Published online Aug 14, 2014. doi: 10.3748/wjg.v20.i30.10355
Revised: January 8, 2014
Accepted: April 1, 2014
Published online: August 14, 2014
Processing time: 322 Days and 21.9 Hours
The efficacy of first- and second-line Helicobacter pylori (H. pylori) eradication regimens varies considerably in West Asian countries, mainly due to the variable prevalence of resistant organisms. However, no review article has yet evaluated and compared the efficacy of different regimens among different countries of this region. Therefore, we conducted a review to select the best options and provide recommendations for H. pylori treatment in this geographic region. A search through PubMed was carried out to obtain relevant randomized clinical trials published in English language up to June 2013. According to the results, among different therapeutic regimens used as the first-line protocols, 10-d Bismuth-Furazolidone/Metronidazole quadruple therapy, 14-d Clarithromycin-containing hybrid therapy and 14-d quadruple therapy including a proton pump inhibitor + Bismuth + Tetracycline (500 mg QID) + Metronidazole (500 mg TDS) seemed to be appropriate options. Among second-line therapeutic regimens, Bismuth-based quadruple therapies containing Tetracycline and Furazolidone/Metronidazole, triple therapy containing Amoxicillin and Gatifloxacin and Quadruple therapy including Bismuth + Azithromycin and Ofloxacin seemed to be effective options. Third-line therapies were not evaluated in West Asia; most guidelines, however, recommend choosing optimal eradication regimen according to the pattern of antibiotic susceptibility of H. pylori. Although we limited our investigation to H. pylori eradication regimens in West Asia, the clinical significance of the results goes beyond the countries situated in this geographic region. In fact, the results are transferrable to any region as long as the patterns of resistance are the same.
Core tip: The efficacy of first- and second-line Helicobacter pylori (H. pylori) eradication regimens varies considerably in West Asian countries, mainly due to the variable prevalence of resistant organisms. The present study evaluates and compares the efficacy of different regimens among different countries of this region. Although we limited our investigation to H. pylori eradication regimens in West Asia, the clinical significance of the results goes beyond the countries situated in this geographic region. In fact, the results are transferrable to any region as long as the patterns of resistance are the same.
-
Citation: Fakheri H, Bari Z, Aarabi M, Malekzadeh R.
Helicobacter pylori eradication in West Asia: A review. World J Gastroenterol 2014; 20(30): 10355-10367 - URL: https://www.wjgnet.com/1007-9327/full/v20/i30/10355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i30.10355
Helicobacter pylori (H. pylori) is among the most common bacterial infections, affecting almost half the world population[1]. It is a known cause of chronic gastritis which may be complicated by peptic ulcer disease (PUD), gastric adenocarcinoma and lymphoma. While the prevalence of H. pylori infection is decreasing in developed countries, it still occurs commonly in developing countries, beginning in early childhood[1]. This suggests the possible role of socio-economic status and living standards in the distribution of the infection.
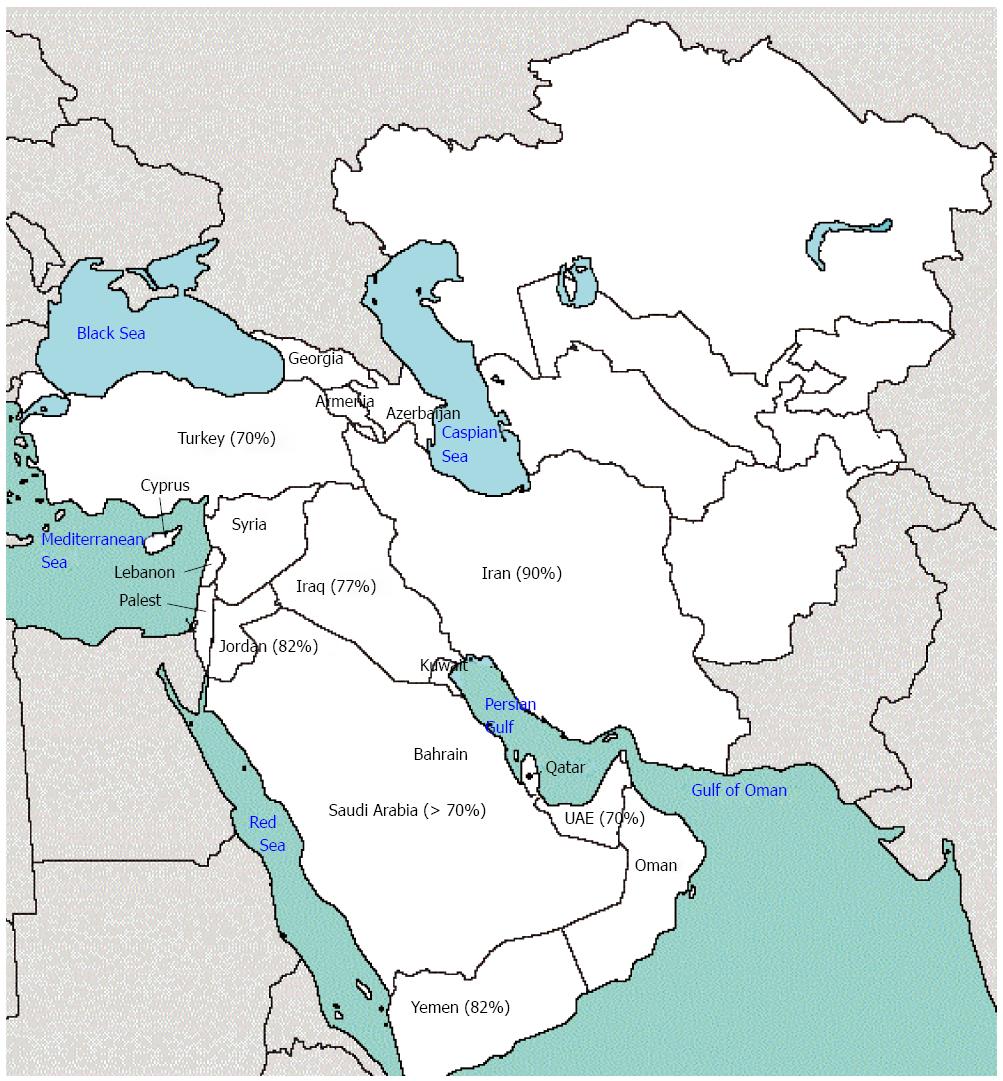
There is little detailed data on the prevalence of H. pylori infection in West Asia; nevertheless, the available data is almost similar among different countries in this region. In Iran, almost 90% of the adult population is infected with the bacterium[2,3]. The prevalence has been reported to be about 82% in Jordan[4] and 78.4% among industrial workers and 64.3% among referent workers in the United Arab Emirates[5]. At the same time, a significant fraction of children are also infected. According to a report from Turkey, 17.4% of children younger than 1 year were infected with the bacterium and the rate exceeded 70% in adults[6]. al-Moagel et al[7] reported the prevalence of H. pylori infection to range from 40% in individuals aged 5-10 years to more than 70% in those aged 20 years or older in Saudi Arabia. Also, 2 different studies in Kuwait and Yemen reported the prevalence of H. pylori infection to be 27% in children younger than 13 years and 12.5% in children 9-10 years old, respectively[8,9] (Figure 1).
The high prevalence of H. pylori infection in West Asia highlights the need for identifying definite H. pylori eradication regimens for the countries situated in this geographic region.
Indications for H. pylori eradication vary by geographic regions. In countries with high H. pylori prevalence rates, it is not cost-effective to treat every person positive for H. pylori infection. Furthermore, H. pylori treatment would often constitute a temporary solution due to high recurrence rates. Therefore, guidelines categorize indications for H. pylori treatment by the geographic region and H. pylori prevalence rates[10].
According to Maastricht IV Consensus Report, eradication of H. pylori is highly recommended in the following situations[11].
Functional dyspepsia, Gastro-esophageal reflux disease, complicated or uncomplicated PUD, mucous-associated lymphoid tissue lymphoma, before starting and during long term nonsteroidal anti-inflammatory drug therapy in patients with a history of PUD, patients receiving long term proton pump inhibitors (PPIs), unexplained iron deficiency anemia, idiopathic thrombocytopenic purpura, gastric cancer prevention in special situations (including first degree relatives, previous history of gastric neoplasia, severe pan-/corpus dominant gastritis, severe gastric atrophy, more than 1 year acid suppression therapy, strong environmental risk factors such as heavy smoking and those who fear gastric cancer) and gastric atrophy.
In addition, advisable indications include presence of intestinal metaplasia and vitamin B12 deficiency.
The prevalence of H. pylori resistance to antibiotics is increasing worldwide, mainly correlated with consumption of antibiotics by the general population[12].
In West Asia, the resistance rates have been increasing over the last 20 years. In Iran, Clarithromycin resistance has increased from 1.4% in 1997[13] to 26.5% in 2013[14]. The rate is even reported higher in Turkey and Bahrain[15-17]. Resistance to other antibiotics, including Metronidazole, Amoxicillin and Tetracycline, has been on the rise, as well. Table 1 demonstrates primary H. pylori resistance to different antibiotics in some West Asian countries. The data highlights the importance of surveillance of antibiotic resistance in clinical practice.
| Country(R) | Year | Number of patients | Testing method | Amoxicillin | Metronidazole | Tetracycline | Clarithromycin | Other drugs |
| Iran[13] | 1997-2000 | 70 | DDM | 1.4 | 33% | 0% | 1.4% | |
| Iran[14] | 2001-2004 | 135 | DDM | 3.7 | 36.3% | 0.7% | 3.7% | |
| Iran[42] | 2005 | 120 | DDM | 1.6 | 57.5% | 0% | 16.7% | |
| Iran[58] | 2005-2008 | 160 | DDM | 7.3 | 55.6% | 38.1% | 7.3% | Furazolidone: 4.5% |
| Iran[44] | 2008 | 124 | DDM | 9.8 | 64.6% | 0% | 17.1% | |
| Iran[43] | 2010 | 42 | DDM | 2.4 | 40.5% | 4.8% | 14.3% | Ciprofloxacin: 2.4% |
| Iran[41] | 2012 | 112 | DDM | 28 | 76.8% | 18.6% | 14.3% | Ciprofloxacin: 33% |
| Rifampin: 28.6% | ||||||||
| Iran[110] | 2013 | 153 | DDM | 7.2 | 63.8% | - | 26.5% | - |
| Turkey[45] | 2003 | 87 | DDM | - | - | - | 27.5% | |
| Turkey[16] | 2004 | 110 | DDM | - | - | - | 48.2% | |
| Turkey[17] | 2006 | 92 | DDM | - | - | - | 40.5% | |
| Turkey[111] | 2007 | 61 | DDM | - | - | - | 16.4% | |
| Turkey[63] | 2012 | 61 | DDM | 0 | 42.6% | 0% | 21.3% | Levofloxain: 3.3% |
| Lebanon[112] | 2002 | 54 | DDM | 0 | 29.5% | 2% | 4% | |
| Bahrain[15] | 1999 | 83 | Epsilometer test | - | 57% | - | 33% | |
| Saudi Arabia[59,61] | 1988 | 71 | DDM | - | 38% | 1.4% | - | |
| Saudi Arabia[59,61] | 1996 | 62 | DDM | - | 85.5% | 0% | - | |
| Saudi Arabia[59] | 1998 | 63 | DDM | - | 78.5% | - | - | |
| Saudi Arabia[60] | 2002 | 223 | DDM | 1.3 | 80% | 0.4% | 4% | |
| Saudi Arabia[62] | 2008 | 46 | DDM | 0 | 69.5% | - | 21% | |
| UAE[113] | 2010 | 26 | E- test | - | - | - | 19.2% | |
| UAE[64] | 2013 | 16 | ? | - | 63% | - | - | |
| Syria[114] | 2012 | 130 | FH | - | - | - | 12.3% |
The present article is a narrative review to critically appraise randomized controlled trials in terms of the efficacy of different treatments for H. pylori eradication conducted in West Asian countries.
To find relevant papers, a search was carried out on PubMed website for studies published in English language up to June 2013. The following keywords were used: (“Helicobacter pylori” or “H. pylori”) and (therapy or treatment or eradication) and country name (Armenia, Azerbaijan, Bahrain, Cyprus, Georgia, Iraq, Iran, Jordan, Kuwait, Lebanon, Oman, Palestine, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates, Yemen). Relevant randomized clinical trials were selected after their abstracts were reviewed by two gastroenterologists. Since the number of second-line therapeutic regimens was very small, non-randomized trials were also included for evaluating second-line therapeutic options for H. pylori eradication in this region. Data on type of patients and therapy regimens in each arm and eradication rate based on intention-to-treat (ITT) and per-protocol (PP) analyses were extracted from the selected studies and recorded in data extraction forms.
The optimal first-line regimen for treatment of H. pylori infection should be considered the one that can achieve > 90% PP eradication rate[18].
According to the Maastricht IV consensus report, the regimen is influenced by the resistance rate to Clarithromycin: thus, standard triple therapy including a PPI + Clarithromycin + Amoxicillin or Metronidazole is recommended for countries with less than 20% resistance to Clarithromycin, while Bismuth-containing quadruple therapies are recommended for those countries with high Clarithromycin resistance[11].
In Iran, a total of 9 studies have investigated the efficacy of standard triple therapy on H. pylori eradication (Table 2). Out of three 7-d regimens, 1 study showed acceptable eradication rates[19-21]. Also, out of three 10-d regimens, 2 were successful in achieving > 90% PP eradication rate[22-24] and out of three 14-d regimens, 1 yielded optimal results[25-27].
| Ref. | Type of patients | Type of triple therapy regimens | Number of patients in each study arm | Eradication rate (%) | |
| Per-protocol | Intention-to-treat | ||||
| Mirzaee et al[21], 2012 | NA | PAC-7, PAC-7 with yoghourt, PAC-7 with probiotic | 34-34-34 | 61.3-73.1-64.5 | NA |
| Sarkeshikian et al[24], 2013 | PUD + NUD | OAC-10, OAAz-10 | 76-89 | 83-75 | NA |
| Keshavarz et al[25], 2007 | NUD | OAC-14, OAC[half dose]-14 | 80-80 | 89-88 | 83.7-85 |
| Malekzadeh et al[19], 2003 | Cancer Screening | OAC-7, OFT-4, OFT-7 | 45-41-42 | 42.1-20.6-29.4 | 35.5-17.1-23.8 |
| Ahmad et al[82], 2013 | PUD + NUD | OAF-7, OAF-7 with probiotic | 33-33 | 70-90 | 70-90 |
| Roghani et al[83], 2003 | DU | OAF-14, OAF-14 [half dose] | 63-61 | 88.9-67.9 | 76.2-62.3 |
| Seyedmajidi et al[26], 2013 | PUD | OAC-14, OABM-14, OCPen-14 | 110-110-110 | 90.8-56-87 | 80.9-50.9-79 |
| Ghadir et al[81], 2011 | PUD | OAF-14, OABF-14 | 43-43 | 61.1-85.3 | 51.2-67.4 |
| Taghavi et al[27], 2009 | NUD | OAC-14, O.Co.Dox.-14, OABF-14 | 53-67-69 | 70-63-56 | 66-61-49 |
| Sotudehmanesh et al[52], 2001 | DU | RBTM-14, RBTM-21, OBCT-14 | 74-73-76 | 73-71-88 | 68-68-80 |
| Malekzadeh et al[49], 2000 | DU | RABM-14, RABF-14 | 53-53 | 56-82 | 55-75 |
| Khatibian et al[48], 2007 | PUD | OABM-14, OABF-14, OABF (first week) M (second week) | 107-104,103 | 83.1-95.2,95.3 | 74.5-87,86.6 |
| Shidfar et al[51], 2012 | NS | OABM-14, OABM-14 + Lycopen | 27-27 | 68-77.8 | 62.9-77.8 |
| Mousavi et al[50], 2006 | PUD, NUD | OABM-14, OAzBM-7 | 65-64 | 75.7-78.1 | 70.4-74.1 |
| Agah et al[47], 2009 | NUD | OABM-14, OABAz[first 7 d]-14 | 30-30 | 69-68 | NA |
| Mirbagheri et al[20], 2006 | NUD | OAC-7, OABM-10, O.Am.BM-10 | 120-120-120 | 91.1-85.5-92.8 | 84.1-86.6-80.8 |
| Shavakhi et al[84], 2013 | PUD | OABC-14, OABC-14 with probiotic | 90-90 | 84.4-82.1 | 81.1-76.6 |
| Fakheri et al[79], 2001 | DU | OABC-14, OABF-14 | 55-63 | 90-90 | 85-84 |
| Daghaghzadeh et al[78], 2007 | PUD, NUD | OABF-7, OABF-14 | 78-78 | 84.8-82.6 | 71.8-73.1 |
| Fakheri et al[80], 2004 | DU | OAF-14, OABF(low dose F)-14, OABF-14 | 50-50-50 | 54-72-92 | 54-72-92 |
| Aminian et al[22], 2010 | Dyspepsia | OA-MC-10, OABM-14, OAC-10, OCCip[first 7 d]-14 | 107-107-107 | 81.1-85.7-90.7-70 | 80.4-84-90.7-65 |
| Fakheri et al[69], 2012 | DU | PA-CT-10, PA-BF[7]-14 | 148-148 | 89.1-88.7 | 83.7-80.4 |
| Sardarian et al[68], 2013 | DU | PA-CT-10, PA-CT-14 (HYBRID) | 210-210 | 79.9-92.9 | 76.7-89.5 |
| Riahizadeh et al[23], 2010 | PUD, NUD | OAB-MF-10, OAC-10, OAB-CF-10 | 107-106-106 | 91.3-90.4-88.7 | 78.5-81.1-82 |
However, out of 12 studies conducted in Turkey to evaluate the effects of 7-, 10- and 14-d standard triple therapies on H. pylori eradication, only one of the 14-d regimens could achieve an acceptable eradication rate[28-39] (Table 3).
| Ref. | Type of patients | Type of triple therapy regimens | Number of patients in each study arm | Eradication rate (%) | |
| Per-protocol | Intention-to-treat | ||||
| Aydin et al[29], 2007 | PUD + NUD | PAC-14, PAC-7 | 40-40 | 73.5-47.1 | 67.6-44.4 |
| Yaşar et al[33], 2010 | Dyspepsia | PAC-14, PAC-14 with probiotic | 38-38 | 53-66 | 53-66 |
| Uygun et al[32], 2004 | Dyspepsia | LAC-14, PAC-14 | 45-45 | 69.2-70 | 62.2-60 |
| Sezikli et al[31], 2012 | NUD | LAC-14, LAT-14 | 40-40 | 47.4-39.3 | 45-37.5 |
| Aladağ et al[28], 2005 | DU | OAC-14, PAC-14 | 64-62 | 90.6-93.5 | 90.6-93.5 |
| Erçin et al[75], 2011 | DU | LALevo-7, LALevo-14 | 51-40 | 34.1-72.7 | 27-65 |
| Seven et al[76], 2004 | PUD-NUD | OALevo-10, OALevo-10[half dose] | 50-60 | 72.7-60 | 72.7-60 |
| Gumurdulu et al[30], 2004 | PUD | OAC-7 , OAC-14, OBTM-10 | 53-59-52 | NA | 24.5-40.6-61.5 |
| Alkim et al[34], 2011 | PUD + NUD | OAC-14, LAC-14, RAC-14, PAC-14, EAC-14, RBAC-14 | 66-66-66-66-66-66 | 65.6-72.1-84.4-62.5-60.3-85.7 | 63.6-66.7-81.8- 60.6-57.6-81.8 |
| Songür et al[35], 2009 | PUD, NUD | LAC-14, LMT-10, LBMT-10, RLBMT-10 | 113-115-119-117 | 35.6-64.4-54.9-64.6 | 32.7-60-47.1-57.3 |
| Uygun et al[36], 2007 | NUD | LAC-14, LBMT-14 | 120-120 | 62.7-82.3 | 57.5-70 |
| Kadayifci et al[56], 2012 | NUD | EABT-14, EAMT-14 | 100-100 | 89.7-80.4 | 79-74 |
| Sezgin et al[55], 2006 | PUD + NUD | RBMT-14, PBMT-14 | 42-40 | 61.9-55 | 61.9-55 |
| Uygun et al[57], 2007 | NUD | RBAT-14, RBAC-14, RBMT-14 | 100-100-100 | 64.4-66.2-58.9 | 58-59-56 |
| Akyildiz et al[53], 2009 | PUD + NUD | RBAT-14, RBADox-14 | 58-57 | 40.8-45.7 | 34.5-36.8 |
| Köksal et al[54], 2013 | Dyspepsia | OBTM-14 , | 45 - 45 | 86.8-90.1 | 73.3-89.9 |
| OBTM-14[Optimal dose] | |||||
| Nadir et al[37], 2011 | Dyspepsia | LA-MT-14, LAC-14, | 144-138 | 77.6-53.6 | 72.2-52.5 |
| Yakut et al[71], 2010 | Dyspepsia | PA-MT-10, RaBAC-14 | 108-75 | 88-95 | 88-95 |
| Uygun et al[39], 2008 | NUD | PA-MT-14, PAC-14 | 150-150 | 80.1-63 | 72.6-58 |
| Cetinkaya et al[70], 2010 | NUD | PA-MT-14, PA-TM-14 (HYBRID) | 56-56 | 83.6-81.4 | 82.1-78.5 |
| Polat et al[38], 2011 | NUD | E-A-[first 7 d]-LevoM-[last 7 d]-14, EAC-14 | 75-75 | 90-57 | 86.6-50.6 |
Overall, it seems that Clarithromycin-containing triple therapies were not successful in studies performed in West Asian countries. This pattern of response is in concordance with the pattern of resistance to Clarithromycin in these countries. As previously reported by Graham et al[40], the success rate with standard triple therapy falls below 90% when the level of resistance to Clarithromycin is above 10% and therefore, should not be used unless proven to be locally effective.
In most studies, resistance to Clarithromycin has been reported to be above 10% in Iran[41-44] and above 20% in Turkey[16,17,45] (Table 1). Furthermore, Clarithromycin is not routinely administered in Iran due to its high cost.
Venerito et al[46] conducted a meta-analysis on 12 randomized clinical trials to compare the efficacy of Bismuth-containing quadruple therapy with standard triple therapy. Per-protocol eradication rates were 77.6% and 68.9%, respectively, and both regimens had suboptimal eradication rates.
Out of nine 7-, 10- and 14-d Bismuth plus Metronidazole-containing quadruple regimens in Iran, none had acceptable eradication rates[20,22,26,47-52] (Table 2). Besides, only one of the 9 studies conducted in Turkey had 90.1% PP eradication rate (Table 3)[30,34-36,53-57].
The efficacy of Bismuth-containing quadruple therapies depends on the pattern of resistance to Metronidazole. In Iran, the resistance rate has been increasing, from 33% in 1997 to 76.8% in 2012[13,14,41-44,58]. The rate has also been rising from 38% to 78.5% over the past 20 years in Saudi Arabia[59-62]. Moreover, in 2 different studies, 42.6% and 63% of H. pylori strains were resistant to Metronidazole in 2012 in Turkey and United Arabia Emirates, respectively[63,64].
Furthermore, the dose and duration of Bismuth therapy are also important variables, especially in case of resistance to Metronidazole[65]. In most previous studies, suboptimal dose and duration have been used and, as expected, unsatisfactory results were achieved[66]. But when administering higher doses, some studies have reported optimal results even in case of high resistance to Metronidazole. Luther et al[66] reported 97.1% PP eradication rate with 2 wk of Omeprazole (20 mg BD), Bismuth subsalicilate (240 mg QID), Tetracycline (500 mg QID) and Metronidazole (500 mg TDS). Also, Bismuth, if given in sufficient duration and doses, may revert metronidazole resistance, and render Metronidazole resistant H. pylori sensitive to metronidazole.
Recently, some studies have shown promising results with the novel sequential therapy. Nevertheless, a meta-analysis performed by Gatta et al[67] on 46 randomized controlled trials including 5666 patients receiving sequential therapy and 7866 patients receiving non-sequential therapies, indicated the overall PP eradication rate with sequential therapy to be 84.3%. According to the result of the mentioned study, sequential therapy was superior to 7-d triple therapy and yielded a marginally higher eradication rate compared to 10-d triple therapy. However, all the three regimens had suboptimal eradication rates.
In Iran, 3 studies evaluated the efficacy of sequential therapy, none of which showed promising results[22,68,69]. Also, 4 studies were performed in Turkey (one 10-d and tree 14-d regimens)[37,56,70,71]. They all contained Metronidazole and Tetracycline in the second half of therapy, but none could achieve optimal eradication rates (Table 3). Another sequential study was conducted in Turkey which contained levofloxacin instead of Clarithromycin. The PP eradication rate was 90%[38].
Concomitant therapy is another therapeutic regimen that has been proposed for areas with high resistance to Clarithromycin. It consists of a PPI plus Amoxicillin, Clarithromycin and Metronidazole prescribed simultaneously during the entire duration of therapy. A meta-analysis comprising of 481 patients showed 90% PP eradication rate with concomitant therapy[72]. An advantage of this regimen over sequential therapy is its simplicity.
One study evaluated the effects of concomitant therapy in Turkey, with less than satisfactory results. In this study, Tetracycline was used instead of Clarithromycin[36].
Hybrid therapy is another novel regimen consisting of dual therapy with a PPI and Amoxicillin over the first 7 d and a concomitant quadruple therapy containing a PPI plus Amoxicillin, Clarithromycin and Metronidazole over the second 7 d. This regimen seems to be effective in areas with dual resistance to Metronidazole and Clarithromycin. A study by Hsu et al[73] reported 99% PP eradication rate with hybrid therapy.
In an Iranian study, 420 patients were randomized to receive either hybrid or sequential therapy[68]. The eradication rates with hybrid therapy were 92.9% and 89.5% on PP and ITT analyses, respectively, while sequential regimen had 79.9% PP eradication and 76.7% ITT eradication rates.
Cetinkaya et al[70] compared the efficacy of a hybrid regimen with sequential therapy in Turkey. They used Tetracycline instead of Clarithromycin in both regimens. The PP eradication rates were 81.4% and 83.6% in hybrid and sequential therapy groups, respectively.
Many clinical trials have shown the efficacy of quinolone-based triple therapies in H. pylori eradication, ranging from 72% to 96% as the first-line therapy[74]. Two studies from Turkey investigated the efficacy of levofloxacin-based triple therapies, but both yielded suboptimal results[75,76]. However, Gatifloxacin-containing triple therapy conferred 92% PP eradication rate in a study performed by Sharara et al[77] in Lebanon (Table 4).
| Ref. | Type of patients | Type of triple therapy regimens | Number of patients in each study arm | Eradication rate (%) | |
| Per-protocol | Intention-to-treat | ||||
| Sharara et al[77], 2004 | Dyspepsia | RAG-7, R[half dose]AG-7 | 52-52 | 92-83 | 92-83 |
Furazolidone has been used in nine quadruple therapies in Iran[23,27,47-49,78-81], four of which showed optimal efficacy, although severe side effects were reported in 14-d regimens. Low dose Furazolidone was used in a further study, which led to significant decline in eradication rate[80]. Therefore, another study was conducted in which Furazolidone was administered only over the first 7 d of therapy, Yielding suboptimal eradication rates[69]. Consequently, 2 other Bismuth-based quadruple therapies were conducted in 10- and 14-d regimens in which Metronidazole was administered over the first half of therapy and was then replaced by Furazolidone over the second half. Both protocols had ideal results with fewer side effects[23,48].
Furazolidone has also been used in triple therapies in Iran[21,80-83]. Five studies have investigated the effects of 4-, 7- and 14-d Furazolidone-containing triple therapies, but only a 7-d protocol, in which a probiotic had been added to the regimen, could achieve > 90% PP eradication rate[82].
In Iran, the resistance of H. pylori to Furazolidone increased from 2003 to 2010, although the rise was not significant (0% to 4.5%) (Table 1). Furazolidone has low cost and is readily available in developing countries. When it was administered in low doses (50 mg BD), the efficacy of regimen was shown to be very low[83]. On the other hand, when administered in high doses (200 mg BD), if tolerated, the efficacy of regimen was comparable to that of Clarithromycin-containing regimens[79]. In order to lower the incidence of adverse effects, it is recommended to eliminate foods high in tyramine and also decrease the duration of Furazolidone administration to 5 or 7 d and replace it with Metronidazole for the remaining days or vice versa. It is obvious that the duration of therapy should not be less than 10 d. Four- or seven-day regimens have not been successful even if the bacteria are susceptible to Furazolidone[21].
Three Iranian studies have investigated the efficacy of 14-d Bismuth- and Clarithromycin-containing quadruple regimens. Only one of the studies could achieve 90% PP eradication rate[52,79,84]. However, if a Clarithromycin-containing 4-drug regimen is to be tried, hybrid therapy may be a better option since it has higher success rates.
Failure of H. pylori treatment depends on multiple factors related to both the bacterium and the host. In fact, the effects of antibiotics in vivo are not the same as those observed in vitro, since the antibiotics must diffuse to the gastric mucosal layer where the bacteria reside. Moreover, low gastric pH may compromise antibiotic activity. Most antibiotics have greatest activity at neutral pH; nevertheless, Clarithromycin especially has greatest activity at high pH (around 8) and metronidazole has greatest activity at lower pH (around 6). Thus, Clarithromycin is the only antibiotic that benefits from a high pH caused by PPI[85]. Furthermore, sometimes H. pylori transforms into coccoid shape, which keeps it from the effects of antibiotics[86]. Also, some strains, including Cag A-negative strains and those carrying Vac As2m2 allele, show resistance to antibiotics[87]. However, the most important factor influencing response to treatment is primary resistance to antibiotics which is increasing all over the world due to extensive use of antibiotics[12].
Among host factors, compliance to treatment plays an important role. Patients may not completely adhere to treatment due to adverse effects or combination of multiple drugs in multiple daily doses.
Besides, the patient’s underlying disease also affects the H. pylori eradication rate. Many studies have shown that patients with non ulcer dyspepsia have lower eradication rates compared to those with PUD[87,88].
Since low gastric pH lowers the effects of antibiotics, PPIs are administered to increase gastric pH. Most PPIs are metabolized by cytochrome P450 in the liver. Therefore, extensive metabolizer patients do not attain sufficient PPI levels to achieve optimal pH level for antibiotic effects[89].
Smoking is also another factor influencing the response to treatment[90]; it reduces gastric mucosal blood flow and increases gastric acid secretion; therefore lowering antibiotics activity.
All the mentioned factors should be kept in mind in patients with failure of treatment.
A second-line H. pylori therapy regimen may be considered optimal that can achieve > 80% PP eradication rate[18]. Few studies have addressed second-line therapeutic regimens in West Asia.
In a study by Minakari et al[91], 220 patients who had failed treatment with OABM were randomized to receive either OABC (C: Clarithromycin) or OBAzOf (Az: Azithromycin, Of: Ofloxacin). Per-protocol eradication rates were 74.7% and 86.7%, respectively.
In a study by Sotoudehmanesh et al[92], 80 patients who had failed treatment with 2 wk of Omeprazole + Amoxicillin + Bismuth + Metronidazole (OABM) received 2 wk of OTBF (F: Furazolidone, T: Tetracycline). Per-protocol eradication rate was 90%.
Fakheri et al[93] investigated the effects of a modified Bismuth- and Furazolidone-containing 14-d quadruple therapy on patients who had failed treatment with classic sequential therapy. The regimen contained Furazolidone only for the first 7 d. The per-protocol eradication rate was optimal (82.9%).
In a study by Sezikli et al[31] 30 patients who had failed treatment with standard triple therapy received Tetracycline instead of Clarithromycin. The per-protocol eradication rate was suboptimal (48.3%).
Another study investigated the effects of 7-d Omeprazole-Amoxicillin-Levofoxacin on 37 patients who had failed treatment with 14-d standard triple therapy. The per-protocol eradication rate was 58.2%[94].
In a study by Köksal et al[95], 56 patients who had failed treatment with 10-d standard triple therapy were randomized to receive either 10-d Ranitidine-Bismuth-Amoxicillin-Clarithromycin (RBAC) or 10-d Ranitidine-Bismuth-Metronidazole-Tetracycline (RBMT). The per-protocol eradication rates were 60.7% and 85.7%, respectively.
Another study investigated the efficacy of 14-d RBMT and 7-d RBAz (Az: Azithromycin) on 52 patients who had failed treatment with 14-d standard triple therapy[96]. The results were unsatisfactory (44.4% and 13% PP eradication rates, respectively).
Second-line therapy was also investigated by Sharara et al[97] in Lebanon. Forty five patients with failed standard triple therapy received Rabeprazole-Amoxicillin-Gatifloxacin for only 7 d. The per-protocol eradication rate was 84.4%.
No study in West Asia had dealt with patients who have failed second-line H. pylori eradication therapy.
According to Maastricht IV Consensus Report, the best therapeutic approach is to obtain gastric biopsy in order to identify suitable antibiotics by culture and antibiogram. However, there are some limitations. The results obtained in vitro are not reproduced exactly in vivo and vice versa. Furthermore, the sensitivity of H. pylori cultures has been reported to be less than 60%[98].
Some regimens have been proposed as third-line therapy. Bismuth-based quadruple therapies including quinolones or Furazolidone + Tetracycline are among the candidates[99]. Rifabutin is another drug introduced to be effective in third-line regimens[100,101]. However, its risks include myelotoxicity and severe ocular adverse effects, and if used extensively, may lead to resistant Mycobacterium tuberculosis strains in the future.
Therefore, it seems that the best option is still to treat based on antibiotic resistance patterns.
The studies evaluating the cost-effectiveness of H. pylori eradication regimens in West Asia are very scarce. In 2006, Sancar et al[102] reported 7-d Lansoprazole-Amoxicillin-Clarithromycin and 14-d Ranitidine-Bismuth-Amoxicillin-Metronidazole regimens as the most cost-effective regimens among 8 therapeutic options in Turkey. That study, however, was conducted in 2001 and therefore reflects the expenses of that time. We could not find a newer study on the pharmaco-economics of H. pylori eradication regimens in Turkey.
In 2013, Sardarian et al[68] compared the effects of hybrid vs sequential regimens in Iran. The PP eradication rates were 92.9% and 79.9%, respectively. The cost of therapy was 30.3$ for the hybrid and 20.5$ for the sequential regimen and Clarithromycin accounted for about 70% of the total price in each arm.
In Iran, Furazolidone, Bismuth, Metronidazole and Tetracycline are very cheap and readily available. A 10-d Pantoprazole-Amoxicillin-Bismuth-Furazolidone therapy costs 8.96$ and a 14-d Omeprazole-Bismuth-Tetracycline-Metronidazole costs 9.85$. Therefore, regarding the results of the present review, the latter 2 regimens seem more cost-effective in Iran.
We found no data about the pharmaco-economics of H. pylori treatment from other countries in West Asia.
Nonetheless, it is logical to assume that the cost-effectiveness of different H. pylori eradication regimens depends largely on the efficacy of the first-line option, since failure of the initial therapy leads to prescription of more expensive drugs with lower success rates and also additional costs of confirming H. pylori eradication.
Treatment of peptic ulcer disease, mucus-associated lymphoid tissue lymphoma and also prevention of peptic ulcer complications and even gastric cancer depends on definite H. pylori eradication. Recurrence of H. pylori infection 1 year after successful treatment is rare in developed countries but goes as high as 41% in developing countries[103]. Investigations have shown that recrudescence mostly accounts for the recurrences during the first year after treatment, while re-infection is responsible for those that occur after one year from the treatment.
Recurrence of H. pylori infection after successful eradication has been investigated in some countries in West Asia. The recurrence rate after 1 year of treatment has been reported to be 34% in Yemen[104], 41.6% in Turkey[105] and 5%-19.1% in Iranian adults and 14.7% in Iranian children[106,107].
Also, 2 other studies from Iran reported 2-year and 3-year recurrence rates to be 13.7% and 20.4%, respectively[108,109].
In conclusion, a significant number of successful H. pylori treatments result in recurrence after 1 year or more in West Asia. This should influence follow up strategies for patients in these countries.
The main limitation of the present narrative review is the unavailability of data about H. pylori culture results in each study.
Furthermore, heterogeneity was present and the studies differed in many aspects, including the underlying peptic disorder, dose of antibiotics, kinds of PPIs and duration of therapy. This could lead to discrepancy in eradication rates, since increased dose and duration of therapy would change eradication rates and the underlying peptic disorder would influence the results. Also, a significant number of studies had less than 100 patients in each therapeutic arm.
Language was another limitation of the present study and our research was restricted to English reports.
Considering the diverse effectiveness of different regimens in West Asian countries, it seems that the ideal therapeutic option must be chosen based on the individual pattern of antibiotic resistance of H. pylori in each single country. According to the results of studies conducted in West Asia, most of which were from Iran, 10-d Bismuth-Furazolidone/Metronidazole quadruple therapy, 14-d Clarithromycin-containing hybrid therapy and 14-d quadruple therapy including a proton pump inhibitor (PPI) + Bismuth + Tetracycline (500 mg QID) + Metronidazole (500 mg TDS) seem to be appropriate options among different therapeutic regimens used as the first-line protocols (Table 5). Among second-line therapeutic regimens, Bismuth-based quadruple therapies containing Tetracycline and Furazolidone/Metronidazole, triple therapy containing Amoxicillin and Gatifloxacin and Quadruple therapy including Bismuth + Azithromycin and Ofloxacin seem to be effective options (Table 5). Third-line therapies have not been evaluated in West Asia, but most guidelines recommend choosing optimal eradication regimen based on the pattern of antibiotic susceptibility of H. pylori. Rifabutin-containing triple therapy may also be a suitable option (Table 5). Although we investigated H. pylori eradication regimens in West Asia only, the clinical significance of the results is not limited to those countries situated in this geographic region. In fact, the results are transferrable to any region provided the patterns of resistance are the same.
| First-line therapeutic options: |
| 10-d Bismuth-Furazolidone Quadruple therapy: |
| Pantoprazole 40 mg BD + Amoxicillin 1 g BD + Bismuth 240 mg BD for 10 d; Metronidazole 500 mg BD just over the first 5 d and Furazolidone 200 mg BD over the second 5 d |
| 14-d Clarithromycin-containing Hybrid therapy: |
| Pantoprazole 40 mg BD + Amoxicillin 1 g BD for 14 d and Clarithromycin 500 mg BD + Tinidazole 500 mg BD just over the last 7 d |
| 14-d Omeprazole-Bismuth-Tetracycline-Metronidazole, if: |
| Omeprazole 20 mg BD + Bismuth 240 mg BD + Tetracycline 500 mg QID + Metronidazole 500 mg TDS for 14 d |
| Second-line therapeutic options: |
| 14-d Omeprazole-Bismuth-Tetracycline-Furazolidone: |
| Omeprazole 20 mg BD + Bismuth 240 mg BD + Tetracycline 500 mg BD + Furazolidone 200 mg BD (if Furazolidone is not used as first-line) |
| 14-d Ranitidine-Bismuth-Tetracycline-Metronidazole: |
| Ranitidine-Bismuth 400 mg BD + Tetracycline 500 mg BD + Metroidazole 500 mg BD |
| 7-d Rabeprazole-Amoxicillin-Gatifloxacin: |
| Rabeprazole 20 mg BD + Amoxicillin 1 g BD + Gatifloxacin 400 mg Daily |
| 14-d Omeprazole-Bismuth-Azithromycin-Ofloxacin: |
| Omeprazole 20 mg BD + Bismuth 240 mg BD + Azithromycin 250 mg BD + Ofloxacin 200 mg BD |
| Third-line therapeutic options: |
| The optimal regimen must be chosen according to the pattern of antibiotic susceptibility of H. pylori1 |
P- Reviewer: Abenavoli L, Andersen LP, Du YQ, Hu R, Jadallah KA S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16 Suppl 1:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 276] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, Yoonessi A, Tavangar M, Abedi BA, Sotoudehmanesh R. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Massarrat S, Saberi-Firoozi M, Soleimani A, Himmelmann GW, Hitzges M, Keshavarz H. Peptic ulcer disease, irritable bowel syndrome and constipation in two populations in Iran. Eur J Gastroenterol Hepatol. 1995;7:427-433. [PubMed] |
| 4. | Bani-Hani KE, Hammouri SM. Prevalence of Helicobacter pylori in Northern Jordan. Endoscopy based study. Saudi Med J. 2001;22:843-847. [PubMed] |
| 5. | Bener A, Uduman SA, Ameen A, Alwash R, Pasha MA, Usmani MA, AI-Naili SR, Amiri KM. Prevalence of Helicobacter pylori infection among low socio-economic workers. J Commun Dis. 2002;34:179-184. [PubMed] |
| 6. | Us D, Hasçelik G. Seroprevalence of Helicobacter pylori infection in an Asymptomatic Turkish population. J Infect. 1998;37:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | al-Moagel MA, Evans DG, Abdulghani ME, Adam E, Evans DJ, Malaty HM, Graham DY. Prevalence of Helicobacter (formerly Campylobacter) pylori infection in Saudia Arabia, and comparison of those with and without upper gastrointestinal symptoms. Am J Gastroenterol. 1990;85:944-948. [PubMed] |
| 8. | Al-Shamahy HA. Seroprevalence of Helicobacter pylori among children in Sana’a, Yemen. Ann Saudi Med. 2005;25:299-303. [PubMed] |
| 9. | Radhakrishnan S, al Nakib B, Kalaoui M, Patric J. Helicobacter pylori-associated gastritis in Kuwait: endoscopy-based study in symptomatic and asymptomatic children. J Pediatr Gastroenterol Nutr. 1993;16:126-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Lee SY. Future candidates for indications of Helicobacter pylori eradication: do the indications need to be revised? J Gastroenterol Hepatol. 2012;27:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1589] [Article Influence: 122.2] [Reference Citation Analysis (5)] |
| 12. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (1)] |
| 13. | Safaralizadeh R, Siavoshi F, Malekzadeh R, Akbari MR, Derakhshan MH, Sohrabi MR, Massarrat S. Antimicrobial effectiveness of furazolidone against metronidazole-resistant strains of Helicobacter pylori. East Mediterr Health J. 2006;12:286-293. [PubMed] |
| 14. | Majlesi A, Sayedin Khorasani M, Khalilian AR, Aslani MM, Jaefari M, Alikhani MY. Antibiotic Susceptibility of Helicobacter pylori Clinical Isolates in Hamadan, West of Iran. Int J Entric Pathog. 2013;1:9-11. [DOI] [Full Text] |
| 15. | Bindayna KM. Antibiotic susceptibilities of Helicobacter pylori. Saudi Med J. 2001;22:53-57. [PubMed] |
| 16. | Onder G, Aydin A, Akarca U, Tekin F, Ozutemiz O, Ilter T. High Helicobacter pylori resistance rate to clarithromycin in Turkey. J Clin Gastroenterol. 2007;41:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Sezgin O, Aslan G, Altintaş E, Tezcan S, Serin MS, Emekdaş G. Detection of point mutations on 23S rRNA of Helicobacter pylori and resistance to clarithromycin with PCR-RFLP in gastric biopsy specimens in Mersin, Turkey. Turk J Gastroenterol. 2008;19:163-167. [PubMed] |
| 18. | Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010;8:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Malekzadeh R, Merat S, Derakhshan MH, Siavoshi F, Yazdanbod A, Mikaeli J, Sotoudemanesh R, Sotoudeh M, Farahvash MJ, Nasseri-Moghaddam S. Low Helicobacter pylori eradication rates with 4- and 7-day regimens in an Iranian population. J Gastroenterol Hepatol. 2003;18:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Mirbagheri SA, Hasibi M, Abouzari M, Rashidi A. Triple, standard quadruple and ampicillin-sulbactam-based quadruple therapies for H. pylori eradication: a comparative three-armed randomized clinical trial. World J Gastroenterol. 2006;12:4888-4891. [PubMed] |
| 21. | Mirzaee V, Rezahosseini O. Randomized control trial: Comparison of Triple Therapy plus Probiotic Yogurt vs. Standard Triple Therapy on Helicobacter Pylori Eradication. Iran Red Crescent Med J. 2012;14:657-666. [PubMed] |
| 22. | Aminian K, Farsad F, Ghanbari A, Fakhreih S, Hasheminasab SM. A randomized trial comparing four Helicobacter pylori eradication regimens: standard triple therapy, ciprofloxacin based triple therapy, quadruple and sequential therapy. Trop Gastroenterol. 2010;31:303-307. [PubMed] |
| 23. | Riahizadeh S, Malekzadeh R, Agah S, Zendehdel N, Sotoudehmanesh R, Ebrahimi-Dariani N, Pourshams A, Vahedi H, Mikaeli J, Khatibian M. Sequential metronidazole-furazolidone or clarithromycin-furazolidone compared to clarithromycin-based quadruple regimens for the eradication of Helicobacter pylori in peptic ulcer disease: a double-blind randomized controlled trial. Helicobacter. 2010;15:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Sarkeshikian SS, Iranikhah A, Ghadir MR. Azithromycin based triple therapy versus standard clarithromycin based triple therapy in eradication of Helicobacter pylori infection in Iran: a randomized controlled clinical trial. Turk J Gastroenterol. 2013;24:10-14. [PubMed] |
| 25. | Keshavarz AA, Bashiri H, Rahbar M. Omeprazole-based triple therapy with low-versus high-dose of clarithromycin plus amoxicillin for H pylori eradication in Iranian population. World J Gastroenterol. 2007;13:930-933. [PubMed] |
| 26. | Seyedmajidi S, Mirsattari D, Zojaji H, Zanganeh E, Seyyedmajidi M, Almasi S, Zali M. Penbactam for Helicobacter pylori eradication: a randomised comparison of quadruple and triple treatment schedules in an Iranian population. Arab J Gastroenterol. 2013;14:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Taghavi SA, Jafari A, Eshraghian A. Efficacy of a new therapeutic regimen versus two routinely prescribed treatments for eradication of Helicobacter pylori: a randomized, double-blind study of doxycycline, co-amoxiclav, and omeprazole in Iranian patients. Dig Dis Sci. 2009;54:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Aladağ M, Kantarçeken B, Karincaoğlu M, Sertkaya A, Cikim K, Harputluoğlu MM, Demirel U, Alan H, Ateş F, Yildirim B. Comparison of pantoprazole- vs. omeprazole- based triple therapy regimens in the treatment of Helicobacter pylori infection and duodenal ulcer healing in a Turkish population. Turk J Gastroenterol. 2005;16:242-243. [PubMed] |
| 29. | Aydin A, Onder G, Akarca U, Tekin F, Tuncyurek M, Ilter T. Comparison of 1- and 2-week pantoprazole-based triple therapies in clarithromycin-sensitive and resistant cases. Eur J Intern Med. 2007;18:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Gumurdulu Y, Serin E, Ozer B, Kayaselcuk F, Ozsahin K, Cosar AM, Gursoy M, Gur G, Yilmaz U, Boyacioglu S. Low eradication rate of Helicobacter pylori with triple 7-14 days and quadriple therapy in Turkey. World J Gastroenterol. 2004;10:668-671. [PubMed] |
| 31. | Sezikli M, Cetinkaya ZA, Güzelbulut F, Yeşil A, Alt Nöz ME, Ulu N, Ovünç Kurdaş AO. Efficacy of the Combination of Tetracycline, Amoxicillin, and Lansoprazole in the Eradication of Helicobacter pylori in Treatment-Naïve Patients and in Patients Who Are Not Responsive to Clarithromycin-Based Regimens: A Pilot Study. Gut Liver. 2012;6:41-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Uygun A, Kadayifçi A, Yeşilova Z, Savaş MC, Ateş Y, Karslioğlu Y, Ciğerim M, Bağci S, Dağalp K. Recent success of pantoprazole -or lansoprazole- based clarithromycin plus amoxicillin treatment in the eradication of Helicobacter pylori. Turk J Gastroenterol. 2004;15:219-224. [PubMed] |
| 33. | Yaşar B, Abut E, Kayadıbı H, Toros B, Sezıklı M, Akkan Z, Keskın Ö, Övünç Kurdaş O. Efficacy of probiotics in Helicobacter pylori eradication therapy. Turk J Gastroenterol. 2010;21:212-217. [PubMed] |
| 34. | Alkim H, Iscan M, Oz F. Effectiveness of ranitidine bismuth citrate and proton pump inhibitor based triple therapies of Helicobacter pylori in Turkey. Libyan J Med. 2011;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Songür Y, Senol A, Balkarli A, Baştürk A, Cerçi S. Triple or quadruple tetracycline-based therapies versus standard triple treatment for Helicobacter pylori treatment. Am J Med Sci. 2009;338:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Uygun A, Kadayifci A, Safali M, Ilgan S, Bagci S. The efficacy of bismuth containing quadruple therapy as a first-line treatment option for Helicobacter pylori. J Dig Dis. 2007;8:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Nadir I, Yonem O, Ozin Y, Kilic ZM, Sezgin O. Comparison of two different treatment protocols in Helicobacter pylori eradication. South Med J. 2011;104:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Polat Z, Kadayifci A, Kantarcioglu M, Ozcan A, Emer O, Uygun A. Comparison of levofloxacin-containing sequential and standard triple therapies for the eradication of Helicobacter pylori. Eur J Intern Med. 2012;23:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Uygun A, Kadayifci A, Yesilova Z, Safali M, Ilgan S, Karaeren N. Comparison of sequential and standard triple-drug regimen for Helicobacter pylori eradication: a 14-day, open-label, randomized, prospective, parallel-arm study in adult patients with nonulcer dyspepsia. Clin Ther. 2008;30:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Graham DY, Dore MP. Helicobacter pylori therapy demystified. Helicobacter. 2011;16:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Milani M, Ghotaslou R, Akhi MT, Nahaei MR, Hasani A, Somi MH, Rafeey M, Sharifi Y. The status of antimicrobial resistance of Helicobacter pylori in Eastern Azerbaijan, Iran: comparative study according to demographics. J Infect Chemother. 2012;18:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Mohammadi M, Doroud D, Mohajerani N, Massarrat S. Helicobacter pylori antibiotic resistance in Iran. World J Gastroenterol. 2005;11:6009-6013. [PubMed] |
| 43. | Shokrzadeh L, Jafari F, Dabiri H, Baghaei K, Zojaji H, Alizadeh AH, Aslani MM, Zali MR. Antibiotic susceptibility profile of Helicobacter pylori isolated from the dyspepsia patients in Tehran, Iran. Saudi J Gastroenterol. 2011;17:261-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Zendedel A, Moradimoghadam F, Almasi V, Zivarifar H. Antibiotic resistance of Helicobacter pylori in Mashhad, Iran. J Pak Med Assoc. 2013;63:336-339. [PubMed] |
| 45. | Tüzün Y, Bayan K, Yilmaz S, Dursun M, Ozekinci T. The prevalence of primary and secondary Helicobacter pylori resistance to clarithromycin and probable contributing cofactors: data from southeastern Anatolia. Hepatogastroenterology. 2008;55:289-293. [PubMed] |
| 46. | Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Agah S, Shazad B, Abbaszadeh B. Comparison of azithromycin and metronidazole in a quadruple-therapy regimen for Helicobacter pylori eradication in dyspepsia. Saudi J Gastroenterol. 2009;15:225-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Khatibian M, Ajvadi Y, Nasseri-Moghaddam S, Ebrahimi-Dariani N, Vahedi H, Zendehdel N, Sotoudehmanesh R, Agah S, Mikaeli J, Pourshams A. Furazolidone-based, metronidazole-based, or a combination regimen for eradication of Helicobacter pylori in peptic ulcer disease. Arch Iran Med. 2007;10:161-167. [PubMed] |
| 49. | Malekzadeh R, Ansari R, Vahedi H, Siavoshi F, Alizadeh BZ, Eshraghian MR, Vakili A, Saghari M, Massarrat S. Furazolidone versus metronidazole in quadruple therapy for eradication of Helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther. 2000;14:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Mousavi S, Toussy J, Yaghmaie S, Zahmatkesh M. Azithromycin in one week quadruple therapy for H pylori eradication in Iran. World J Gastroenterol. 2006;12:4553-4556. [PubMed] |
| 51. | Shidfar F, Agah S, Ekhlasi G, Salehpour A, Ghourchian S. Lycopene an adjunctive therapy for Helicobacter pylori eradication: a quasi-control trial. J Complement Integr Med. 2012;9:Article 14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Sotudehmanesh R, Malekzadeh R, Fazel A, Massarrat S, Ziad-Alizadeh B, Eshraghian MR. A randomized controlled comparison of three quadruple therapy regimens in a population with low Helicobacter pylori eradication rates. J Gastroenterol Hepatol. 2001;16:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | Akyildiz M, Akay S, Musoglu A, Tuncyurek M, Aydin A. The efficacy of ranitidine bismuth citrate, amoxicillin and doxycycline or tetracycline regimens as a first line treatment for Helicobacter pylori eradication. Eur J Intern Med. 2009;20:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Köksal AS, Onder FO, Torun S, Parlak E, Sayilir A, Tayfur O, Yildiz H, Ozbalci GS, Kayaçetin E. Twice a day quadruple therapy for the first-line treatment of Helicobacter pylori in an area with a high prevalence of background antibiotic resistance. Acta Gastroenterol Belg. 2013;76:34-37. [PubMed] |
| 55. | Sezgin O, Altintaş E, Uçbilek E, Tataroğlu C. Bismuth-based therapies for the first step eradication of Helicobacter pylori. Turk J Gastroenterol. 2006;17:90-93. [PubMed] |
| 56. | Kadayifci A, Uygun A, Polat Z, Kantarcioğlu M, Kılcıler G, Başer O, Ozcan A, Emer O. Comparison of bismuth-containing quadruple and concomitant therapies as a first-line treatment option for Helicobacter pylori. Turk J Gastroenterol. 2012;23:8-13. [PubMed] |
| 57. | Uygun A, Kadayifci A, Yesilova Z, Ates Y, Safali M, Ilgan S, Bagci S, Dagalp K. Poor efficacy of ranitidine bismuth citrate-based triple therapies for Helicobacter pylori eradication. Indian J Gastroenterol. 2007;26:174-177. [PubMed] |
| 58. | Siavoshi F, Saniee P, Latifi-Navid S, Massarrat S, Sheykholeslami A. Increase in resistance rates of H. pylori isolates to metronidazole and tetracycline--comparison of three 3-year studies. Arch Iran Med. 2010;13:177-187. [PubMed] |
| 59. | Al-Qurashi AR, El-Morsy F, Al-Quorain AA. Evolution of metronidazole and tetracycline susceptibility pattern in Helicobacter pylori at a hospital in Saudi Arabia. Int J Antimicrob Agents. 2001;17:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Eltahawy AT. Prevalence of primary Helicobacter pylori resistance to several antimicrobials in a Saudi Teaching Hospital. Med Princ Pract. 2002;11:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Larbi EB. The changing sensitivity of Helicobacter pylori to metronidazole. Saudi J Gastroenterol. 1999;5:124-128. [PubMed] |
| 62. | Marie MAM. Patterns of Helicobacter pylori Resistance to Metronidazole, Clarithormycin and Amoxicillin in Saudi Arabia. J Bacteriol Virol. 2008;38:173-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Ozbey G, Bahcecioglu IH, Nuri Acik M. Resistance Rates to Various Antimicrobial Agents of Helicobacter pylori Isolates in Eastern Turkey. Inter J Mol Clin Microbiol. 2012;2:148-152. |
| 64. | Yasser AS, Haneen Y. Treatment of helicobacter pylori, comparison of three regimens, a double blind randomized trial. J Gastroenterol Hepatol Research. 2013;2:699-702. |
| 65. | van der Wouden EJ, Thijs JC, van Zwet AA, Sluiter WJ, Kleibeuker JH. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol. 1999;94:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol. 2010;105:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 67. | Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 2013;347:f4587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (95)] |
| 68. | Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter. 2013;18:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Fakheri H, Taghvaei T, Hosseini V, Bari Z. A comparison between sequential therapy and a modified bismuth-based quadruple therapy for Helicobacter pylori eradication in Iran: a randomized clinical trial. Helicobacter. 2012;17:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Cetinkaya ZA, Sezikli M, Güzelbulut F, Coşgun S, Düzgün S, Kurdaş OO. Comparison of the efficacy of the two tetracycline-containing sequential therapy regimens for the eradication of Helicobacter pylori: 5 days versus 14 days amoxicillin. Helicobacter. 2010;15:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Yakut M, Çinar K, Seven G, Bahar K, Özden A. Sequential therapy for Helicobacter pylori eradication. Turk J Gastroenterol. 2010;21:206-211. [PubMed] |
| 72. | Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012;5:23-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Berning M, Krasz S, Miehlke S. Should quinolones come first in Helicobacter pylori therapy? Therap Adv Gastroenterol. 2011;4:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Erçin CN, Uygun A, Toros AB, Kantarcioğlu M, Kilciler G, Polat Z, Bağci S. Comparison of 7- and 14-day first-line therapies including levofloxacin in patients with Helicobacter pylori positive non-ulcer dyspepsia. Turk J Gastroenterol. 2010;21:12-16. [PubMed] |
| 76. | Seven G, Cinar K, Yakut M, Idılman R, Ozden A. Assessment of Helicobacter pylori eradication rate of triple combination therapy containing levofloxacin. Turk J Gastroenterol. 2011;22:582-586. [PubMed] |
| 77. | Sharara AI, Chaar HF, Racoubian E, Moukhachen O, Barada KA, Mourad FH, Araj GF. Efficacy of two rabeprazole/gatifloxacin-based triple therapies for Helicobacter pylori infection. Helicobacter. 2004;9:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Daghaghzadeh H, Emami MH, Karimi S, Raeisi M. One-week versus two-week furazolidone-based quadruple therapy as the first-line treatment for Helicobacter pylori infection in Iran. J Gastroenterol Hepatol. 2007;22:1399-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Fakheri H, Malekzadeh R, Merat S, Khatibian M, Fazel A, Alizadeh BZ, Massarrat S. Clarithromycin vs. furazolidone in quadruple therapy regimens for the treatment of Helicobacter pylori in a population with a high metronidazole resistance rate. Aliment Pharmacol Ther. 2001;15:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 80. | Fakheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2004;19:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Ghadir MR, Shafaghi A, Iranikhah A, Pakdin A, Joukar F, Mansour-Ghanaei F. Furazolidone, amoxicillin and omeprazole with or without bismuth for eradication of Helicobacter pylori in peptic ulcer disease. Turk J Gastroenterol. 2011;22:1-5. [PubMed] |
| 82. | Ahmad K, Fatemeh F, Mehri N, Maryam S. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr. 2013;23:79-84. [PubMed] |
| 83. | Roghani HS, Massarrat S, Shirekhoda M, Butorab Z. Effect of different doses of furazolidone with amoxicillin and omeprazole on eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2003;18:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Shavakhi A, Tabesh E, Yaghoutkar A, Hashemi H, Tabesh F, Khodadoostan M, Minakari M, Shavakhi S, Gholamrezaei A. The effects of multistrain probiotic compound on bismuth-containing quadruple therapy for Helicobacter pylori infection: a randomized placebo-controlled triple-blind study. Helicobacter. 2013;18:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Morini S, Zullo A, Hassan C, Lorenzetti R, Stella F, Martini MT. Gastric cardia inflammation: role of Helicobacter pylori infection and symptoms of gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:2337-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Kusters JG, Gerrits MM, Van Strijp JA, Vandenbroucke-Grauls CM. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65:3672-3679. [PubMed] |
| 87. | van Doorn LJ, Schneeberger PM, Nouhan N, Plaisier AP, Quint WG, de Boer WA. Importance of Helicobacter pylori cagA and vacA status for the efficacy of antibiotic treatment. Gut. 2000;46:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Gisbert JP, Marcos S, Gisbert JL, Pajares JM. Helicobacter pylori eradication therapy is more effective in peptic ulcer than in non-ulcer dyspepsia. Eur J Gastroenterol Hepatol. 2001;13:1303-1307. [PubMed] |
| 89. | Hokari K, Sugiyama T, Kato M, Saito M, Miyagishima T, Kudo M, Nishikawa K, Ishizuka J, Komatsu Y, Mizushima T. Efficacy of triple therapy with rabeprazole for Helicobacter pylori infection and CYP2C19 genetic polymorphism. Aliment Pharmacol Ther. 2001;15:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Labenz J, Stolte M, Blum AL, Jorias I, Leverkus F, Sollböhmer M, Bertrams J, Börsch G. Intragastric acidity as a predictor of the success of Helicobacter pylori eradication: a study in peptic ulcer patients with omeprazole and amoxicillin. Gut. 1995;37:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Minakari M, Davarpanah Jazi AH, Shavakhi A, Moghareabed N, Fatahi F. A randomized controlled trial: efficacy and safety of azithromycin, ofloxacin, bismuth, and omeprazole compared with amoxicillin, clarithromycin, bismuth, and omeprazole as second-line therapy in patients with Helicobacter pylori infection. Helicobacter. 2010;15:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Sotoudehmanesh R, Malekzadeh R, Vahedi H, Dariani NE, Asgari AA, Massarrat S. Second-line Helicobacter pylori eradication with a furazolidone-based regimen in patients who have failed a metronidazole-based regimen. Digestion. 2001;64:222-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Fakheri H, Bari Z, Sardarian H. A modified bismuth-containing quadruple therapy including a short course of furazolidone for Helicobacter pylori eradication after sequential therapy failure. Helicobacter. 2012;17:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Ermis F, Akyuz F, Uyanikoglu A, Kurt R, Pinarbasi B, Nazik H, Kaymakoglu S, Mungan Z. Second-line levofloxacin-based triple therapy’s efficiency for Helicobacter pylori eradication in patients with peptic ulcer. South Med J. 2011;104:579-583. [PubMed] |
| 95. | Köksal AS, Parlak E, Filik L, Yolcu OF, Odemiş B, Ulker A, Saşmaz N, Ozden A, Sahin B. Ranitidine bismuth citrate-based triple therapies as a second-line therapy for Helicobacter pylori in Turkish patients. J Gastroenterol Hepatol. 2005;20:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Altintaş E, Ulu O, Sezgin O, Aydin O, Camdeviren H. Comparison of ranitidine bismuth citrate, tetracycline and metronidazole with ranitidine bismuth citrate and azithromycin for the eradication of Helicobacter pylori in patients resistant to PPI based triple therapy. Turk J Gastroenterol. 2004;15:90-93. [PubMed] |
| 97. | Sharara AI, Chaar HF, Aoun E, Abdul-Baki H, Araj GF, Kanj SS. Efficacy and safety of rabeprazole, amoxicillin, and gatifloxacin after treatment failure of initial Helicobacter pylori eradication. Helicobacter. 2006;11:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Hsu PI, Peng NJ. H. pylori Eradication Therapy. Gastroenterol Res Pract. 2013;2013:935635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 99. | Gisbert JP, Gisbert JL, Marcos S, Jimenez-Alonso I, Moreno-Otero R, Pajares JM. Empirical rescue therapy after Helicobacter pylori treatment failure: a 10-year single-centre study of 500 patients. Aliment Pharmacol Ther. 2008;27:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 101. | Van der Poorten D, Katelaris PH. The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment Pharmacol Ther. 2007;26:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Sancar M, Izzettin FV, Apikoglu-Rabus S, Besisik F, Tozun N, Dulger G. Pharmacoeconomic comparison of Helicobacter pylori eradication regimens. Pharm World Sci. 2006;28:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 103. | Xia HX, Talley NJ, Keane CT, O’Morain CA. Recurrence of Helicobacter pylori infection after successful eradication: nature and possible causes. Dig Dis Sci. 1997;42:1821-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Gunaid AA, Hassan NA, Murray-Lyon IM. Recurrence of Helicobacter pylori infection 1 year after successful treatment: prospective cohort study in the Republic of Yemen. Eur J Gastroenterol Hepatol. 2004;16:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 105. | Gürel S, Beşişk F, Demir K, Mungan Z, Kaymakoğlu S, Boztaş G, Cakaloğlu Y, Yeğinsü O, Okten A. After the eradication of Helicobacter pylori infection, relapse is a serious problem in Turkey. J Clin Gastroenterol. 1999;28:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Mansour-Ghanaei F, Taefeh N, Joukar F, Besharati S, Naghipour M, Nassiri R. Recurrence of Helicobacter pylori infection 1 year after successful eradication: a prospective study in Northern Iran. Med Sci Monit. 2010;16:CR144-CR148. [PubMed] |
| 107. | Najafi M, Sobhani M, Khodadad A, Farahmand F, Motamed F. Reinfection Rate after Successful Helicobacter pylori Eradication in Children. Iran J Pediatr. 2010;20:58-62. [PubMed] |
| 108. | Tirgar Fakheri H, Eshqi F. The Recurrence Rate of Helicobacter Pylori Infection 2 Years After Eradication. J Mazand Univer Med Sci. 2007;17:72-78. |
| 109. | Zendehdel N, Nasseri-Moghaddam S, Malekzadeh R, Massarrat S, Sotoudeh M, Siavoshi F. Helicobacter pylori reinfection rate 3 years after successful eradication. J Gastroenterol Hepatol. 2005;20:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Siavoshi F, Safari F, Doratotaj D, Khatami GR, Fallahi GH, Mirnaseri MM. Antimicrobial resistance of Helicobacter pylori isolates from Iranian adults and children. Arch Iran Med. 2006;9:308-314. [PubMed] |
| 111. | Baglan PH, Bozdayi G, Ozkan M, Ahmed K, Bozdayi AM, Ozden A. Clarithromycin resistance prevalence and Icea gene status in Helicobacter Pylori clinical isolates in Turkish patients with duodenal ulcer and functional dyspepsia. J Microbiol. 2006;44:409-416. [PubMed] |
| 112. | Sharara AI, Chedid M, Araj GF, Barada KA, Mourad FH. Prevalence of Helicobacter pylori resistance to metronidazole, clarithromycin, amoxycillin and tetracycline in Lebanon. Int J Antimicrob Agents. 2002;19:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Alfaresi MS, Elkoush AA. Characterization of clarithromycin resistance in isolates of Helicobacter pylori from the UAE. Indian J Gastroenterol. 2010;29:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Zefenkey Z, Naser M, Balach O. Primary clarithromycin resistence of helicobacter pylori by fluorescent in situ hybridization in Aleppo-Syria. Inter J Pharm Pharm Sci. 2012;4:217-221. |