Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9314
Revised: January 16, 2014
Accepted: April 8, 2014
Published online: July 28, 2014
Processing time: 278 Days and 0.7 Hours
Infection with Helicobacter pylori (H. pylori) is a worldwide problem. Endoscopic observation of H. pylori infection in vivo would be helpful to obtain an immediate diagnosis. The aim of this review is to describe recent advances in endoscopic technology and to review the available literature pertaining to its clinical application in H. pylori infection. Endoscopic visualization of H. pylori infection is not always feasible using conventional endoscopy. Thus, advanced endoscopic techniques have been developed with the aim of providing a precise and ‘‘real-time’’ endoscopic diagnosis. Recently, new endoscopic techniques such as magnifying endoscopy, narrow band imaging, I-Scan, endocytoscopy and endomicroscopy help focus examination of the stomach to diagnose disease in a time-efficient manner, and the analysis of mucosal surface details is beginning to resemble histologic examination. The new detailed images have enabled endoscopists to observe microscopic structures, such as gastric pit patterns, microvessels and cell morphology. Accordingly, endoscopic prediction of H. pylori infection is possible by analysis of surface architecture of the mucosa, which influences the clinical management. These endoscopic techniques might lead us to easier diagnosis and treatment of H. pylori-related diseases.
Core tip: Attempts to diagnose Helicobacter pylori (H. pylori) infection directly during endoscopy have been made in the past. With the aid of novel endoscopic techniques, it becomes easier to observe indirect and direct evidence of the presence of a H. pylori infection. Thus, the endoscopists can assess the abnormal mucosa in vivo and can generate smart biopsy samples rather than random biopsy samples.
-
Citation: Ji R, Li YQ. Diagnosing
Helicobacter pylori infectionin vivo by novel endoscopic techniques. World J Gastroenterol 2014; 20(28): 9314-9320 - URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9314
Helicobacter pylori (H. pylori) infects about half of the world’s population. It is now well accepted as a crucial factor in the multistep carcinogenic process of gastric cancer[1,2]. There is considerable interest in diagnostic methods for H. pylori infection both before and after treatment. Generally, H. pylori infection can be diagnosed by invasive (endoscopy and biopsy) and non-invasive techniques (e.g., serology, urea breath test, stool test). For patients with dyspepsia, alarm symptoms or tumor history, prompt endoscopy is recommended to clarify the underlying disease. At the time of endoscopy certain mucosal findings may be highly suggestive of H. pylori infection, and biopsy specimens can be assessed for the presence of organisms directly by histological staining, or indirectly by urease testing. However, multiple non-targeted biopsies usually add to the cost and time of the procedures, and infections may be missed based on tests in random biopsy samples in which H. pylori is not present.
In recent years, various new endoscopic techniques have been developed that allow a clear visualization of minute mucosal structures. The new detailed images have enabled endoscopists to observe microscopic structures, such as gastric pit patterns, microvessels, cell morphology, and even microbes. Accordingly, more and more specific endoscopic features suggestive of H. pylori infection were recognized, which were useful for immediate and less invasive endoscopic diagnosis, being closer to the pathological diagnosis. In this article, we will review the capabilities of novel imaging techniques in the diagnosis of H. pylori infection from the perspective of endoscopic practice.
Endoscopic visualization of H. pylori infection is not always feasible using conventional endoscopy. On the basis of many earlier studies, the diagnosis of non-bleeding duodenal ulcer by endoscopy has a > 90% positive predictive value for the diagnosis of H. pylori infection, while gastric ulcer has a positive predictive value of 60%-90%[3]. Unfortunately, standard endoscopic features often labeled as gastritis such as erythema, area gastricae, clefts and nodularity have a poor correlation with pathologic findings. This may be due to features of erythema are hard to adequately define and obtain a consensus. It is worth noting that antral nodularity which shows a chicken-skin appearance has a high specificity (96%) for diagnosis of H. pylori infection, but the sensitivity is only 32%[4].
On standard endoscopy, H. pylori-negative mucosa usually shows numerous minute points throughout the gastric body. On closer observation, the points are shown to be star-fish like arrangements of vessels, and this endoscopic finding was termed “regular arrangement of collecting venules (RAC)”. It is often observed in the gastric body, especially in young patients. RAC was considered a characteristic endoscopic feature of H. pylori-negative normal stomach. In a study by Hidaka et al[5], the sensitivity and specificity of RAC-negative pattern in gastric body for predicting a H. pylori-infected stomach were 100% and 90%, respectively. However, the antrum does not show the generalized features of the H. pylori-negative normal stomach, and close observation or magnified view is essential for more exact diagnosis.
Chromoendoscopy also can be used for diagnosis of H. pylori infection. H. pylori has strong urease activity and produces abundant ammonia on the surface of the gastric mucosa. Therefore, an endoscopic procedure using phenol red, a pH indicator, to assess the distribution of H. pylori has been developed. Cho et al[6] used phenol red staining to reveal the intragastric distribution of H. pylori. A 0.1% phenol red solution was sprayed on the gastric mucosa, and the extent of staining was positively correlated with the urea breath test values and with H. pylori density by histology.
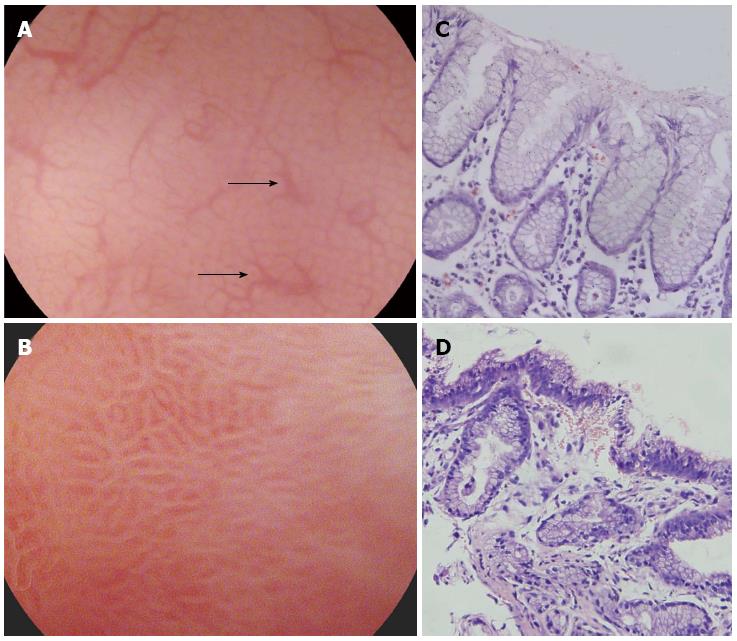
Magnifying endoscopy allows the structure of the mucosa to be observed in detail. The resolution of most magnifying endoscope is less than 10 µm, and it is easy to visualize the subepithelial capillary network surrounding gastric pits (Figure 1). Hence, the correlation between histopathologic and magnified endoscopic features of H. pylori-associated gastritis was reported as satisfactory with a good interobserver agreement[7].
According to Yagi’s classification, the normal body mucosa shows collecting venules and true capillaries that form a network surrounding gastric pits with a pinhole-like appearance (Z0 pattern), while other three types (Z1, Z2, and Z3) corresponded to the H. pylori-positive mucosa. Z0 pattern had a 93.8% sensitivity and a 96.2% specificity for predicting normal gastric mucosa without H. pylori infection[8]. Similarly, Nakagawa’s classification divided the morphology of collecting venules into three patterns: regular (R), irregular (I), and obscured (O). The sensitivity and specificity of R pattern gastric mucosa as an indicator of the absence of H. pylori infection were 63.9% and 100%, respectively[9]. Recently, Kawamura et al[10] focused on the white gastric mucosal crypt openings. They classified the whiteness of the crypt openings as the “white-edged dark spot” type, the “white” type and the “dense white pit” type. The sensitivity and specificity of the “white” and “dense white pit” types for predicting H. pylori infection were 78.5% and 81.7%, respectively.
The majority of the studies to evaluate gastric mucosal patterns have been conducted by Japanese investigators. Anagnostopoulos et al[11] tested the feasibility of magnifying endoscopy in a Western population, and the gastric body was categorized into four types: type 1, regular arrangement of collecting venules and regular, round pits; type 2, regular, round pits, but loss of collecting venules; type 3, loss of normal collecting venules, with enlarged white pits surrounded by erythema; and type 4, loss of normal round pits, with irregular arrangement of collecting venules. The sensitivity and specificity of types 2 and 3 patterns for predicting H. pylori infection were 100% and 92.7%, respectively. The type 4 pattern corresponded to atrophic gastritis with a sensitivity of 90% and a specificity of 96%. A prospective study of 129 patients performed in Turkey confirmed that high resolution magnifying endoscopy is superior to standard endoscopy for the diagnosis of H. pylori-associated gastritis[12].
H. pylori infection almost invariably causes chronic inflammation of the gastric mucosa, and collecting venules are not seen probably because infiltrated inflammatory cells, degenerated epithelium, and disruption of the microvascular systems prevent a clear endoscopic view. Currently, there are no comparative data among these classifications concerning the diagnostic accuracy for the H. pylori infection. It is therefore difficult to objectively comment on the advantage of each of these classifications. Generally, observation of regular collecting venules and normal round pits by magnifying endoscopy suggests that a biopsy is unnecessary.
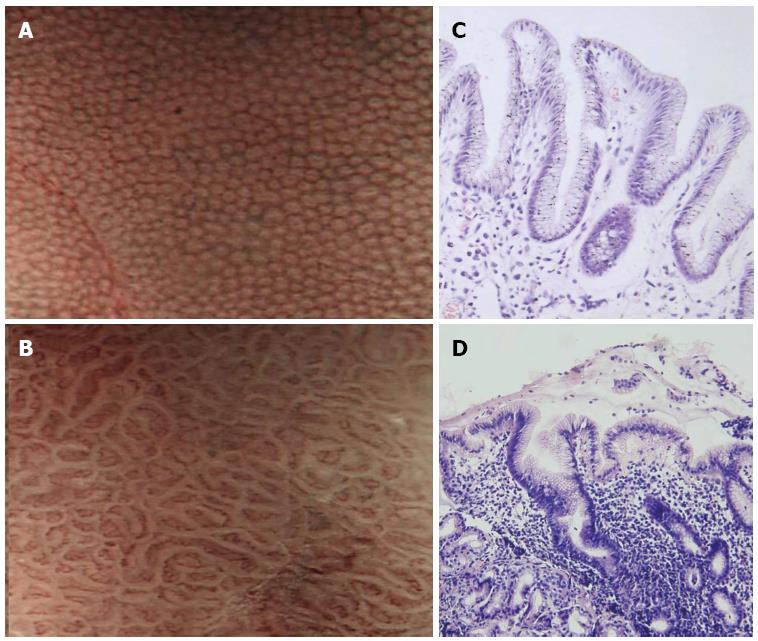
Narrow band imaging (NBI) is based on the principle that depth of light penetration into tissues is directly proportional to the wavelength, which implies that the shorter the wavelength, the more superficial the penetration. The NBI images look like chromoendoscopy without dye focusing on capillaries. Combining the NBI system and magnifying endoscopy brings a better visualization of superficial gastric mucosal and capillary patterns. NBI identifies the gastric pits and vascular network similar to previous reports with magnifying endoscopy. The normal pattern shows small, round pits surrounded by honeycomb-like subepithelial capillary networks (SECNs) (Figure 2). Abnormal patterns were classified into three types: type 1: slightly enlarged, round pits with unclear or irregular SECNs; type 2: obviously enlarged, oval or prolonged pits with increased density of irregular vessels; and type 3: well-demarcated oval or tubulovillous pits with clearly visible coiled or wavy vessels. The sensitivity and specificity of types 1 + 2 + 3 for detection of H. pylori infection were 95.2% and 82.2%, respectively[13]. In the antrum the SECN was seen without the collecting venules. This may be due to the presence of collecting venules in the antrum at a deeper level of the gastric epithelium. Yagi et al[14] compared the diagnostic value of conventional endoscopy and magnifying NBI in patients after endoscopic resection, and the interobserver agreement was moderate (0.56) for conventional endoscopy and substantial (0.77) for magnifying NBI. The sensitivity and specificity were 79% and 52% for conventional endoscopy and 91% and 83% for magnifying NBI endoscopy, respectively.
Magnifying NBI was also used to investigate the changes of gastric mucosal patterns before and 12 wk after H. pylori eradication. Patients who had been successfully treated showed remarkable changes of gastric mucosal patterns: enlarged or elongated pits were improved to small oval or pinhole-like round pits, and the density of fine irregular vessels was decreased. In patients without severe atrophy and intestinal metaplasia, the diagnostic efficacy of magnifying NBI for predicting the results of H. pylori eradication was excellent (sensitivity and specificity, 100%). However, no change occurred in patients with severe gastric atrophy and intestinal metaplasia, regardless of the H. pylori eradication[15]. NBI enhances the quality of microstructure imaging without the need of a dye. However, the light intensity of NBI is so weak that it is hard to observe the whole stomach only with NBI mode, and it is generally used in combination with magnifying endoscopy to further characterize mucosal changes detected by white light endoscopy.
Along with the NBI system, I-Scan and Fuji Intelligent Chromoendoscopy are also the recent development in computed virtual chromoendoscopy imaging. I-Scan is based on the post processing of reflected light. Because of a new computed spectral estimation technology, it is not dependent on optical filters[16]. Qi et al[17] compared the diagnostic value and image quality of white light magnifying endoscopy and magnifying endoscopy with I-Scan for H. pylori infection. No significant difference in diagnostic sensitivity was observed between white light magnifying endoscopy and magnifying I-Scan, but the accuracy and specificity were significantly higher for magnifying I-Scan than for white light magnifying endoscopy (accuracy: 94.0% vs 84.5%, and specificity: 93.5% vs 80.6%)[17]. The study suggested that I-Scan may provide a better image quality for H. pylori infection. However, I-Scan consists of three types of algorithms: surface enhancement, contrast enhancement, and tone enhancement (TE). TE contains a variety of modes such as TE-g for gastric lesion, TE-v for vessel changes, and TE-e for pit patterns. The best optimal mode of the I-Scan for H. pylori infection should be determined in future trials.
Raman spectroscopy is a vibrational spectroscopic technique which can be used to probe biomolecular structures and conformations of tissues. With the use of near-infrared (NIR) excitation light, NIR Raman spectroscopy could interrogate a deeper tissue penetration. In the stomach, more than 90% diagnostic accuracies have been achieved for discriminating neoplastic lesions from normal gastric mucosa tissues. In a feasibility study, normal, H. pylori-infection and intestinal metaplasia gastric tissue samples from 56 patients were measured using a rapid-acquisition NIR Raman spectroscopic system. The principal components analysis and linear discriminant analysis techniques were implemented to develop effective diagnostic algorithms for classification of different gastric tissues. The sensitivity and specificity for detection of H. pylori infection tissues were 80.0% and 100%, respectively[18]. The Raman band at around 1542 cm-1 representing proteins and porphyrin biomolecules showed higher percentage signals for H. pylori-infection tissue compared to the normal tissue, indicating that inflammatory mediators (e.g., monocytes, lymphocytes) were increased in the stromal layer of the tissue. Recently, the latest rapid NIR Raman endoscopy that is capable of working concurrently with white light imaging has been used[19], and additional tests should be carried out to validate the results.
Endocytoscopy is a novel optical imaging technique that enables real-time surface imaging (about 25 μm deep) of gastric mucosa under 450-fold magnification. The probe-based endocytoscope can be passed over the working channel of a standard endoscope[20], and a distal plastic cap is attached to the endoscope to reduce motion artefacts. In a pilot study, Kimura et al[21] cultured H. pylori ex vivo from gastric mucus of three gastric ulcer patients. Staphylococcus aureus and red blood cells were used as controls. Live, moving bacteria can be observed directly without staining in the culture medium using a probe-based endocytoscope. However, this was a pilot ex vivo study, and further in vivo human studies during endoscopy are needed.
Confocal laser endomicroscopy allows subsurface analysis of the gastric mucosa at 1000-fold magnification during endoscopy. Currently, two endomicroscopy systems are available including an integrated endoscopy system (Pentax Endomicroscopy System) and a probe-based system (Cellvizio Endomicroscopy System). The lateral resolution of integrated endomicroscopy is up to 0.7 μm and the range of the Z-axis was 0-200 μm below the surface layer. Fluorescent contrast agents are necessary for endomicroscopic imaging. Fluorescein sodium shows the characteristic morphology of mucosal tissue. Whereas topically used acriflavine strongly labels the superficial epithelial cells and nuclei.
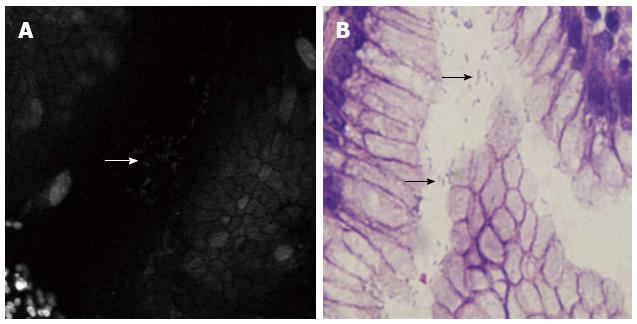
H. pylori was for the first time detected by endomicroscopy in 2005[22]. The bacteria were stained with topical acriflavine and appeared as white dots within the superficial gastric epithelium, and ex vivo examination of cultures proved the presence of H. pylori and the active uptake of acriflavine into the bacteria. Ji et al[23] evaluated the diagnostic efficacy of endomicroscopy in a prospective study. They refined the confocal imaging of H. pylori infection into 3 features: white spots resembling H. pylori organisms (Figure 3), neutrophils and microabscesses. The diagnosis based on the 3 features has a sensitivity of 89.2% and a specificity of 95.7%, and the mean kappa value for inter-observer agreement was 0.78. Subsequently, Wang et al[24] focused on the histological severity of H. pylori-associated gastritis, especially atrophy and intestinal metaplasia, also with a rather good diagnostic accuracy. Until now, there were no side effects reported regarding acriflavine, but acriflavine accumulates in nuclei and carries therefore a potential mutagenic risk. Other contrast agents, such as fluorescein sodium and cresyl violet, need to be evaluated in further controlled trials.
Using endomicroscopy, the endoscopists can assess the abnormal mucosa in vivo and can generate smart biopsy samples for rapid urease testing and histology examination rather than random biopsy samples. Accordingly, Kiesslich et al[22] reported that H. pylori infection was detected in a patient who had negative urease test results. However, further studies are needed to exactly specify the diagnostic yield of endomicroscopy compared with blind biopsies.
Attempts to diagnose H. pylori infection directly during endoscopy have been made in the past. Endoscopic visualization of H. pylori infection is not always feasible using conventional endoscopy. The goal of ideal diagnostic endoscopy is virtual histology or “on-line’’in vivo histology. Many newly developed endoscopic methods offer features that allow more and new mucosal details to be seen, and it becomes easier to observe indirect or direct evidence of the presence of a H. pylori infection (Table 1). Furthermore, these techniques are important in alerting subtle abnormalities such as intestinal metaplasia and neoplasia that may be missed on routine endoscopy. However, we should note that the majority of the studies performed are descriptive, and large prospective trials comparing current techniques are needed to allow clear recommendations regarding which endoscopic imaging technique should be used in different clinical settings. User-friendly diagnostic criteria which can be reproducible in clinical application are important. In addition, the diagnostic accuracy of novel endoscopy is affected by the learning curve, and training and experience are needed to become competent with image interpretation and allow routine clinical use.
| Endoscopic method | Endoscopic features | Sensitivity | Specificity | Limitation |
| Standard endoscopy | Antral nodularity | 32.0% | 96.0% | Low sensitivity |
| Magnifying endoscopy | Gastric pits/collecting venules | 63.9%-93.2% | 96.2%-100% | Lack of uniform diagnostic criteria |
| NBI | SECNs | 95.2% | 82.2% | Low light intensity, and magnification is usually required |
| I-Scan | Gastric pits/collecting venules | 94.0% | 93.5% | Magnification is usually required |
| NIR Raman endoscopy | Raman spectra | 80.0% | 100.0% | Inconvenient for clinical practice |
| Endocytoscopy | Observe organisms directly | - | - | Lack of clinical validation |
| Endomicroscopy | Observe organisms directly/neutrophils infiltration | 89.2% | 95.7% | Depend upon knowledge of pathology |
P- Reviewer: Buzas GM, Gangl A, Karatapanis S, Luzza F, Mach TH, Ozen H, Vorobjova T, Xia HHX S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Perez-Perez GI, Rothenbacher D, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2004;9 Suppl 1:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 829] [Article Influence: 46.1] [Reference Citation Analysis (3)] |
| 3. | Cohen H, Laine L. Endoscopic methods for the diagnosis of Helicobacter pylori. Aliment Pharmacol Ther. 1997;11 Suppl 1:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Laine L, Cohen H, Sloane R, Marin-Sorensen M, Weinstein WM. Interobserver agreement and predictive value of endoscopic findings for H. pylori and gastritis in normal volunteers. Gastrointest Endosc. 1995;42:420-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Hidaka N, Nakayama Y, Horiuchi A, Kato S, Sano K. Endoscopic identification of Helicobacter pylori gastritis in children. Dig Endosc. 2010;22:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Cho YS, Chae HS, Jang SN, Kim JS, Son HS, Kim HK, Kim BW, Han SW, Choi KY, Lee HK. Comparison of the 13C-urea breath test and the endoscopic phenol red mucosal pH test in the quantification of Helicobacter pylori infection loading. Korean J Intern Med. 2008;23:134-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Okubo M, Tahara T, Shibata T, Nakamura M, Kamiya Y, Yoshioka D, Maeda Y, Yonemura J, Ishizuka T, Arisawa T. Usefulness of magnifying narrow-band imaging endoscopy in the Helicobacter pylori-related chronic gastritis. Digestion. 2011;83:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Nakagawa S, Kato M, Shimizu Y, Nakagawa M, Yamamoto J, Luis PA, Kodaira J, Kawarasaki M, Takeda H, Sugiyama T. Relationship between histopathologic gastritis and mucosal microvascularity: observations with magnifying endoscopy. Gastrointest Endosc. 2003;58:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kawamura M, Sekine H, Abe S, Shibuya D, Kato K, Masuda T. Clinical significance of white gastric crypt openings observed via magnifying endoscopy. World J Gastroenterol. 2013;19:9392-9398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Gonen C, Simsek I, Sarioglu S, Akpinar H. Comparison of high resolution magnifying endoscopy and standard videoendoscopy for the diagnosis of Helicobacter pylori gastritis in routine clinical practice: a prospective study. Helicobacter. 2009;14:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Tahara T, Shibata T, Nakamura M, Yoshioka D, Okubo M, Arisawa T, Hirata I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Yagi K, Saka A, Nozawa Y, Nakamura A. Prediction of Helicobacter pylori status by conventional endoscopy, narrow-band imaging magnifying endoscopy in stomach after endoscopic resection of gastric cancer. Helicobacter. 2014;19:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Okubo M, Tahara T, Shibata T, Nakamura M, Yoshioka D, Maeda Y, Yonemura J, Ishizuka T, Arisawa T, Hirata I. Changes in gastric mucosal patterns seen by magnifying NBI during H. pylori eradication. J Gastroenterol. 2011;46:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Cho WY, Jang JY, Lee DH. Recent Advances in Image-enhanced Endoscopy. Clin Endosc. 2011;44:65-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Qi QQ, Zuo XL, Li CQ, Ji R, Li Z, Zhou CJ, Li YQ. High-definition magnifying endoscopy with i-scan in the diagnosis of Helicobacter pylori infection: a pilot study. J Dig Dis. 2013;14:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Teh SK, Zheng W, Ho KY, Teh M, Yeoh KG, Huang Z. Near-infrared Raman spectroscopy for optical diagnosis in the stomach: identification of Helicobacter-pylori infection and intestinal metaplasia. Int J Cancer. 2010;126:1920-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Huang Z, Teh SK, Zheng W, Mo J, Lin K, Shao X, Ho KY, Teh M, Yeoh KG. Integrated Raman spectroscopy and trimodal wide-field imaging techniques for real-time in vivo tissue Raman measurements at endoscopy. Opt Lett. 2009;34:758-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Neumann H, Fuchs FS, Vieth M, Atreya R, Siebler J, Kiesslich R, Neurath MF. Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther. 2011;33:1183-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Kimura S, Inoue H, Sato Y, Aoyama Y, Shimojima M, Masuyama T, Kudo SE. Ex vivo visualization of Helicobacter pylori using an endocytoscopic probe. Biomed Res. 2006;27:255-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Kiesslich R, Goetz M, Burg J, Stolte M, Siegel E, Maeurer MJ, Thomas S, Strand D, Galle PR, Neurath MF. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology. 2005;128:2119-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Ji R, Li YQ, Gu XM, Yu T, Zuo XL, Zhou CJ. Confocal laser endomicroscopy for diagnosis of Helicobacter pylori infection: a prospective study. J Gastroenterol Hepatol. 2010;25:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Wang P, Ji R, Yu T, Zuo XL, Zhou CJ, Li CQ, Li Z, Li YQ. Classification of histological severity of Helicobacter pylori-associated gastritis by confocal laser endomicroscopy. World J Gastroenterol. 2010;16:5203-5210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |