Published online Jul 28, 2014. doi: 10.3748/wjg.v20.i28.9286
Revised: February 15, 2014
Accepted: April 5, 2014
Published online: July 28, 2014
Processing time: 280 Days and 10.9 Hours
The prevention of a disease process has always been superior to the treatment of the same disease throughout the history of medicine and surgery. Local recurrence and peritoneal metastases occur in approximately 8% of colon cancer patients and 25% of rectal cancer patients and should be prevented. Strategies to prevent colon or rectal cancer local recurrence and peritoneal metastases include cytoreductive surgery and hyperthermic perioperative chemotherapy (HIPEC). These strategies can be used at the time of primary colon or rectal cancer resection if the HIPEC is available. At institutions where HIPEC is not available with the treatment of primary malignancy, a proactive second-look surgery is recommended. Several phase II studies strongly support the proactive approach. If peritoneal metastases were treated along with the primary colon resection, 5-year survival was seen and these results were superior to the results of treatment after peritoneal metastases had developed as recurrence. Also, prophylactic HIPEC improved survival with T3/T4 mucinous or signet ring colon cancers. A second-look has been shown to be effective in two published manuscripts. Unpublished data from MedStar Washington Cancer Institute also produced favorable date. Rectal cancer with peritoneal metastases may not be so effectively treated. There are both credits and debits of this proactive approach. Selection factors should be reviewed by the multidisciplinary team for individualized management of patients with or at high risk for peritoneal metastases.
Core tip: The prevention of a disease process has always been superior to the treatment of the same disease throughout the history of medicine and surgery. Local recurrence and peritoneal metastases occur in approximately 8% of colon cancer patients and 25% of rectal cancer patients and should be prevented. If peritoneal metastases are treated along with the primary colon resection, improved 5-year survival was seen. These results are superior to the results of treatment after peritoneal metastases developed as recurrence. A second-look in selected patients has been shown to be effective.
- Citation: Sugarbaker PH. Update on the prevention of local recurrence and peritoneal metastases in patients with colorectal cancer. World J Gastroenterol 2014; 20(28): 9286-9291
- URL: https://www.wjgnet.com/1007-9327/full/v20/i28/9286.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i28.9286
Common sense values prevention over treatment. Benjamin Franklin said, “An ounce of prevention is worth a pound of cure.” Also, it is self evident that the most remarkable improvements in healthcare have come about as a result of prevention. Sanitation, vaccination, behavior modification such as smoking cessation, reduction in incidence of stroke through the use of antihypertensive medicines, and endoscopic screening are examples. Colonoscopy has been successful in preventing colon cancer in the West and esophagogastroduodenoscopy effective preventing esophagus cancer and gastric cancer in Japan and Korea. This manuscript attempts to establish that hyperthermic perioperative chemotherapy (HIPEC) in properly selected primary and colon rectal cancer patients can prevent local recurrence and peritoneal metastases that would otherwise develop in the follow-up of these patients.
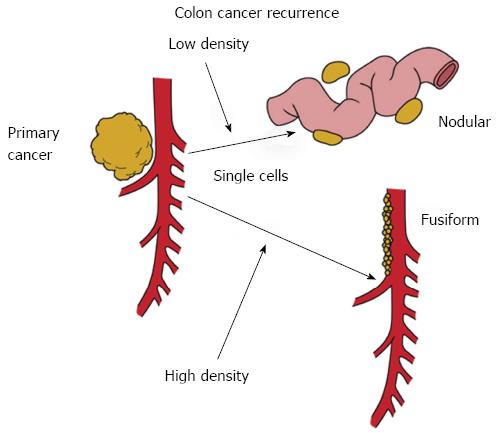
An important concept concerns the fact that local recurrence and peritoneal metastases have the same natural history (Figure 1). Free cancer cells or tiny nodules that are disseminated from the primary colorectal malignancy will enter the free peritoneal space. At low density, these cancer cells will implant at a distance from the primary malignancy and develop into peritoneal metastases. At higher density within the resection site, these free cancer cells or tiny nodules will implant and grow within the colon or rectal resection site. This high density seeding near the primary cancer resection will result in a fusiform distribution of metastases. In rectal cancer, this fusiform distribution of metastases will most likely occur on the hollow of the sacrum. In right colon cancer, the local recurrence will be on the second portion of the duodenum, and along the right ureter. In left colon cancer, the disease recurrence is seen in the left paracolic sulcus and in and around the first portion of the jejunum. Cancer trapped above or below the duodenal mesocolic fold is frequently seen with recurrence of a primary colon cancer of the splenic flexure.
When patients present with primary colon cancer there is an approximate 8.5% incidence of peritoneal metastases observed at the time of diagnosis or at the time of colon cancer resection[1,2]. The incidence of involvement of the peritoneum is higher with rectal cancer and estimated at about 25%[3,4]. These poor prognosis patients are usually not approached with a curative treatment option. They are given a palliative surgical procedure and then systemic chemotherapy. Their survival is very limited and consistently less than one year.
At a few peritoneal surface oncology centers around the world, an individualized management plan that includes cytoreductive surgery and hyperthermic perioperative chemotherapy may be initiated with the primary cancer resection. An individualized treatment plan for this group of patients will be defined at a later time.
Realizing that the first and foremost goal of cancer surgery is to provide local control, it is appropriate that we would survey the incidence of local recurrence and peritoneal metastases observed at the time of colon or rectal cancer resection. It is important to note that local control has improved greatly for colon and rectal cancer in recent years as a result of total mesocolic resection and total mesorectal resection. Currently, local recurrence rates are approximately 20% for colon malignancy and 10% for rectal cancer. Although this is a great improvement over previous statistics, there is still a group of patients that may greatly profit from individualized management strategies.
It has been established that local control can be improved by the use of intraperitoneal cytotoxic agents used either at the time of or soon after the performance of a colon or rectal cancer resection. Sugarbaker presented data suggesting that peritoneal metastases can be prevented with intraperitoneal 5-fluorouracil treatment. Randomized patients who received the intravenous 5-fluorouracil had a statistically significant higher incidence of local recurrence or peritoneal metastases than those patients treated with the intraperitoneal 5-fluorouracil[5]. The data are presented in Table 1.
| Sites of treatment failure for IV or IP 5-FU | ||||
| No. of patients | No. of recurrences | Peritoneal surface | Liver | |
| IP 5-FU | 36 | 13 (36) | 2/10 | 3/11 |
| IV 5-FU | 30 | 11 (37) | 10/11 | 4/11 |
| Statistical analysis P2 | 0.003 | 1.000 | ||
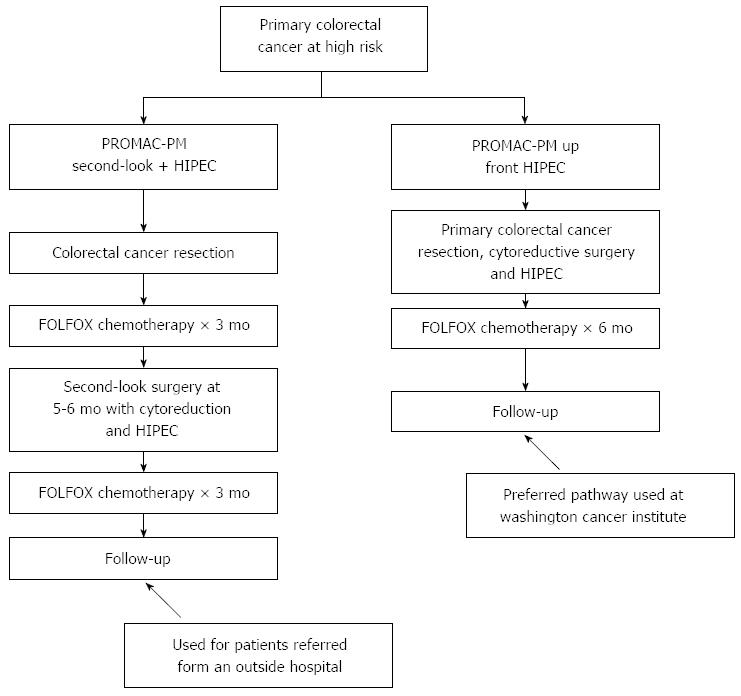
Currently, the multidisciplinary team at the Medstar Washington Cancer Institute is pursuing a clinical pathway that provides a proactive management of primary colon cancer that either has or may have a high risk of peritoneal metastases. In order to fulfill the requirements of this clinical pathway, patients must have all of their treatments for the primary malignancy and for the peritoneal metastases within nine months of diagnosis of the primary disease. The three requirements of complete treatment are 12 cycles of systemic chemotherapy with folinic acid, 5-fluorouracil, and oxaliplatin (FOLFOX). These cycles of chemotherapy may be administered in a neoadjuvant manner or as adjuvant treatment following colon cancer resection. Secondly, with the primary cancer resection or at a second-look, patients must have a complete cytoreduction of all the visible peritoneal metastases. Finally, with the major resection which is the cytoreduction, patients must receive HIPEC using mitomycin C, 5-fluorouracil, and systemic 5-FU plus leucovorin.
Figure 2 shows two ways that a patient may enter the proactive management clinical pathway. If patients are found to have peritoneal metastases at an outside institution, they are referred for definitive treatment after the primary colon cancer has been either resected or bypassed. Patients are to receive FOLFOX chemotherapy for 3 mo and then after a 5-6 wk, they come back for definitive cytoreductive surgery and a CC-1 cytoreduction combined with HIPEC. Following the second-look cytoreduction with HIPEC, the FOLFOX chemotherapy is completed for a total of 12 cycles. This pathway is used at institutions where HIPEC is not available at the time of the primary colorectal cancer resection.
At institutions where HIPEC is available at the time of primary colorectal cancer resection, the primary resection is augmented intraoperatively by complete cytoreductive surgery. Not only the bowel resection, but also greater and lesser omentectomy and oophorectomy in women are required. Prior to the intestinal reconstruction, HIPEC is administered. After HIPEC, the bowel anastomosis is performed and the abdomen is closed. These patients then receive their 12 cycles of FOLFOX chemotherapy. It should be emphasized that in either the second-look HIPEC or the up-front HIPEC, all treatments are to be completed within 9 mo of the cancer diagnosis.
Current indications for the proactive management of local recurrence and peritoneal metastases for colon cancer are listed in Table 2. Three groups of patients are considered for second-look plus HIPEC. These are patients with a biopsy-proven cancer nodule on any peritoneal surface including the primary malignancy, a positive biopsy or pathologic enlargement of an ovary, or cancer at a surgical margin of resection (R-1 resection).
| 1Biopsy-proven cancer nodule(s) on any peritoneal surface including the primary cancer (T4) |
| 1Positive biopsy or pathologic enlargement of an ovary |
| 1Cancer at the surgical margin of resection (R-1) |
| Perforation through the malignancy (iatrogenic by colonoscopy or spontaneous) |
| Positive peritoneal cytology |
| Adjacent organ involvement or fistula formation |
| Lymph nodes positive at the margins of resection |
| Rupture of the primary cancer during resection |
For the up-front HIPEC, the eligibility requirements are not as strict. It is thought that the intraoperative chemotherapy combined with a primary colon cancer resection is of very low morbidity and carries no mortality. Patients who should have up-front HIPEC include those with a perforation through the malignancy either iatrogenic by colonoscopy or spontaneous, a positive peritoneal cytology, adjacent organ involvement or fistula formation, lymph nodes positive at the margins of resection as determined by frozen section, and rupture of the primary cancer during the resection.
Phase II data exists to support these proactive management strategies of colon cancer at high risk for local recurrence or peritoneal metastases. Pestieau and Sugarbaker looked at their results in patients treated with peritoneal metastases simultaneously with the primary resection. In five patients who had concomitant colon resection, cytoreductive surgery, and perioperative chemotherapy, there were no survivors less than five years. These results were compared to patients who were treated after the peritoneal metastases had occurred in follow-up. The results of the proactive management were superior with a P value of 0.0001[6].
Sammartino and colleagues reported on the prevention of peritoneal metastases from colon cancer patients at high risk with the use of a prophylactic HIPEC. Twenty-five patients with colon cancer with clinical T3/T4, mucinous, or signet ring cell histology underwent hemicolectomy, omentectomy, and HIPEC. The control group was 50 patients treated by a standard surgical resection. The incidence of peritoneal metastases was reduced from 28% to 4% (P < 0.03). Also, overall survival was increased (P < 0.03)[7] (Figure 3).
Elias and colleagues had three requirements for a systemic second-look in patients with colon malignancy. Patients with perforated cancer, ovarian metastases or peritoneal metastases underwent a second-look procedure after receiving a full course of modern systemic chemotherapy. Of the 29 patients who underwent second-look, 16 (55%) were found to have peritoneal metastases at second-look. With a median follow-up of 2 years, 50% of these patients remained disease-free. In those 13 patients who did not have peritoneal metastases at second-look, 9 patients (69%) remained disease-free. In this study, there was no mortality and a 14% incidence of grade III/IV morbidity. Elias and colleagues concluded that an early intervention to prevent peritoneal metastases was superior to a watch-and-wait policy operating after the peritoneal metastases become evident in CT scan follow-up[8]. Currently, a randomized trial is being performed in order to further establish the efficacy of this treatment plan.
At the Medstar Washington Cancer Institute, we have early results with 20 patients who have had the proactive management with colon cancer. Upon reoperation in these 20 patients, 62% had a peritoneal cancer index between 1 and 10. Also, 85% had a complete cytoreduction with the second-look surgery. In our patients who had only 4 cycles of chemotherapy prior to the second-look surgery, all 20 patients (100%) were found to have progressive peritoneal metastases (submitted for publication).
Delhorme et al[9] have published data on a mandatory second-look surgery (MSLS) for the treatment of histologically confirmed peritoneal metastases present with the primary colon cancer resection. At their MSLS, 71% of patients were found to have persistent or progressive disease and the median peritoneal carcinomatosis index was 10. There was no postoperative mortality and a 7% incidence of grade III/IV complications. The 2-year overall survival and disease-free survival rates were 91% and 38%, respectively. Following MSLS with HIPEC, peritoneal recurrence was observed in only 8% of patients vs 100% of the patients treated in a standardized fashion.
A valid question regards recommendations for rectal cancer patients at high risk for local recurrence or peritoneal metastases. In these patients, the up-front proactive management may be the only option. Verwaal and colleagues showed that rectal cancer patients rarely show long-term survival with cytoreductive surgery and HIPEC[10].
Da Silva and Sugarbaker[11] reported on rectal cancer patients with peritoneal metastases treated with CRS and HIPEC had a 17 mo median survival and a 0% 5-year survival as compared to colon cancer patients who had a 35 mo median survival and a 30% 5-year survival. These authors postulated that pelvic peritonectomy after an anterior resection or abdomino-perineal resection makes an adequate peritonectomy difficult or impossible. With this incomplete cytoreduction in the pelvis after a rectal cancer resection, the results of CRS and HIPEC are poor.
The expected credits and debits of a proactive approach to the management of colon and rectal local recurrence and peritoneal metastases are presented in Table 3. The anticipated results of treatment with a PCI of less than 10 is 50% or perhaps even 70% at 5 years. The selection factors for treating patients proactively have been well defined. These selection factors should be used by the multidisciplinary team for individualized management of colorectal cancer patients. With up-front HIPEC or second-look HIPEC, the morbidity should be 10% or less and a less than 1% mortality at experienced centers.
| Credits | Debits |
| Long-term survival in 50% of patients | Many patients treated for a few to benefit |
| Selection factors have been well defined | The surgical technology is complex and requires an extended learning curve |
| An important addition to the MDT for management of colorectal malignancy | Referral by medical oncologist are often late with a large extent of disease |
| Morbidity (10%) and mortality (1%) at experienced centers is acceptable | So far a uniform HIPEC treatment has not emerged; perioperative chemotherapy needs to be optimized |
There are some debits that remain to globally introduce this treatment strategy. The surgical technology for primary cancer resection combined with HIPEC is complex and there is a more extended learning curve. Also, to this point in time, a uniform HIPEC treatment has not emerged. The perioperative chemotherapy management plans need to optimized. Finally, CRS and HIPEC are not generally available at the institutions performing primary colon or rectal cancer surgery. Also, many patients who are in need of the proactive approach are not referred.
The most important outcome to result from this update concerns a change in the evaluation of the clinical features and histopathology (including peritoneal cytology) of patients with primary colon and rectal cancer. Every patient needs to be carefully considered by the multidisciplinary team for upfront HIPEC at the time of primary colorectal cancer resection or second-look HIPEC as a planned part of the overall treatment strategy. An estimated 30% of patients with primary disease should have a change in the primary cancer treatment strategy in order to prevent local recurrence and/or peritoneal metastases. Including the gastrointestinal pathologist, who would review the resected specimen in the operating room, is an essential member of the multidisciplinary team. This change in treatment strategy for primary colorectal cancer comes about as a result of the large number of manuscripts that document extent of peritoneal metastases as a major determinant of the success of cytoreductive surgery with HIPEC. Maximum benefit cannot be achieved through clinical follow-up or radiologic follow-up to treat patients who have a moderate to large extent of disease. The goals of HIPEC to prevent local recurrence and peritoneal metastases is to treat minimal residual disease or prevent subsequent disease progression altogether.
P- Reviewer: Moldovan R, Simmons RG S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
| 1. | Lennon AM, Mulcahy HE, Hyland JM, Lowry C, White A, Fennelly D, Murphy JJ, O’Donoghue DP, Sheahan K. Peritoneal involvement in stage II colon cancer. Am J Clin Pathol. 2003;119:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Sjo OH, Berg M, Merok MA, Kolberg M, Svindland A, Lothe RA, Nesbakken A. Peritoneal carcinomatosis of colon cancer origin: highest incidence in women and in patients with right-sided tumors. J Surg Oncol. 2011;104:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Ludeman L, Shepherd NA. Serosal involvement in gastrointestinal cancer: its assessment and significance. Histopathology. 2005;47:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Mitchard JR, Love SB, Baxter KJ, Shepherd NA. How important is peritoneal involvement in rectal cancer? A prospective study of 331 cases. Histopathology. 2010;57:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 5. | Sugarbaker PH, Gianola FJ, Speyer JC, Wesley R, Barofsky I, Meyers CE. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery. 1985;98:414-422. [PubMed] |
| 6. | Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management. Dis Colon Rectum. 2000;43:1341-1346; discussion 1347-1348. [PubMed] |
| 7. | Sammartino P, Sibio S, Biacchi D, Cardi M, Accarpio F, Mingazzini P, Rosati MS, Cornali T, Di Giorgio A. Prevention of Peritoneal Metastases from Colon Cancer in High-Risk Patients: Preliminary Results of Surgery plus Prophylactic HIPEC. Gastroenterol Res Pract. 2012;2012:141585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Elias D, Goéré D, Di Pietrantonio D, Boige V, Malka D, Kohneh-Shahri N, Dromain C, Ducreux M. Results of systematic second-look surgery in patients at high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2008;247:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Delhorme JB, Triki E, Romain B, Meyer N, Rohr S, Brigand C; Mandatory second-look surgery after surgical treatment of peritoneal carcinomatosis of colonic origin. In press. . |
| 10. | Verwaal VJ, van Tinteren H, van Ruth S, Zoetmulder FA. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg. 2004;91:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |