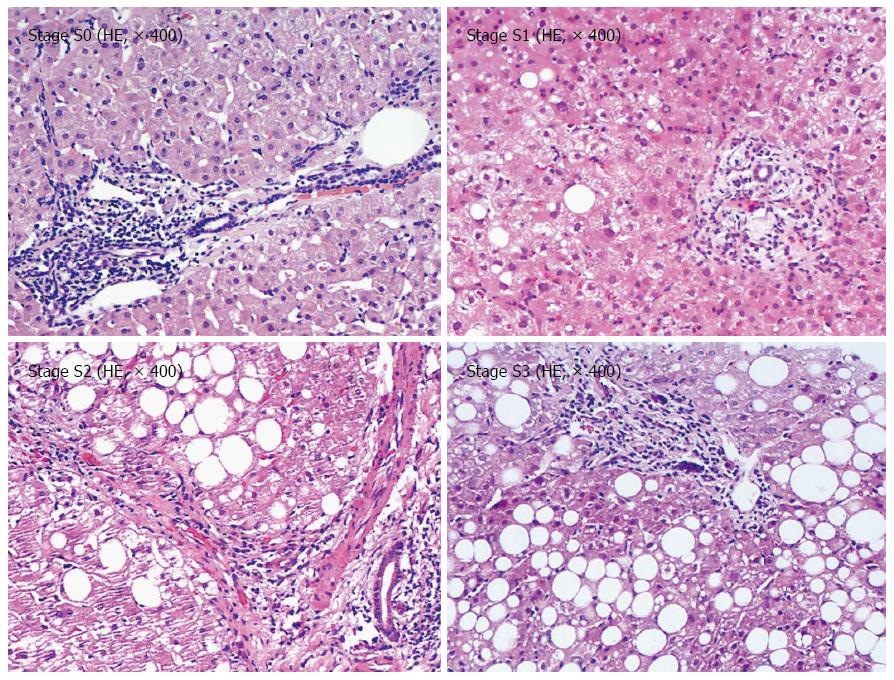
Liver biopsy specimens ≥ 1.5 cm in length were obtained. Liver biopsy specimens were fixed using 10% neutral formalin and embedded in paraffin. Hepatic steatosis was quantified as follows: S0: liver fat content/liver wet weight ratio ≤ 10%; S1: 11% to 33%; S2: 34% to 66%; and S3: 67% to 100%. Two pathologists then independently read the films by using a blind method. If they reached different diagnosis results, the films were read again to reach a consensus.
Statistical analyses were carried out using data analysis software STATISTICA version 8.0 (Statsoft Inc., United States). Using positive results as a diagnostic criterion, we illustrated a receiver operating characteristic (ROC) curve. Linear correlations between CAP index and hepatic steatosis stage, inflammation degree, and fibrosis degree were evaluated with the Pearson correlation coefficient (r). The correlation was evaluated based on Spearman’s coefficient. P-values < 0.05 were considered statistically significant.
Biochemical parameters for the 88 patients are shown in Table 1.
| Parameter | Value |
| Alanine transaminase (U/L) | 61.8 ± 37.3 |
| Aspartate aminotransferase (U/L) | 73.6 ± 40.1 |
| Albumin (g/L) | 40.5 ± 22.8 |
| Total bilirubin (μmol/L) | 16.4 ± 7.8 |
| Direct bilirubin (μmol/L) | 9.4 ± 5.4 |
| γ-glutamyl transpeptadase (U/L) | 102.6 ± 65.3 |
| Alkaline phosphatase (U/L) | 99.8 ± 49.2 |
| Body mass index (kg/m2) | 24.16 ± 4.97 |
| Triglyceride (mmol/L) | 1.8 ± 1.0 |
| Total cholesterol (mmol/L) | 5.0 ± 1.7 |
Among the 88 patients, the following cases were obtained (Figure 1): 32 at the steatosis stage S0 (36.36%); 32 at stage S1 (36.36%); 16 at stage S2 (19.05%); and eight cases at stage S3 (9. 09%).
CAP values for the 88 patients varied from 124 dB/m to 349 dB/m, with an average of 232.13 ± 48.82 dB/m. The mean CAP value at S0 was 209.59 ± 41.25 dB/m, 223.84 ± 35.28 dB/m at S1, 274.17 ± 43.69 dB/m at S2, and 312.50 ± 25.44 dB/m at S3. CAP showed an increasing trend from S0 to S4. The differences among the four stages showed statistical significance (F = 17.79, P < 0. 01).
CAP was positively correlated with hepatic steatosis (r = 0.582, P < 0.05), but was not correlated with inflammation degree or fibrosis degree (r = -0. 025, P > 0. 05; r = 0. 068, P > 0. 05) (Table 2).
| CAP | Hepatic steatosisdegree | Hepatic inflammation degree | Hepatic fibrosisdegree |
| r | 0.582 | -0.025 | 0.068 |
| P value | < 0.05 | > 0.05 | > 0.05 |
The areas under the curves (AUCs) for CAP at stages S1, S2, and S3 were 0.711 (0.592 to 0. 870), 0.868 (0.748 to 0.989), and 0.974 (0.922 to 1.026), respectively. The optimal cut-off points were selected according to the ROC curves (Table 3 and Figure 2).
| Decision point | No. | Optimal cut-off point | Sensitivity | Specificity | NPV | PPV | Degree of accuracy |
| S1 | 32 | 219.5 | 0.694 | 0.718 | 71.7% | 63.3% | 69.5% |
| S2 | 16 | 230.0 | 0.833 | 0.781 | 89.3% | 65.0% | 77.3% |
| S3 | 8 | 283.5 | 1 | 0.969 | 100% | 85.7% | 97.4% |
In previous studies, approximately 27% to 51% of the total number of patients with CHB suffered from hepatic steatosis[4,5]. However, the morbidity of nonalcoholic fatty liver disease increased in years, thereby increasing the number of patients with hepatic steatosis and CHB. Current studies consider that hepatic steatosis is closely related to the inflammation degree. Multivariate analysis has shown that the advancement of hepatic steatosis is an independent factor related to hepatic fibrosis development. In general, the improvement of hepatic steatosis occurs simultaneously with the reduction of the fibrosis degree[6]. Other studies have demonstrated that hepatic steatosis elicits specific effects on the progress of fibrosis in patients with CHB[7]. Hence, the evaluation of steatosis degree among patients with hepatitis B virus infection is considered an important method to facilitate treatment and determine postoperative effects and treatment efficacy.
For healthy people, intrahepatic fat accounts for 2% to 4% of the liver weight. If fat weight (mainly triglycerides) exceeds 5% of the total liver weight or > 5% of cells in hepatic tissues exhibit fat deposition, the condition is considered as hepatic steatosis[8]. The causes of hepatic steatosis are generally correlated with alcoholism, viral hepatitis, or metabolic syndrome. The prevalence rate has reached 16% to 31%, 50.9%, 60% to 100%, and 86% to 96% in healthy individuals, patients with chronic hepatitis C, alcoholic individuals, and individuals with severe obesity, respectively[9-13]. Although pure steatosis is benign and reversible, the incidence of hepatic steatosis is often observed with other hepatotoxic factors; as a result, oxidative stress and hepatic cell apoptosis occur. In addition, hepatic steatosis is closely related to the progress of inflammation and fibrosis. These factors influence the postoperative effects in hepatic steatosis at different degrees[14]. Hence, the precise quantification of hepatic steatosis degree and dynamic monitoring are of great importance.
At present, liver pathological examination is the main method used to evaluate hepatic steatosis. However, liver pathological examination is invasive and shows several disadvantages, such as sampling error and complications[15,16].
Moreover, current non-invasive tests, such as serum markers and imaging technology, exhibit some limitations. Different combinations of serum biochemical markers can be used to evaluate hepatic steatosis degree. Such methods include steato test, fatty liver index, and hepatic steatosis index. In addition, indicators are available to evaluate nonalcoholic steatohepatitis. However, the effectiveness of similar non-invasive indexes in clinical diagnosis should be further improved, and these indexes are less feasible in clinical practice.
Other methods, such as ultrasound, computed tomography (CT), and magnetic resonance imaging, can be applied to diagnose fatty liver and evaluate hepatic steatosis degree[17,18]. Although ultrasound is widely used in clinical diagnosis of fatty liver because this technique entails low costs and exhibits easy operation, ultrasound can only detect hepatic steatosis greater than 30%. This technique also relies greatly on operators and devices. By comparison, CT can be performed to assess the occurrence of hepatic steatosis based on decreased hepatic density. However, hepatic density is possibly influenced by Fe content in the liver. Intrahepatic iron deposition is commonly observed in patients with adrenoleukodystrophy disease, non-alcoholic fatty liver disease, or chronic hepatitis C. Meanwhile, CT is radioactive, which limits the clinical diagnosis of fatty liver[19]. The recently developed magnetic resonance mass spectrometry (MRS) is relatively promising; however, MRS is expensive and not an exclusive targeting technique in the diagnosis of fatty liver. A great amount of clinical experiments are needed to validate results obtained using this technique.
In clinical practice, CAP is determined by transient elastography for the non-invasive evaluation of hepatic steatosis degree[8,20-23]; transient elastography can also be performed to diagnose hepatic steatosis and assess patients with this condition during their follow-up visits. Fibroscan based on vibration-controlled transient elastography is applied on the basis of the significant attenuation of ultrasound generated and transmitted in the media.
CAP is expressed as dB/m when ultrasonic frequency is fixed. CAP is a newly re-defined parameter based on Fibroscan. Considering that CAP is positively correlated with hepatic steatosis degree, we can use this parameter to detect the degree of hepatic steatosis quantitatively in the human body. In theory, the volume measured by CAP is 100 times that of the tissue volume of liver biopsy. CAP detection shows higher sensitivity, which can distinguish hepatic steatosis greater than 10%, than ultrasound and other imageological examinations. This technique greatly improved the detection rate of hepatic steatosis and provided possibilities of early diagnosis, early treatment, and prevention of hepatic steatosis. In addition, this method can be used to monitor and evaluate the condition of diseases at different stages. Transient elastography is non-invasive and can quantitatively evaluate hepatic steatosis. This technique also requires minimum operator dependence and does not emit radiation. Transient elastography is also appropriate for detection and follow-up visits.
The findings of our study demonstrated that (1) CAP was consistent with the pathological results of liver biopsy and can be used to detect fatty liver and identify fatty liver degree (P < 0.05). CAP index showed statistical significance in the qualitative diagnosis of fatty liver and staging of fatty liver (P < 0.01). CAP is also important for the early diagnosis of steatosis; (2) AUC values for CAP were 0.711 (0.592 to 0.870), 0.868 (0.748 to 0.989), and 0.974 (0.922 to 1.026) for S1, S2, and S3, respectively. According to the maximum sum of sensitivity and specificity, the obtained optimal cut-off values were 219.5, 230.0, and 283.5 dB/m. Similar to the related findings of Sasso[21], the results of the present study suggested that CAP could accurately diagnose intermediate and severe hepatic steatosis. Our results could be extensively used to preliminarily exclude patients with severe hepatic steatosis in clinical practice; (3) The negative predictive values for stages S1, S2, and S3 were relatively higher, indicating that CAP is an optimal tool that can be used to screen steatosis; and (4) The study also revealed that CAP values were irrelevant in liver inflammation and fibrosis rate. CAP is a stable and independent tool that can be used to predict hepatic steatosis.
In conclusion, CAP detection is a highly effective method to evaluate the steatosis rate in patients with hepatitis B virus infection. For patients who suffer from mild steatosis with hepatitis B virus infection, the accuracy rate of diagnosis based on CAP measurement was relatively lower. In clinical practice, CAP can be integrated with other techniques used to detect steatosis to improve the accuracy rate of diagnosis. Liver pathological examination should be conducted if necessary.
The authors would like to thank Xiao-Fen Yue and Jia-Fen Zhao, who collected the data or performed Fibroscan examinations. We would like to thank Ming Luo for his help with the statistical analysis.
In recent years, numerous patients suffer from steatosis combined with hepatitis B virus infection. Hence, the amount of fat in the liver of these patients should be evaluated. As the gold standard for steatosis assessment, liver biopsy has a potential sampling error, is an invasive and often painful procedure and can result in severe complications. Furthermore, it cannot readily repeated for the follow-up of patients. Transient elastography is a non-invasive, painless, rapid, simple, and objective quantitative detection method.
At present, controlled attenuation parameter (CAP) can be mostly applied to assess the degree of liver steatosis in patients with fatty liver. Thus, this study aimed to investigate the diagnostic importance of CAP, evaluated by transient elastography, for liver steatosis in patients with chronic hepatitis B.
To evaluate hepatic steatosis degree, medical practitioners employ advanced transient elastography as an optimal technique. This technique can be used to determine CAP to evaluate the degree of hepatic steatosis. Transient elastography is also a non-invasive, painless, rapid, simple, and objective quantitative detection method. At present, the study on assessment of liver steatosis degree in patients with chronic hepatitis B by transient elastography is less, and the authors applied transient elastography to determine CAP and evaluate the degree of hepatic steatosis among patients with chronic hepatitis B.
Transient elastography is a non-invasive, painless, rapid, simple, and objective quantitative detection method, and it is a highly effective method to evaluate the steatosis rate in patients with hepatitis B virus infection. It will be widely used in clinical application.
The authors demonstrated that CAP detection is a highly effective method to evaluate the steatosis rate in patients with hepatitis B virus infection. This study is interesting and worthy to be published.
P- Reviewer: Ozenirler S, Tovo CV, Zhang SJ S- Editor: Gou SX L- Editor: Wang TQ E- Editor: Liu XM
| 1. | Farrell GC. Fatty liver disease: NASH and related disorders. Malden (MA): Blackwell Publishing 2004; . |
| 2. | Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 635] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 3. | The guideline of prevention and treatment for chronic hepatitis B (Version 2010). Zhonghua Shiyan He Linchuang Ganranbing Zazhi. 29:65-80. |
| 4. | Lefkowitch JH, Schiff ER, Davis GL, Perrillo RP, Lindsay K, Bodenheimer HC, Balart LA, Ortego TJ, Payne J, Dienstag JL. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. The Hepatitis Interventional Therapy Group. Gastroenterology. 1993;104:595-603. [PubMed] |
| 5. | Czaja AJ, Carpenter HA. Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology. 1993;105:1824-1832. [PubMed] |
| 6. | Shi JP, Fan JG. Advances in researches on relationship of steatosis with hepatitis B virus infection. Guoji Xiaohuabing Zazhi. 2008;2:100-102. |
| 7. | Fassio E, Alvarez E, Domínguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820-826. [PubMed] |
| 8. | Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 9. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2695] [Article Influence: 128.3] [Reference Citation Analysis (3)] |
| 10. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 863] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 11. | Sussman S, Dent CW, Skara S, de Calice P, Tsukamoto H. Alcoholic liver disease (ALD): a new domain for prevention efforts. Subst Use Misuse. 2002;37:1887-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 13. | Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399-408. [PubMed] |
| 14. | Powell EE, Jonsson JR, Clouston AD. Steatosis: co-factor in other liver diseases. Hepatology. 2005;42:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 16. | Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45 Suppl 4:IV1-IV11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 18. | Mancini M, Prinster A, Annuzzi G, Liuzzi R, Giacco R, Medagli C, Cremone M, Clemente G, Maurea S, Riccardi G. Sonographic hepatic-renal ratio as indicator of hepatic steatosis: comparison with (1)H magnetic resonance spectroscopy. Metabolism. 2009;58:1724-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Wallace DF, Subramaniam VN. Co-factors in liver disease: the role of HFE-related hereditary hemochromatosis and iron. Biochim Biophys Acta. 2009;1790:663-670. [PubMed] |
| 20. | Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, Duarte-Rojo A, Wong D, Crotty P, Elkashab M. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 22. | Boursier J, Calès P. Controlled attenuation parameter (CAP): a new device for fast evaluation of liver fat? Liver Int. 2012;32:875-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | de Lédinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |