Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10553
Revised: February 8, 2014
Accepted: March 8, 2014
Published online: August 14, 2014
Processing time: 248 Days and 16.1 Hours
AIM: To test the ability of adult-derived human liver stem/progenitor cells (ADHLSC) from large scale cultures to conjugate bilirubin in vitro and in bilirubin conjugation deficient rat.
METHODS: ADHLSC from large scale cultures were tested for their phenotype and for their capacity to conjugate bilirubin in vitro after hepatogenic differentiation. In vivo, Gunn rats [uridine diphosphate-glucuronosyltransferase 1A1 (UGT1A1) deficient animal] were injected with ADHLSC and cryopreserved hepatocytes (positive control). Two, 4, 13 and 27 wk post-transplantation, transplanted Gunn rat bilirubin serum levels were determined by high performance liquid chromatography. Human transplanted cell engraftment was assessed 27 wk post-transplantation using immunohistochemistry and RTqPCR.
RESULTS: Large scale culture conditions do not modify ADHLSC phenotype, ADHLSC were able to specifically conjugate bilirubin. ADHLSC were intraportally injected into Gunn rats and blood UCB was measured at different times post-transplantation, infused-Gunn rats exhibited a metabolic effect 3 mo post-transplantation and maintained over a 6 mo period. ADHLSC engraftment into Gunn rat’s liver was demonstrated by RTqPCR and immunohistochemistry against albumin and UGT1A1.
CONCLUSION: ADHLSC from large scale cultures are efficient in conjugating bilirubin in vitro and in restoring a deficient metabolic function (reducing bilirubin level) in hyperbilirubinemic rats.
Core tip: In this study we demonstrated the ability of adult-derived human liver stem/progenitor cells (ADHLSC) to specifically conjugate bilirubin after intraportal injection into Gunn rats a model presenting a deficient bilirubin conjugation function (homologous to human Crigler-Najjar type I syndrome). Infused-Gunn rats exhibited a metabolic effect 3 mo post-transplantation and maintained over a 6 mo period. ADHLSC engraftment into Gunn rat’s liver was demonstrated by immunohistochemistry and RTqPCR. These results suggest the potential of ADHLSC to restore a deficient metabolic function in situ.
- Citation: Maerckx C, Tondreau T, Berardis S, Pelt JV, Najimi M, Sokal E. Human liver stem/progenitor cells decrease serum bilirubin in hyperbilirubinemic Gunn rat. World J Gastroenterol 2014; 20(30): 10553-10563
- URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10553.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10553
Bilirubin is a toxic derivative of the protoporphyrin moiety of haem proteins such as haemoglobin. For its clearance from serum, bilirubin is conjugated in hepatocytes with glucuronic acid thanks to uridine diphosphate-glucuronosyltransferase 1A1 (UGT1A1) activity.
Genetic variations in the gene encoding UGT1A1 lead to complete or partial inactivation of the bilirubin glucuronidation, which causes unconjugated bilirubin accumulation in the serum. This can result in hyperbilirubinemic conditions such as the human Crigler-Najjar syndrome type I and II.
Gunn rat, derived from Wistar parent strain, has constantly elevated concentrations of serum bilirubin which causes unconjugated hyperbilirubinaemia. Gunn rat inherently lacks all glucuronidation activities catalysed by the UGT1 isoforms and is therefore used as animal model for Crigler-Najjar syndrome type I[1,2].
Clinically, Crigler-Najjar syndrome type I is associated with progressive damage of the central nervous system leading to bilirubin encephalopathy (kerincterus)[3-5]. The current treatment induces biliary clearance of bilirubin by photoactivation under light exposure[6]. Unfortunately the treatment becomes inefficient due to progressive skin thickening and hence is insufficient to prevent brain damage. So far, orthotopic or auxiliary liver transplantation remains the only cure[7,8]. However, donor shortage, life-long immunosuppression to prevent rejection and risks related to surgery constitute major hurdles to an extensive use of this treatment[9].
As a mean to circumvent complications associated with organ transplantation, liver cell transplantation (LCT) has emerged as a new strategy to treat patients with chronic and acute liver disease and to restore metabolic liver functions in inherited liver disorders[10-16]. Hepatocyte transplantation has namely been used for the treatment of Crigler-Najjar syndrome type I. The first demonstration of its efficacy in Crigler-Najjar type I patient was provided by Fox et al[15]; the effect was a significant decrease of bilirubin levels for up to 11 mo. We and others[17] reported four additional patients in which the reduction of serum bilirubin reached up to 50% over a follow-up of 7 mo. LCT is confronted to limited availability of human hepatocytes, their incapacity to proliferate in vitro and their poor resistance to cryopreservation which prevent their wide use in the clinic[18,19]. In addition, mature hepatocytes do not provide long term support as these mature cells have lost their repopulation capacity.
Therefore researchers have turned towards adult stem/progenitor cells as alternative cell sources more suitable for regenerative medicine and more likely to integrate the regenerative niche and provide long term allogeneic cell renewal. Adult stem or progenitor cells which are resident in mature organs and tissues are rather tissue specific and lineage restricted. They have either the self-renewal capacity to proliferate indefinitely (stem cells) or display a limited proliferation potential as their daughter cells spontaneously differentiate into mature cells (progenitor cells) but altogether have advantages to proliferate in vitro and to resist to cryopreservation.
Our group[20] has isolated adult stem/progenitor cells from adult human liver (ADHLSC). These cells express mesenchymal (CD29, CD73, CD90, α-smooth muscle actin, Vimentin) and hepatic markers (albumin, Multidrug resistance-associated protein 2 (MRP2), Hepatocyte Nuclear Factor 4 (HNF4), cytochrome P450 (CYP)1B1 and CYP3A4) but no biliary markers[20]. Moreover, these cells have the capacity to differentiate in hepatocyte-like cells under selective culture conditions and are not only able to engraft into the liver of immunodeficient mice and remained stable up to 60 d post-transplantation but to differentiated in situ and participate to liver regeneration after hepatectomy stimuli[20,22].
This study demonstrates that ADHLSC can be cultivated in large scale conditions without phenotype alterations and provides the proof of concept that ADHLSC transplantation can reverse hyperbilirubinemic symptoms in a relevant animal model of Crigler-Najjar disease. We demonstrate here the ability of ADHLSC to engraft in recipient rat livers where they participate in restoration of liver function by a significant reduction of the serum bilirubin levels. Our findings support ADHLSC as a promising candidate for liver cell-based therapy developments.
All experiments using human material in the present study were done under the approval of the Institution Ethical committee and donor informed consent.
Hepatocytes were recovered post mortem (cerebral hemorrhage) from a healthy 11 years old male donor after 2 steps collagenase perfusion as described by Seglen[23]. The procedure was performed within the tissue bank of the hospital. Cells were tested negative for microbiological, viral and mycoplasma contaminations. Viable isolated hepatocytes, 20 millions, (79%, trypan blue exclusion), were seeded onto Cell Bind 175 cm² flasks (Corning, Lasne, Belgium) in Williams’E medium (Invitrogen, Merelbeke, Belgium) supplemented with 10% fetal bovine serum (FBS) (AE scientific, Marcq, Belgium), 10 μg/mL human insulin (Lilly, Brussels, Belgium), 1 μmol/L dexamethasone (Sigma, Bronem, Belgium), and 1% penicillin/streptomycin (P/S) (Invitrogen) at 37 °C in a fully humidified atmosphere containing 5% CO2 according to Najimi et al[20] with some modifications. On days 7-12 hepatocytes died and small colonies emerged and proliferated. At this time, culture medium was switched to complete DMEM medium (DMEM high glucose with 10% FBS and 1% P/S) in order to accelerate the emerging cells proliferation. When cell cultures reached 90% confluence, cells were trypsinized with 0.05% trypsin-1 mmol/L EDTA solution (Invitrogen) and replated on Cell Bind flasks at a density of 5 × 103 cells/cm2. Large scale culture using Cell Stack 10 (6360 cm²) (Corning) was carried out in clean-room facility.
According to Najimi et al[20], hepato-mesenchymal character was analyzed by FACS and immunocytochemistry using CD29, CD44, CD73, CD90, Vimentin, α-smooth muscle actin (ASMA), Albumin and Pan-CK antibodies (BD, Erembodegem, Belgium). In order to assess the homogeneity and to exclude hematopoietic contamination CD45 marker was also controlled as well as stem cell markers CD117 and CD133. Karyotype analysis as described by Scheers et al[24] showed no structural or numerical chromosomal aberrations.
Hepatic differentiation has been performed on ADHLSC at different passages as described by Najimi et al[20] with some modifications. Briefly, cells were seeded at 104 cells/cm2 in 6-well plates in expansion medium for 48 h. Differentiation consisted of 4 steps protocol lasting 32 d: Culture medium was replaced by IMDM (Invitrogen) containing 1% PS, 20 ng/mL epithelial growth factor (Peprotech, London United Kingdom) and 10 ng/mL basic fibroblast growth factor (bFGF; Peprotech) for 48 hours (1 step). During step 2, cells were cultured in IMDM supplemented with 1% PS, 20 ng/mL hepatocyte growth factor (Peprotech), 10 ng/mL bFGF, 0.61 g/L nicotinamide (Sigma) and 1% insulin-transferrin-selenium premix (ITS; Invitrogen) for 10 d. In 3rd step, bFGF was replaced by 20 ng/mL oncostatin M (OSM; Peprotech) for 10 additional days. Maturation step was completed by changing the medium to IMDM containing 1% PS, 20 ng/mL OSM, 1 μmol/L dexamethasone (Sigma) and 1% ITS for 10 d.
The undifferentiated cells (control) were kept during the whole process of differentiation in IMDM supplemented with 1% PS and 2% FBS. Medium was changed twice a week.
In vitro differentiation was controlled at morphological level, by CYP3A4 activity (Promega, Madison, Wisconsin) and by the ability to specifically conjugate bilirubin as described below.
6 × 105 undifferentiated and differentiated ADHLSC were seeded per 10 cm² collagen coated well and incubated with William’s medium, 1% FBS, containing 100 μmol/L (= 5.84 mg/dL) unconjugated bilirubin (UCB) (Sigma) for 24 h and 48 h. Supernatant was harvested at the end of incubation period and mixed with 2 μg/mL Xantobilirubinic acid used as internal standard (IS). The reaction product was submitted to an alkaline methanolysis followed by nitrogen evaporation as described by Muraca et al[25]. Precipitates were resuspended with chloroform and dimethyl sulfoxide (Sigma). The solution was injected into the liquid chromatograph [Waters 515 high-performance liquid chromatography (HPLC) pump] for separation on C18 column (Macherey-Nagel, Düren, Germany). Elutriation flow started with methanol/water/tetrabutylammonium (solvent A) and ended after 11 min with methanol/ethanol/water/tetrabutylammonium (solvent B). The absorbance was monitored at 436 nm using a 996 photodiode array detector (Waters, Zellik, Belgium) and the area under peak was integrated electronically with Millennium software (Waters, Zellik, Belgium). The concentration, in micromoles per liter, of each bilirubin fraction in samples was calculated as follow: (areapigment/areaIS) × (IS/SV) × RF, in which IS corresponds to micrograms of IS added to the sample, SV to the volume of sample (mL), and RF to the response factor. Conjugation rate (CR) was evaluated as follow: [(Conjugated bilirubin concentration)/(Total bilirubin concentration)] × 100, in which total bilirubin concentration is the sum of unconjugated and conjugated bilirubin.
Animal procedures were conducted according to the guidelines established by the Université Catholique de Louvain, in accordance with European Union Regulation and approval of the protocols by the local animal ethical and welfare committees (UCL/MD/2009/003). In order to confirm serum UCB level stability 11 naive Gunn rats were followed over 6 mo. Non-differentiated ADHLSC cultured on Cell-Bind Corning plastic in clean-room facility were detached using 0.05% trypsin-EDTA (Invitrogen) solution. ADHLSC used in this study were obtained from passages 4-6. After centrifugation cells were washed in PBS and recovered in PBS containing 4% N-acetylcystein (Lysomucyl, Zambon, Jette, Belgium). Cryopreserved hepatocytes, used as positive control were thawed in clean room, centrifuged, washed and also resuspended in PBS-4% N-acetylcystein suspension. 2.5 × 106 millions ADHLSC (n = 5 rats) or hepatocytes (n = 3 rats) were injected into the portal vein of 9-11 wk-old male Gunn rats. This corresponds, for a 200 g rat, to an estimate of 0.25% of his total liver cell mass of 5 billion cells/kg body weight[15]. In order to limit cell rejection, rats were injected every 2 d with immunosuppressor medication: cyclosporine A (Sandimmum, Novartis, Vilvoorde, Belgium) at a dose of 5 mg/kg of body weight. Two, 4, 13 and 27 wk post-transplantation, transplanted Gunn rat UCB serum levels were determined by High performance liquid chromatography (HLPC) as described above. Human cell engraftment of transplanted cells was assessed 27 wk post-transplantation. Liver was then recovered, fixed in formaldehyde 3.5% (VWR International, Leuven, Belgium) and then embedded in paraffin for immunohistochemistry assessment of the engrafted cells.
Five μm liver sections were deparaffinized and rehydrated in xylene and graded alcohol series. Antigen retrieval was performed by incubating the sections in citric acid monohydrate solution (pH 6.0). Cells were permeabilized by incubating the sections into 0.1% triton X-100. Non-specific immunostaining was prevented by incubation in PBS buffer containing 1.5% normal goat serum at room temperature (RT). Sections were incubated overnight with anti-human Albumin (Calbiochem, United Kingdom) (1/1000) or anti-human UGT1A1 (Santa Cruz biotechnology, Heidelberg, Germany) (1/100), in 2% normal goat serum at 4 °C. Staining detection was visualized with 1/500 AlexaFluor 594 anti-rabbit antibody solution (Invitrogen) incubation at RT. The nuclei were counterstained using DAPI (1/500). Slices were scanned using a Mirax digital slide system (Zeiss, Zaventem, Belgium) and analyzed thanks to Mirax viewer version 112220 (Zeiss). To determine cell repopulation, 10000 nuclei were counted over two randomly chosen area per section. Repopulation ratio was obtained as follow: (marked cells/total cells) × 100.
Cell culture: 6 × 105 control and in vitro differentiated cells were lyzed in Tripure followed by phenol chloroform extraction. The aqueous phase was recovered after centrifugation (11500 rpm) and total RNA precipitated with isopropanol (Sigma) and quantified using a nanodrop spectrophotometer (ThermoFisher, Erembodegem, Belgium). Two μg of total RNA was DNase-treated to eliminate genomic DNA and reverse transcribed using the Super Thermoscript kit (Invitrogen). Real time PCR was performed in 25 μL reaction with TaqMan® Master mix containing Taq DNA Polymerase (Applied Biosystems), 300 nmol/L concentration of forward and reverse primers (UGT1A and the house keeping gene: Cyclophilin A) and 5 μL of cDNA samples. The PCR reaction was performed using StepOnePlus Real Time instrument (Applied Biosystems). Time and temperature profile of the PCR reaction consisted of the following steps: 2 min at 50 °C, 10 min at 95 °C, followed by 40 step cycles of 15 s of denaturation at 95 °C followed by 1 min of annealing/elongation at 60 °C. For analysis, UGT1A Ct was normalized to Cyclophilin A (delta Ct) for both differentiated and undifferentiated status. The double delta Ct (ΔΔCt) is then calculated as the difference between delta Ct for differentiated and undifferentiated cells. Fold increase or relative quantification was obtained using the following formula: RQ = 2-ΔΔCt.
Rat liver analysis: Four mg liver tissue soaked in Tripure were disaggregated using tissue homogenizer (IKA Ultra-Turrax, Qlab, Vilvoorde, Belgium) and followed by phenol chloroform extraction. Total RNA was quantified using a nanodrop spectrophotometer. One μg of total RNA was DNase-treated and reverse transcribed using the Super Thermoscript™ kit (Invitrogen). Real time PCR was performed in 25 μL reaction with TaqMan® Master mix containing Taq DNA Polymerase (Applied Biosystems), 300 nmol/L concentration of forward and reverse primers (Human Albumin and Human GAPDH) and 5 μL of cDNA samples.
PCR reaction was performed using StepOnePlus Real Time instrument as described above.
Analyses were done in the GraphPad Prism software program (GraphPad, San Diego, California). Comparison for UCB mean between following times for a same group was analyzed using a paired t test. Means statistical analyses was carried out using Student test.
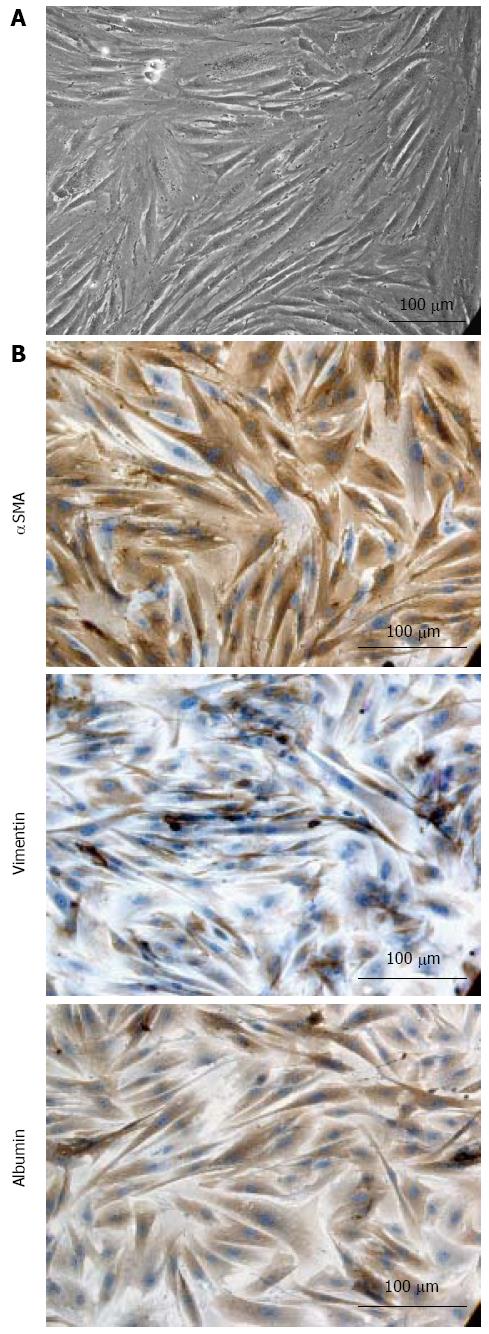
The parenchymal cell suspension fraction obtained after human liver dissociation with a viability of 79% was plated on Corning CellBind surface flasks. After 2 wk of culture in serum supplemented medium, ADHLSC with fibroblast-like morphology and prominent cytoplasm (Figure 1A) started to emerge. From the second passage, cells were cultivated in large culture conditions (CellStack-10 corresponding to a surface area of 6360 cm²) and phenotype of the growing cells was analyzed. Flow cytometry and immunocytochemistry confirmed that these cells shared the phenotype described previously for ADHLSC grown at smaller scale and on rat collagen[20]. At passage 2 already, cells were shown to be CD73, CD90, CD29 and CD44-positive. As expected the cells also expressed αSMA and vimentin, two additional markers of mesenchymal status, as demonstrated by immunocytochemistry (Figure 1B). In addition immunocytochemistry confirmed their immuno-positivity for albumin as evidence of the hepatic-lineage commitment of ADHLSC. Hematopoietic stem cell contamination was excluded based on the lack of expression of CD45, CD117 and CD133. As illustrated in Table 1, phenotypical characteristics were maintained at least until passage 6. These results confirmed that large scale culture provided cells displaying identical characteristics as the hepato-mesenchymal ADHLSC previously isolated and described.
| Surface marker | Positive cells |
| CD73 | 64.1% |
| CD90 | 94.1% |
| CD44 | 41.6% |
| CD29 | 87.4% |
| CD45 | 0.1% |
| CD117 | 0.7% |
| CD133 | 0% |
| Albumin | 61.4% |
| Pan CK | 1% |
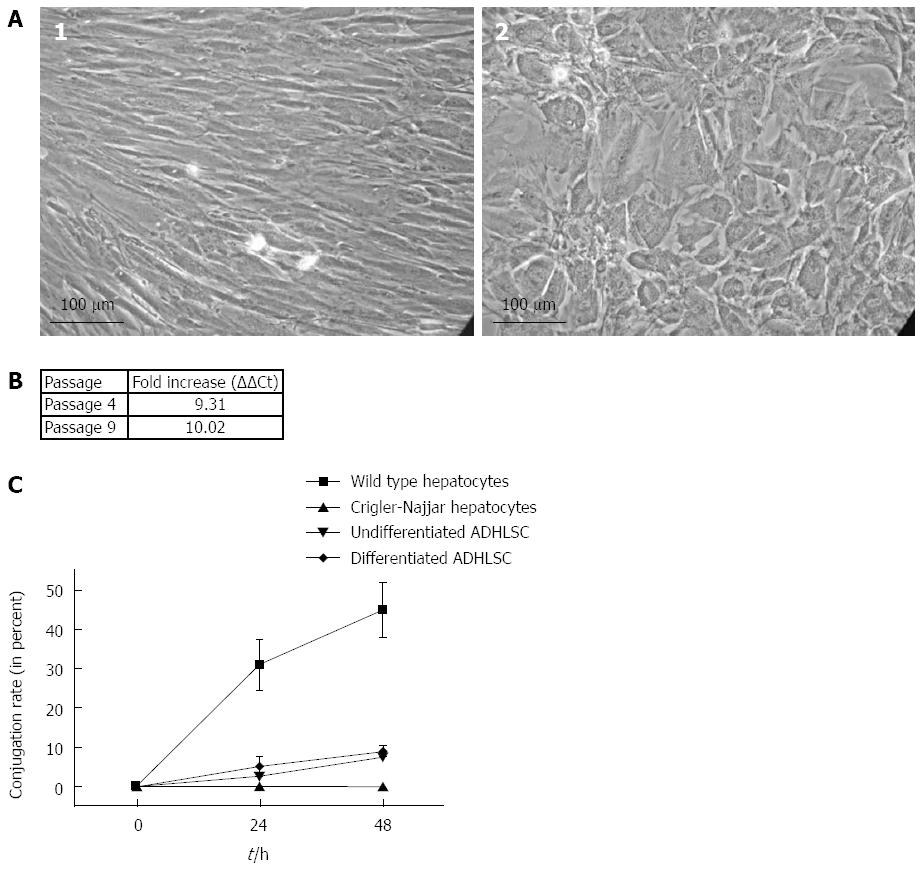
We evaluated the capacity of ADHLSC large scale cultured to differentiate into tissue-specific cell types. To do so, cells were cultured for 32 d in hepatocyte differentiation culture conditions as described by Najimi et al[20] with some modifications. At the end of the protocol, differentiated cells adopted an hepatocyte-like morphology as shown in Figure 2A in addition we noticed an 36 fold increase in CYP3A4 activity between undifferentiated and hepatogenic differentiated cells at passage 6 (data not shown).
In addition, we evaluated the ability of ADHLSC to express UGT1A mRNA and to conjugate bilirubin in vitro.
We assayed using QPCR technology, the expression of UGT1A mRNA constituted of the first 4 common exons of UGT1A family in total RNA extracts. Indeed, hepatogenic differentiation induced a 9.31 and 10.02 fold increase in UGT1A mRNA levels at passage 4 and 9 respectively (Figure 2B). Expression of UGT1A mRNA was constant between passage 4 and 9 (no significant difference between UGT1A fold increase was noted).Thereafter, we assessed the ability of differentiated ADHLSC to conjugate bilirubin. For that we incubated both undifferentiated and differentiated ADHLSC with 100 μmol/L UCB for 24 and 48 h after which supernatants were recovered to analyze the glucuronidation of bilirubin. We could determine that differentiated ADHLSC were able to specifically conjugate bilirubin as illustrated in Figure 2C. Undifferentiated ADHLSC exhibited a low bilirubin conjugation potential in vitro after 24 h (2.5% ± 0.4%) which increased with time reaching 7.4% ± 0.5% after 48 h. Conjugation rate of differentiated ADHLSC reached 5.1% ± 1.8% and 9.0% ± 1.1% after 24 h and 48 h respectively. Wild type hepatocytes used as positive control showed a conjugation percentage of 31.0% ± 6.6% and 45.0% ± 7.1% after 24 h and 48 h respectively. In contrast Crigler-Najjar hepatocytes, used as negative control were not able to conjugate bilirubin indicating that the activity of either undifferentiated or differentiated ADHLSC is partial as compared to mature hepatocytes activity (16% to 20% of hepatocytes activity after 48 h incubation) but highly significant.
Building on the capacity of ADHLSC to conjugate bilirubin in vitro, we investigated their potential benefit after transplantation in providing UGT1A1 activity in the Crigler-Najjar animal model, the Gunn Rat[26]. We performed transplantation of Gunn rats either by infusing 2.5 millions freshly trypsinized ADHLSC or cryopreserved and thawed hepatocytes into the portal vein of five and three rats respectively. Three additional control rats were subjected to surgery and received intraportal injection of vehicle only (4% NAC in PBS). Starting on day one after cell infusion, all rats received cyclosporine A via ip route once every two days. Rats were followed up for a total period of 27 wk with weekly weighing. Body weight of transplanted rats during the experiment was comparable to weight evolution of normal Gunn rats used as controls (2 wk post transplantation: 229 ± 9 g and 230 ± 6 g for transplanted and control rats respectively, 27 wk post transplantation: 399 ± 15 g for transplanted rats and 400 ± 1 g for control rats).
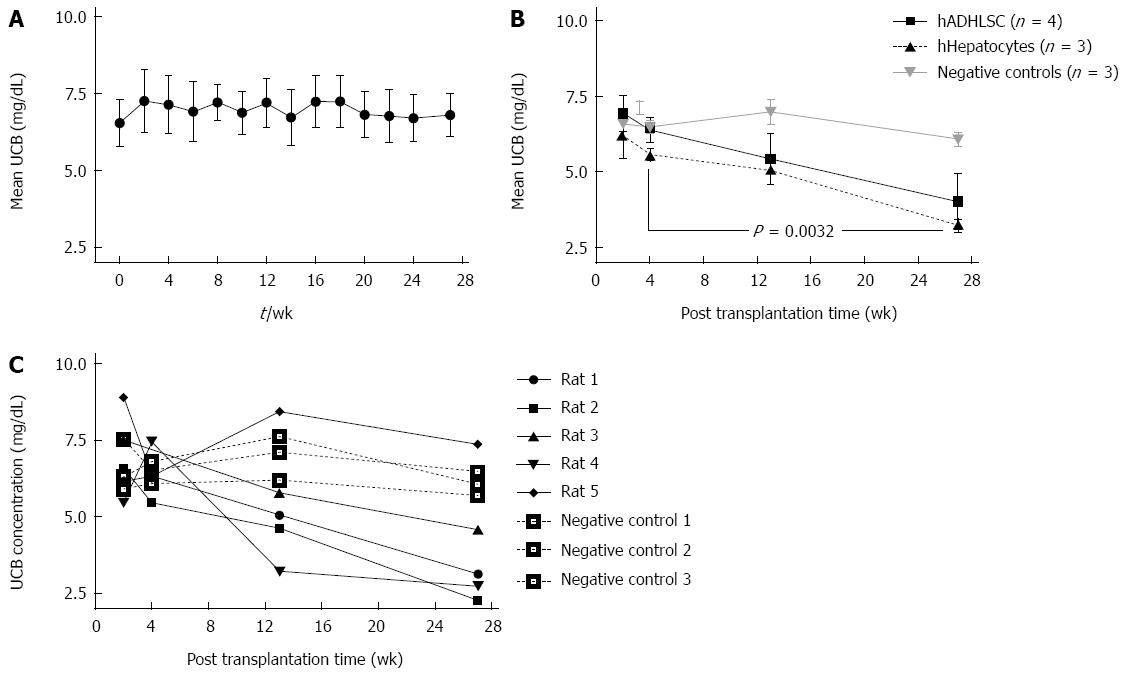
Blood drawings were used to measure serum UCB concentrations by a very sensitive HPLC method. Beforehand we had demonstrated that serum UCB levels in 11 naive Gunn rats colony were stable over 6 mo (Figure 3A). In addition, to avoid any bias due to sublocalization of individual cages, one cage with two rats was moved to all parts of the room to measure a possible influence of light sources on serum UCB concentrations. As for the previous test, no significant modification in serum UCB was observed (data not shown). Comparison of serum UCB levels measured 27 wk after transplantation with levels measured 2 wk post-transplantation revealed a 50% drop of UCB levels in rats transplanted with ADHLSC or hHepatocytes (Figure 3B). As illustrated in Figure 3C, when individual results were examined, it appeared that 4 of the 5 ADHLSC-transplanted rats showed a remarkable reduction of serum UCB levels after 3 mo. UCB concentration reached 4.7 ± 0.5 mg/dL in average for the four responsive rats instead of 7.0 ± 0.4 mg/dL in average for the vehicle-infused Gunn rats. Further correction of UCB levels was achieved after 6 mo when UCB levels reached 3.2 ± 0.5 mg/dL vs 6.1 ± 0.2 mg/dL in the vehicle injected rats. One rat out of five transplanted with ADHLSC did not respond to the treatment. Paired t test analysis revealed a significant difference (P = 0.0032) between the serum UCB levels when compared at 2 and 27 wk post-transplantation in the four responsive animals. Adding the fifth animal to the statistics caused the P to increase to 0.0625, just below statistical significance. Gunn rats transplanted with hHepatocytes also saw their serum UCB levels decrease with similar kinetics as the rats transplanted with ADHLSC from 6.3 ± 0.8 mg/dL to 3.2 ± 0.2 mg/dL at 6 mo post-transplantation (P = 0.0658).
In order to confirm that metabolic improvement was due to ADHLSC engraftment, RTqPCR and immuno-fluorescence staining were performed on transplanted rat liver.
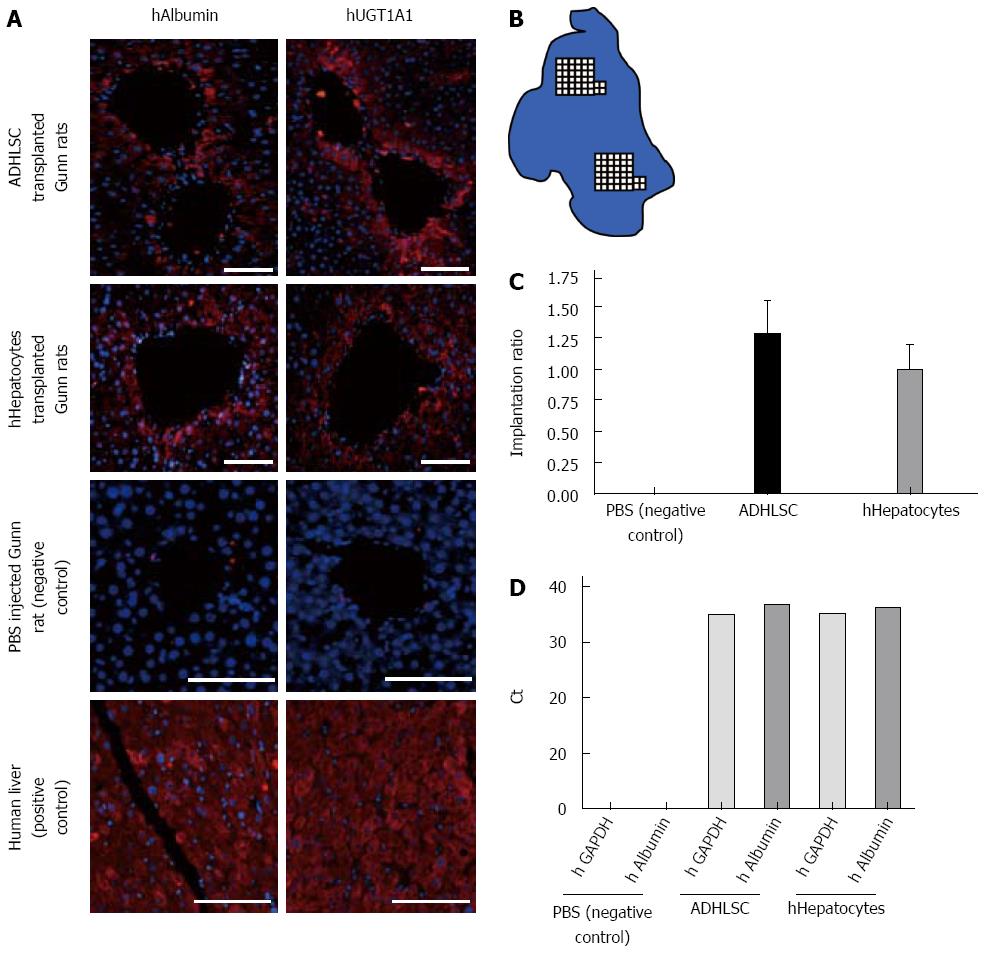
Engraftment of infused ADHLSC and hepatocytes was revealed by RTqPCR positive signal for human albumin and GAPDH (Figure 4D). ADHLSC injected rat livers revealed a positive signal for human albumin and human GAPDH after 37.68 ± 0.11 and 34.98 ± 0.38 cycles respectively whereas hepatocytes infused rat livers exhibit a positive signal after 36.77 ± 0.42 and 35.62 ± 0.17 cycles for human albumin and human GAPDH respectively.
In the four rats where UCB decrease after treatment, engraftment of infused ADHLSC was revealed by positive staining for human albumin (Figure 4A) using a specific antibody which does not cross-react with rat albumin as illustrated in PBS injected rat liver parenchyma (negative control). Since ADHLSC constitutively express albumin, human albumin positivity is not sufficient to infer about the differentiation status achieved by ADHLSC in vivo. Thus in addition to recognizing cells from human origin within the rat parenchyma, we also wanted to confirm that these cells expressed UGT1A1 in vivo and that improvement of hyperbilirubinemia observed in four rats could be correlated with implantation of functional cells. To do, we processed serial sections by immunofluorescence for human albumin detection as described above and for human UGT1A1 detection (Figure 4A). Such serial sections allowed us to demonstrate localisation of human albumin and UGT1A1 expression in the four rats where UCB decrease after treatment. As shown in Figure 4A, 27 wk post-transplantation, human cells not only appeared integrated into rat liver parenchyma but also exhibited a typical hepatocyte morphology which is consistent with their expression of UGT1A1, a marker of mature hepatocytes. Interestingly all human albumin- and UGT1A1-positive cells were for a majority found implanted in periportal zones.
We were also interested to evaluate the engraftment level of cells in a model of metabolic liver that was not subjected to any chemical or surgical insult as regeneration stimulus since this is closest to the clinical setting for CN patients. A rough estimation was done by counting albumin-positive cells over two randomly chosen areas per section and per liver (see the schematic in Figure 4B). Total cells in these areas were counted based on nuclear DAPI staining. Areas corresponding to 104 total cells were counted visually on a scanned image of each section. Such quantification was used to provide a gross evaluation of engraftment levels 27 wk post-transplantation. We found repopulation varies from 0% to 2.11% and from 0.58% to 1.31% for ADHLSC and hepatocytes respectively (Figure 4C); mean of repopulation ratio rates 1.3% ± 0.3% and 1.0% ± 0.2% for ADHLSC and hepatocytes respectively (Figure 4D).
In the current study, we demonstrate that ADHLSCs from large scale cultures are able to engraft into the host liver parenchyma and to partially restore one important hepatic function (bilirubin conjugation) into the Crigler-Najjar animal model, the Gunn rat.
Hepatocyte transplantation experience in Crigler-Najjar disease patients had shown a significant reduction in bilirubin levels allowing to reduce the phototherapy requirement[15,17], but the clinical improvement remains limited in time as these mature cells have a limited self-renewal capacity[17]. In addition, human hepatocytes shortage, poor cryopreservation resistance[18,19] and absence of in vitro proliferation capacity constitute a major hurdle in large application of this mature liver cell therapy.
Therefore, other cell sources become mandatory. Stem/progenitor cells can be expanded in vitro, can engraft, proliferate and participate to host liver repopulation, and may modulate the host immune response[27,28]. In this study carried out in large scale culture conditions, we confirm the stable ADHLSC characteristics: expression of mesenchymal markers (CD73, CD90, CD29, CD44, vimentin and ASMA) and hepatic markers expression (albumin). Cells can also differentiate in tissue specific cell type when exposed to a specific hepatogenic culture medium as demonstrated by the (1) acquisition of polygonal shape; (2) increase in CYP3A4 activity (data not shown); (3) ability to increase the bilirubin conjugation activity (5.1% ± 1.8% and 9.0% ± 1.1% after 24 h and 48 h respectively); and (4) increase in UGT1A mRNA (mRNA constituted of the 4 first common exons of UGT1A family).
Moreover, as demonstrated by HPLC and immunohistochemistry (IHC) our study provides long-term activity and engraftment of ADHLSC in xenogenic transplantation model for Crigler-Najjar metabolic liver disorder. HPLC results indicate that 3 mo post-transplantation, 4 of the 5 ADHLSC intraportally injected Gunn rats, exhibit a non significant decrease in unconjugated bilirubin (UCB) blood level: 4.7 ± 0.5 mg/dL in transplanted rats instead of 7.0 ± 0.4 mg/dL for the vehicle infused Gunn rats (negative control group). Twenty-seven weeks after transplantation the four responsive injected Gunn rats, exhibit a higher decrease in UCB blood level: 3.2 ± 0.5 mg/dL vs 6.1 ± 0.2 mg/dL in the negative control group. A similar decrease was also observed in the positive control group (rats injected with hHepatocytes).
RTqPCR results indicated the presence of human cells into the Gunn rat liver parenchyma and thanks to IHC we were able to specifically discriminate human cells into the ADHLSC intraportally injected rat liver. IHC against human albumin and UGT1A1, allows us to determine that injected human cells seem localized in periportal zone. No ADHLSC were identified in the single rat which did not respond to the therapy. This may be due to the loss of cells due to bleeding during the portal vein injection or to an immune rejection in this animal.
Transplantation data confirm our recently published report on ADHLSC and the kinetic of engraftment into liver of immunodeficient mouse as well as their participation in tissue regeneration after 20% of hepatectomy. In this study on mice, analyses were performed only after 2 mo post transplantation, and do not allow drawing conclusion on long term engraftment and differentiation[22]. In this experiment rats were injected with a relative low dose of 2.5 millions cells, i.e. 10 to 12.5 million/kg body weight, without regeneration stimulus and this dose led to a UCB decrease and engraftment demonstrated 6 mo later. Moreover UCB decrease obtained in ADHLSC and hepatocytes transplanted Gunn rats is in accordance with results obtained in transplanted rats[29] and in human[17], while animals or patients exhibited a progressive decrease of serum bilirubin 2-4 mo after liver cell transplantation.
In our experimental model, we could detect around 1.265% ± 0.288% and 0.973% ± 0.212% for ADHLSC and hepatocytes in transplanted Gunn rats respectively which is still in agreement from what is generally accepted that a repopulation of 1%-5% of the hepatic mass is required to restore liver disease in most injury models[29-33]. Recently; human clinical hepatocyte transplantation engraftment rates have been documented to range from 0% to a maximum of 12% leading in most cases to a transitory quality of life improvement for metabolic liver disease patients[34].
This repopulation rate is likely due to in vivo expansion, as the infused cell mass corresponded to only 0.25% of the total liver mass, assuming that the total supposed liver cell mass is 5 billion per kg body weight[15]. No regeneration stimulus was applied in these animals, and such stimuli using radiotherapy of chemicals might improve the engraftment[35]. In parallel, amelioration of immunosuppression treatment will be interesting (use of microsomal encapsulated clodronate, low liver irradiation…).
In conclusion, ADHLSC large scale cultured were able to differentiate into hepatocyte-like cells in vitro, exhibiting hepatic functions (CYP3A4 activity, glucuronidation activity…). In vivo these cells were also able to engraft and repopulate the liver parenchyma and led to partially correct a bilirubin conjugation defect in a homologous rodent model of Crigler-Najjar syndrome.
All together, these results make ADHLSC an attractive candidate for cell therapy treatment of Crigler-Najjar type I syndrome.
Liver cell transplantation using hepatocytes was successfully performed in patients with inborn errors of metabolism. However, the success of such a therapeutic approach remains limited by the quality of transplanted cells. To overcome these problems several approaches to isolate and propagate liver stem or progenitor cells have been developed. The capacity of those cells to restore a liver metabolic function must be demonstrated.
Crigler-Najjar type I syndrome is characterized by a high unconjugated bilirubin blood level due to uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) deficiency. Orthotopic liver transplantation remains the only curative treatment but the procedure does not guarantee lifelong complication free survival. Hepatocyte infusion has established that metabolic control is possible, but the procedure remains limited by several organ shortage and transient survival of cells. Stem cells look promising to overcome these problems and adult-derived human liver stem/progenitor cells (ADHLSC) are tested for their ability to engraft and differentiate in a homologous animal model (Gunn rat) of human liver Crigler-Najjar type I metabolic disease.
In this study, authors demonstrate the ability of ADHLSC to engraft in recipient rat livers where they participate in restoration of liver function by a significant reduction of the serum bilirubin levels. Our findings support ADHLSC as a promising candidate for liver cell-based therapy developments.
In this study, authors demonstrated that large scale culture conditions do not modify adult-derived human liver stem/progenitor cells phenotype, ADHLSC were able to specifically conjugate bilirubin when intraportally injected into Gunn rats as demonstrated by blood unconjugated bilirubin measurement at different times post-transplantation; infused-Gunn rats exhibited a metabolic effect 3 mo post-transplantation and maintained over a 6 mo period. ADHLSC engraftment into Gunn rat’s liver was demonstrated by immunohistochemistry. All the results suggest the ADHLSC potential to restore a deficient metabolic function in situ.
Crigler-Najjar syndrome type I and II are characterized by unconjugated bilirubin accumulation in the serum. These syndromes are due to genetic variations in the gene encoding uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) leading to complete or partial inactivation of the bilirubin glucuronidation. Clinically, Crigler-Najjar syndrome type I is associated with progressive damage of the central nervous system leading to bilirubin encephalopathy (kerincterus). Gunn rat, derived from Wistar parent strain, has constantly elevated concentrations of serum bilirubin which causes unconjugated hyperbilirubinaemia. Gunn rat inherently lacks all glucuronidation activities catalysed by the UGT1 isoforms and is therefore used as animal model for Crigler-Najjar syndrome type I.
This study shows that ADHLSC were able to specifically conjugate bilirubin when intraportally injected into Gunn rats as demonstrated by blood unconjugated bilirubin measurement at different times post-transplantation; infused-Gunn rats exhibited a metabolic effect 3 mo post-transplantation and maintained over a 6 mo period. ADHLSC engraftment into Gunn rat’s liver was demonstrated by immunohistochemistry. All the results suggest the ADHLSC potential to restore a deficient metabolic function in situ.
P- Reviewer: Cong WM S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Chowdhury JR, Kondapalli R, Chowdhury NR. Gunn rat: a model for inherited deficiency of bilirubin glucuronidation. Adv Vet Sci Comp Med. 1993;37:149-173. [PubMed] |
| 2. | Sato H, Aono S, Koiwai O. [Genetic defect of the hyperbilirubinemic Gunn rat, a model for Crigler-Najjar syndrome type I]. Nihon Rinsho. 1993;51:501-506. [PubMed] |
| 3. | CRIGLER JF, NAJJAR VA. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics. 1952;10:169-180. [PubMed] |
| 4. | CRIGLER JF, NAJJAR VA. Congenital familial nonhemolytic jaundice with kernicterus; a new clinical entity. AMA Am J Dis Child. 1952;83:259-260. [PubMed] |
| 5. | van der Veere CN, Sinaasappel M, McDonagh AF, Rosenthal P, Labrune P, Odièvre M, Fevery J, Otte JB, McClean P, Bürk G. Current therapy for Crigler-Najjar syndrome type 1: report of a world registry. Hepatology. 1996;24:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Yohannan MD, Terry HJ, Littlewood JM. Long term phototherapy in Crigler-Najjar syndrome. Arch Dis Child. 1983;58:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Sokal EM, Silva ES, Hermans D, Reding R, de Ville de Goyet J, Buts JP, Otte JB. Orthotopic liver transplantation for Crigler-Najjar type I disease in six children. Transplantation. 1995;60:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Schauer R, Stangl M, Lang T, Zimmermann A, Chouker A, Gerbes AL, Schildberg FW, Rau HG. Treatment of Crigler-Najjar type 1 disease: relevance of early liver transplantation. J Pediatr Surg. 2003;38:1227-1231. [PubMed] |
| 9. | Shneider BL. Pediatric liver transplantation in metabolic disease: clinical decision making. Pediatr Transplant. 2002;6:25-29. [PubMed] |
| 10. | Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 271] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Najimi M, Sokal E. Update on liver cell transplantation. J Pediatr Gastroenterol Nutr. 2004;39:311-319. [PubMed] |
| 12. | Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, Bernard Otte J, Evrard V, Latinne D, Vincent MF. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Stéphenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317-1323. [PubMed] |
| 14. | Stéphenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 716] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 16. | Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, Arya R, Wade JJ, Verma A, Heaton ND. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812-1814. [PubMed] |
| 17. | Lysy PA, Najimi M, Stephenne X, Bourgois A, Smets F, Sokal EM. Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J Gastroenterol. 2008;14:3464-3470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Stéphenne X, Najimi M, Ngoc DK, Smets F, Hue L, Guigas B, Sokal EM. Cryopreservation of human hepatocytes alters the mitochondrial respiratory chain complex 1. Cell Transplant. 2007;16:409-419. [PubMed] |
| 19. | Stéphenne X, Najimi M, Sokal EM. Hepatocyte cryopreservation: is it time to change the strategy. World J Gastroenterol. 2010;16:1-14. [PubMed] |
| 20. | Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, Sokal EM. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes. Cell Transplant. 2007;16:717-728. [PubMed] |
| 21. | Khuu DN, Scheers I, Ehnert S, Jazouli N, Nyabi O, Buc-Calderon P, Meulemans A, Nussler A, Sokal E, Najimi M. In vitro differentiated adult human liver progenitor cells display mature hepatic metabolic functions: a potential tool for in vitro pharmacotoxicological testing. Cell Transplant. 2011;20:287-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Khuu DN, Nyabi O, Maerckx C, Sokal E, Najimi M. Adult human liver mesenchymal stem/progenitor cells participate in mouse liver regeneration after hepatectomy. Cell Transplant. 2013;22:1369-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 3877] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 24. | Scheers I, Maerckx C, Khuu DN, Marcelle S, Decottignies A, Najimi M, Sokal E. Adult-derived human liver progenitor cells in long-term culture maintain appropriate gatekeeper mechanisms against transformation. Cell Transplant. 2012;21:2241-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Muraca M, Blanckaert N. Liquid-chromatographic assay and identification of mono- and diester conjugates of bilirubin in normal serum. Clin Chem. 1983;29:1767-1771. [PubMed] |
| 26. | Gunn CK. Hereditary Acholuric Jaundice in the Rat. Can Med Assoc J. 1944;50:230-237. [PubMed] |
| 27. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3279] [Article Influence: 156.1] [Reference Citation Analysis (0)] |
| 28. | El Haddad N, Heathcote D, Moore R, Yang S, Azzi J, Mfarrej B, Atkinson M, Sayegh MH, Lee JS, Ashton-Rickardt PG. Mesenchymal stem cells express serine protease inhibitor to evade the host immune response. Blood. 2011;117:1176-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, Roy-Chowdhury N, Vikram B, Roy-Chowdhury J. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology. 2002;36:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Joseph B, Malhi H, Bhargava KK, Palestro CJ, McCuskey RS, Gupta S. Kupffer cells participate in early clearance of syngeneic hepatocytes transplanted in the rat liver. Gastroenterology. 2002;123:1677-1685. [PubMed] |
| 32. | Grompe M. Principles of therapeutic liver repopulation. J Inherit Metab Dis. 2006;29:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Wang LJ, Chen YM, George D, Smets F, Sokal EM, Bremer EG, Soriano HE. Engraftment assessment in human and mouse liver tissue after sex-mismatched liver cell transplantation by real-time quantitative PCR for Y chromosome sequences. Liver Transpl. 2002;8:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, Ericzon BG. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272:201-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Bahde R, Kapoor S, Bandi S, Bhargava KK, Palestro CJ, Gupta S. Directly acting drugs prostacyclin or nitroglycerine and endothelin receptor blocker bosentan improve cell engraftment in rodent liver. Hepatology. 2013;57:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |