Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9626
Revised: January 7, 2014
Accepted: May 23, 2014
Published online: July 28, 2014
Processing time: 271 Days and 17 Hours
Primary malignant melanoma originating in the colon is an extremely rare disease. Herein, we report a case of primary melanoma of the ascending colon. The patient was a 57-year-old male who was admitted to our hospital for persistent abdominal pain and episodes of bloody stool, nausea and vomiting. A computed tomography scan revealed lower intestinal intussusception and enlarged lymph nodes in the abdominal cavity and retroperitoneum. During laparoscopic operation, multiple enlarged lymph nodes were found. Several segments of the proximal small intestine were incarcerated into the distal small intestine, forming an internal hernia and obstruction. The necrotic terminal ileum was invaginated into the ascending cecum. Subsequently, adhesive internal hernia reduction and palliative right hemicolectomy were performed. Pathologic examination of the excised specimen revealed a polypoid mass in the ascending colon. Histological examination showed epithelioid and spindle tumor cells with obvious cytoplasmic melanin deposition. Immunohistochemical staining revealed that the tumor cells were positive for S-100, HMB-45 and vimentin, confirming the diagnosis of melanoma. The patient history and a thorough postoperative investigation excluded the preexistence or coexistence of a primary lesion elsewhere in the skin, anus or oculus or at other sites. Thus, we consider our case to represent an aggressive primary colon melanoma presenting as ileocecal intussusception and intestinal obstruction.
Core tip: Primary malignant melanoma originating in colon is an extremely rare disease. Herein, we report a case of primary colon melanoma causing ileocecal intussusception. A misdiagnosis of intestinal lymphoma, with widespread abdominal lymph nodes metastasis, was made before and during the operation. Immunohistochemical staining confirmed the diagnosis of melanoma.
- Citation: Li WX, Wei Y, Jiang Y, Liu YL, Ren L, Zhong YS, Ye LC, Zhu DX, Niu WX, Qin XY, Xu JM. Primary colonic melanoma presenting as ileocecal intussusception: Case report and literature review. World J Gastroenterol 2014; 20(28): 9626-9630
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9626.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9626
In the clinic, malignant gastrointestinal melanomas are relatively rare and frequently metastasize from cutaneous primaries. However, primary melanomas can also originate from certain regions within the gastrointestinal tract (GIT), including the esophagus, small intestine, rectum and anus[1]. The small intestine has the highest predilection for the development of metastatic melanoma due to its abundant blood supply to the GIT, and the anorectum is the most common site for primary GIT melanomas due to the presence of melanocytes[2,3]. The colon is deemed to be an especially uncommon site for melanoma, the incidence of which is extremely low compared with that of other types of colon cancers. Only 12 cases of colon melanoma have been reported, based on a recent literature survey[4]. Until now, the existence of primary melanoma in the colon has remained controversial, as the colon does not naturally harbor any melanocytes. Due to its low incidence, there is no comprehensive understanding of the pathogenesis and natural history of colon melanoma. Moreover, large databases have not been well established, and a standardized treatment strategy is yet to be determined. Despite the poor prognosis, surgical operation remains the mainstay of treatment for patients with colon melanoma, with either curative or palliative intent. In this paper, a case of solitary malignant melanoma of the ascending colon, presenting as ileocecal intussusception and intestinal obstruction, is presented, and a review of the relevant literature is provided.
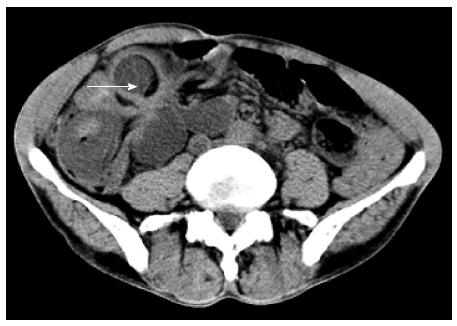
The patient was a 57-year-old male who was referred to our hospital with a complaint of persistent lower right abdominal pain for 5 d and episodes of bloody stool, nausea and vomiting. The patient’s personal and family medical histories were unremarkable, except that he had suffered from hypertension for many years and that his father had died of lymphoma. A plain abdominal computed tomography (CT) scan revealed extensively dilated intestines and right lower intestinal intussusception involving a long segment of the small intestine. Enlarged lymph nodes were revealed within the abdominal cavity and retroperitoneum, part of which had merged into large blocks, with an imaging appearance similar to that of lymphoma (Figure 1). Supine abdominal radiography displayed pneumatic and dilated convolutions, and upright abdominal radiography disclosed air-fluid levels in the left upper abdomen, suggestive of lower intestinal obstruction. On physical examination, the abdomen was extensively distended, with no visible intestinal pattern or peristaltic waves. Tenderness and rebound tenderness could be felt in the right lower quadrant on deep palpation.
Laparotomy exploration of the patient was performed. No palpable mass was found on the liver. Multiple enlarged intraperitoneal, retroperitoneal and mesenteric lymph nodes were discovered, parts of which had merged into large blocks. The wall of the small intestine was also studded with miliary lymph nodes and edematous with exudates. Several segments of the proximal small intestine were incarcerated into the distal small intestine, forming an internal hernia. However, there were no obvious signs of necrotic changes of the incarcerated intestines. Approximately 10 cm of the necrotic terminal ileum was invaginated into the cecum and the ascending colon. Judging from intraoperative findings and preoperative imaging results, an intraoperative diagnosis of intestinal lymphoma with widespread abdominal lymph node metastasis and secondary ileocecal intussusception and multiple internal hernias was made. At last, the patient underwent adhesive internal hernia reduction and palliative right hemicolectomy.
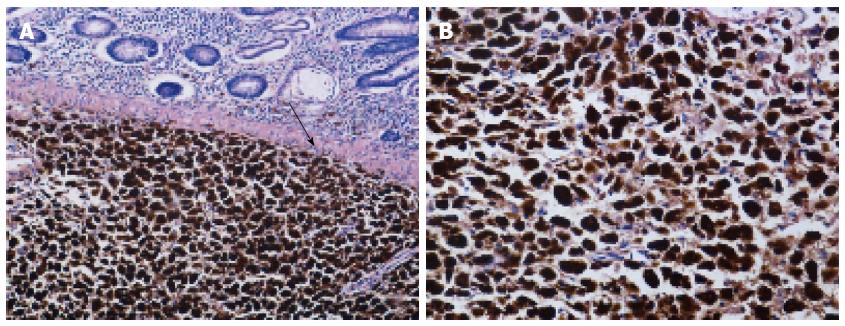
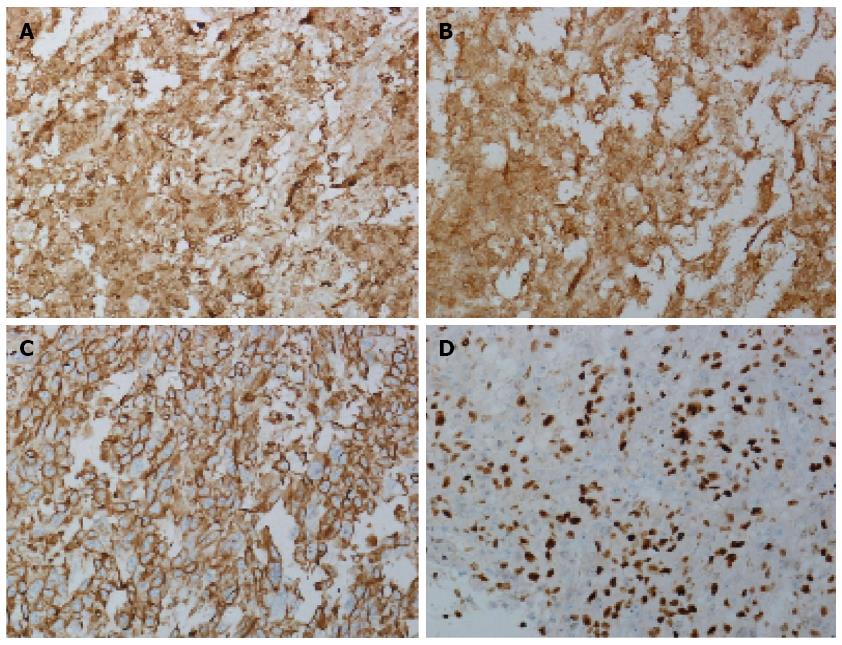
Macroscopic examination of the excised specimen showed that approximately 10 cm of the necrotic terminal ileum was invaginated into the colon. A 4 cm × 2.5 cm × 2.5 cm polypoid mass was located in the ascending colon, with a dull-gray cut surface. Microscopic examination revealed that tumor cells had invaded into the submucosa, accompanied by mucous hyperemia and mucosa necrosis, suggesting pathological changes due to acute intestinal obstruction (Figure 2A). Three of eight resected mesenteric lymph nodes were positive for metastases (3/8), and a total of 16 intestinal lymph nodes were all negative (0/16). Microscopic analysis revealed epithelioid and spindle cells with obvious melanin granules in the cytoplasm. The melanin was so rich in most cells that the nucleolus could not be distinguished, even at higher magnification (Figure 2B). A definite diagnosis of colon melanoma was based on immunohistochemical examination, revealing that nearly all of the tumor cells were positive for S-100, HMB-45 and vimentin (Figure 3A-C). Ki-67, a cell cycle-associated antigen that is a marker of proliferation, was present in 80% of cells (Figure 3D).
To exclude the possibility of metastasis, the patient underwent thorough postoperative dermatologic and ophthalmologic examinations, including ophthalmoscopy and a whole-body CT scan, with no suspicious primary lesions found at other sites. Postoperative blood investigations revealed anemia and hypoproteinemia, so the patient received blood transfusion and nutritional support treatment. After 1 wk of hospitalization, the patient was discharged with a passable performance status and appetite. No severe complications related to the operation occurred in the patient during either hospitalization or follow-up. However, the patient was in the final phase of a progressive life-limiting illness, and he died only 1 mo after discharge due to a worsening condition.
Malignant GIT melanoma is an uncommon clinical entity with a poor prognosis. The vast majority are frequently metastases from cutaneous primaries. Approximately more than 4% of patients dying from melanoma will have certain parts of their GIT involved during their lifetime, and the percentage reaches 60% by autopsy[5]. The small intestine is the most common site for metastatic melanoma, followed by the large intestine and stomach[6]. Compared with metastatic melanoma, primary melanomas originating in the small and large intestines are relatively rare events[7]. Only few cases with primary intestinal melanoma have been reported so far.
More than half of adult intestinal intussusceptions are caused by malignant tumors, such as lymphomas and benign tumors, including lipoma and leiomyoma. In contrast, adult intestinal intussusceptions attributed to melanoma are extremely scarce. In the small intestine, the most common clinical presentation of primary melanoma is intestinal obstruction secondary to intussusception[8,9]. Although metastatic melanoma in the small intestine is more frequent than primary tumors, these metastases seldom present as intestinal intussusception[2]. Due to its special anatomical features, the ileocecum has a predilection for the formation of intussusception under certain pathological conditions. Regarding cases of ileocecal intussusception caused by colon or ileocecal melanoma, only two studies can be found in the literature[10,11]. The case described above is an aggressive primary melanoma of the ascending colon, presenting as ileocecal intussusception and multiple small intestine incarcerations. To the best of our knowledge, this represents the first complex case under such special circumstances so far.
In their early stages, GIT melanomas are usually asymptomatic. Diagnosis is frequently delayed until the occurrence of a potentially life-threatening complication, such as intestinal intussusception, obstruction, bleeding or perforation[12]. For most patients with metastatic GIT melanoma, a surgical procedure is performed on an emergency basis[13]. The patient in our case mainly presented typical symptoms associated with intestinal obstruction. Although a CT scan revealed lower intestinal intussusception and extensive intestinal dilatation, no obvious intestinal tumor was visible due to lack of contrast-enhanced CT. However, the enlarged intraperitoneal and retroperitoneal lymph nodes were in accordance with the manifestations of lymphoma. During intraoperative exploration, gray-white blocks of merged lymph nodes located at the root of the mesentery were found, with an appearance also hinting of lymphoma. Thus, a misdiagnosis of intestinal lymphoma, with widespread abdominal lymph nodes metastasis and secondary ileocecal intussusception, was made. Based on our findings and experience in the process of treatment, we can conclude that even in exigent circumstances, for a patient suspected of having malignant tumors, contrast-enhanced CT or other more effective radiological examinations are requisite if the patient’s general condition permits. In an attempt to achieve higher rates of diagnosis, intraoperative frozen-section analysis of the metastatic node should be performed, especially when the suspected primary tumor could not be found.
Patients with primary GIT melanoma have associated with a worse prognosis than do patients with cutaneous melanoma. Melanomas arising from mucosal surfaces, including the intestinal mucosa, tend to be more aggressive due to the rich lymphatic and vascular supplies of the mucosa. According to a study of metastatic colon melanoma, the median survival times of patients undergoing curative or palliative resection was 48.9 mo and 5.7 mo, respectively[14]. This poor prognosis may be related to delayed diagnosis and severe complications that have already occurred in most cases. One study involving a smaller number of patients noted that intestinal perforation and obstruction were directly associated with the dismal prognosis of primary colon melanoma[10]. However, a systematic review of retrospective case series to elucidate the factors influencing prognosis is lacking.
The actual existence of primary melanoma in the intestine remains a controversial topic. Different theories concerning the origin of malignant melanoma of the intestine have been proposed. According to current theories of embryogenesis, melanocytes develop from neural crest-derived precursors and migrate along the branchial arches. Although neural crest-derived cells are abundant within the intestine, factors released from the branchial arches deprive these cells of potential melanocyte differentiation[15]. Another argument is that APUD cells might account for the cellular origin of primary melanoma in the intestine because these cells can differentiate into melanocytes[16]. Given that most GIT melanomas are metastatic, comprehensive medical history investigation, relevant physical examination and imaging studies are required for a definitive diagnosis. In the setting of GIT melanomas with unknown primaries, the most accepted explanation is the model of tumor regression. Certain scholars hold that GIT melanomas with unknown primaries may be metastatic, with either unidentified or spontaneously regressed cutaneous malignancy. In a study consisting of 437 cutaneous melanoma cases, 12.3% manifested at least partial regression[17]. Although we consider our case to be primary disease, the possibility of metastasis from an otherwise regressed cutaneous melanoma cannot be completely excluded.
As in cutaneous melanoma, melanoma cells of the GIT are composed of different proportions of epithelioid and spindle cells. In most cases, melanin pigment deposition in the cytoplasm is helpful in diagnosis. However, approximately 30% of tumor cells are completely amelanotic. A panel of immunohistochemical stains, including stains for S-100, HMB-45 and vimentin, are required for amelanotic cells. In our case, over 90% of the cells were positive for S-100, HMB-45 and vimentin, further confirming our diagnosis. Moreover, 80% of cells were positive for Ki-67, suggesting that the tumor was mitotically active.
It is generally accepted that patients with limited local disease should be managed with curative surgical resection with wide margins[4]. For patients with multiple sites involved, palliative surgery can alleviate acute symptoms, such as intestinal obstruction and intussusception. A pooled analysis revealed that beyond being palliative, surgery is also a significant predictor of improved survival for patients with primary GIT melanomas[18]. Although chemotherapy may aid in controlling disseminated disease and has played an important role in improving the quality of life of patients and reducing local recurrence, neither single-agent nor combination regimens seem to significantly improve survival[19]. Postoperative radiotherapy and immunotherapy have also been shown to ameliorate residual disease and lymphadenopathy. These adjuvant therapies are worthy of further investigation.
In conclusion, primary malignant melanoma of the colon, causing intussusception, is an extremely rare clinical phenomenon. Comprehensive preoperative investigation, intraoperative exploration and postoperative analysis are necessary for definitive diagnosis and experience accumulation.
A 57-year-old male presented with persistent abdominal pain and episodes of bloody stool, nausea and vomiting.
Tenderness and rebound tenderness could be felt in the right lower quadrant on deep palpation.
Lymphoma with widespread abdominal lymph nodes metastasis.
Hemoglobin, 83.0 g/L; white blood cells count, 16.1 × 109/L. A metabolic panel showed that electrolyte levels, liver and kidney function were all within normal limits.
A computed tomography scan revealed lower intestinal intussusception, and enlarged lymph nodes in the abdominal cavity and retroperitoneum.
Immunohistochemical staining revealed that the tumor cells were positive for S-100, HMB-45 and vimentin, confirming the diagnosis of melanoma.
The patient underwent adhesive internal hernia reduction and palliative right hemicolectomy.
Intraoperative frozen-section analysis of the metastatic node should be performed when the suspected primary tumor could not be found.
This is a rare presentation of colonic melanoma with ileocecal intussusception. In the abstract, the authors have mentioned laparoscopic surgery whereas in the text it is open exploration - needs clarification.
P- Reviewer: Kate V S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Schuchter LM, Green R, Fraker D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr Opin Oncol. 2000;12:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Alghamdi S, Omarzai Y. Metastatic melanoma presenting as intussusception in an 80-year-old man: a case report. Case Rep Pathol. 2013;2013:672816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Weyandt GH, Eggert AO, Houf M, Raulf F, Bröcker EB, Becker JC. Anorectal melanoma: surgical management guidelines according to tumour thickness. Br J Cancer. 2003;89:2019-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Khalid U, Saleem T, Imam AM, Khan MR. Pathogenesis, diagnosis and management of primary melanoma of the colon. World J Surg Oncol. 2011;9:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Blecker D, Abraham S, Furth EE, Kochman ML. Melanoma in the gastrointestinal tract. Am J Gastroenterol. 1999;94:3427-3433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Ricaniadis N, Konstadoulakis MM, Walsh D, Karakousis CP. Gastrointestinal metastases from malignant melanoma. Surg Oncol. 1995;4:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | De Palma GD, Persico G. Primary malignant melanoma of the right colon. Clin Gastroenterol Hepatol. 2007;5:A28. [PubMed] |
| 8. | Schoneveld M, De Vogelaere K, Van De Winkel N, Hoorens A, Delvaux G. Intussusception of the small intestine caused by a primary melanoma? Case Rep Gastroenterol. 2012;6:15-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Karmiris K, Roussomoustakaki M, Tzardi M, Romanos J, Grammatikakis J, Papadakis M, Polychronaki M, Kouroumalis EA. Ileal malignant melanoma causing intussusception: report of a case. Surg Today. 2007;37:506-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Takahashi-Monroy T, Vergara-Fernandez O, Aviles A, Morales JM, Gatica E, Suarez E. Primary melanoma of the colon presenting as ileocecal intussusception. Am J Gastroenterol. 2006;101:676-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Alvarez FA, Nicolás M, Goransky J, Vaccaro CA, Beskow A, Cavadas D. Ileocolic intussusception due to intestinal metastatic melanoma. Case report and review of the literature. Int J Surg Case Rep. 2011;2:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Atmatzidis KS, Pavlidis TE, Papaziogas BT, Papaziogas TB. Primary malignant melanoma of the small intestine: report of a case. Surg Today. 2002;32:831-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ihde JK, Coit DG. Melanoma metastatic to stomach, small bowel, or colon. Am J Surg. 1991;162:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Ollila DW, Essner R, Wanek LA, Morton DL. Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg. 1996;131:975-979; 979-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Jacobs-Cohen RJ, Wade PR, Gershon MD. Suppression of the melanogenic potential of migrating neural crest-derived cells by the branchial arches. Anat Rec. 2002;268:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Krausz MM, Ariel I, Behar AJ. Primary malignant melanoma of the small intestine and the APUD cell concept. J Surg Oncol. 1978;10:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | McGovern VJ. Spontaneous regression of melanoma. Pathology. 1975;7:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Cheung MC, Perez EA, Molina MA, Jin X, Gutierrez JC, Franceschi D, Livingstone AS, Koniaris LG. Defining the role of surgery for primary gastrointestinal tract melanoma. J Gastrointest Surg. 2008;12:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Yang AS, Chapman PB. The history and future of chemotherapy for melanoma. Hematol Oncol Clin North Am. 2009;23:583-97, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |