Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.9185
Revised: February 28, 2014
Accepted: April 15, 2014
Published online: July 21, 2014
Processing time: 256 Days and 15.9 Hours
AIM: To evaluate the effectiveness of thiopurines in maintaining steroid-free remission in routine clinical practice.
METHODS: The multi-center Pediatric Inflammatory Bowel Disease Network (PIBDNet) cohort study prospectively collected data on thiopurine naïve patients initiating mercaptopurine (6MP) or azathioprine. Patients with a diagnosis of Crohn’s disease (CD) were included in our study upon entering remission as determined by physician global assessment (PGA) within 365 d of initiation of thiopurines. The primary outcome of the study was maintenance of steroid-free remission (SFR) at each follow up visit. Patients were considered treatment failures if there had been a change in PGA from remission to mild, moderate or severe disease; disease relapse between visits; need for rescue therapy (biologic therapy, methotrexate, steroids); thiopurine discontinuation, hospitalization or surgical intervention. A secondary outcome defined treatment failure as a change from remission to moderate or severe (not mild) in addition to the previously defined criteria.
RESULTS: Sixty-five of 182 patients in the PIBDNet registry met criteria for inclusion in this study. Forty-five of 65 (69%) of included patients achieved remission within 180 d of thiopurine initiation. For the primary outcome, 47% and 23% of patients remained in SFR at 6 and 12 mo. The mean thiopurine dose at initiation for the 65 included patients was 0.89 ± 0.31 mg/kg per day. Metabolite levels were obtained in 48% (31/65) of the included patients with a mean 6TG level of 258 pmole/8 × 108 RBC ± 147. For the secondary outcome, 65% and 42% of patients remained in SFR at 6 and 12 mo.
CONCLUSION: Thiopurines were less effective in maintaining remission for pediatric CD in this “real world” cohort than has been previously described. Variation in thiopurine dosing and metabolite measurement was found among practitioners.
Core tip: This manuscript describes the real world effectiveness of thiopurines in maintaining remission for pediatric Crohn’s disease. The outcomes differ in comparison to the initially published randomized controlled trial for pediatric Crohn’s but are similar to more recently published studies evaluating the effectiveness of thiopurines. The study evaluates data from a pediatric inflammatory bowel disease registry and is representative of real world clinical care. Varitation in practitioner approach to thiopurine dosage and metabolite measurement was found. Implementation of a more standardized approach to use of thiopurines could impact clinical outcomes.
- Citation: Boyle BM, Kappelman MD, Colletti RB, Baldassano RN, Milov DE, Crandall WV. Routine use of thiopurines in maintaining remission in pediatric Crohn’s disease. World J Gastroenterol 2014; 20(27): 9185-9190
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/9185.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.9185
The incidence of pediatric Crohn’s disease (CD) is increasing and nearly 25% of patients with inflammatory bowel disease (IBD) present during childhood[1-3]. Thiopurines have been shown to be effective in the treatment of children and adults with CD[4-6] and are associated with a steroid sparing effect[7]. However, studies describing the effectiveness in maintaining steroid-free remission (SFR) with thiopurines have been inconsistent[6-13]. The efficacy of thiopurines was previously evaluated by a randomized controlled trial (RCT) in pediatric patients with moderate to severe CD. This study found that greater than 90% of patients achieving clinical remission remained in clinical remission for 18 mo[7]. Subsequent retrospective pediatric studies have found thiopurines to be less effective than the initial RCT[14,15], as has the more recent SONIC trial performed in adults[16].
Variation in the delivery of chronic illness care and its negative impact upon outcomes has been previously described[17]. Evaluation of pediatric IBD registry data identified significant variation between care centers in the initial management of patients with CD including the frequency of thiopurine use, thiopurine dosing, and thiopurine methyl transferase (TPMT) measurement[18,19]. Efforts to reduce variation in pediatric IBD care using quality improvement methods have led to improved patient outcomes[20].
The aim of our study was to examine maintenance of SFR in patients who achieved clinical remission after initiating thiopurine therapy. The multi-center Pediatric Inflammatory Bowel Disease Network (PIBDNet) prospectively collected data on patients initiating thiopurine therapy, thus presenting an opportunity to investigate this research question using data from a large number of diverse clinical practices and care approaches representative of real world clinical care.
The PIBDNet multi-center cohort study prospectively collected data from thiopurine naïve patients initiating mercaptopurine (6MP) or azathioprine (AZA) therapy. Forty-eight practice sites enrolled patients in the cohort study. Participating practitioners included pediatric gastroenterologists from both university and private practice settings of various sizes[19]. Evaluation of data from patients enrolled from 2004-2008 was performed. Enrolled patients were aged 1-17 years with a diagnosis of CD. Data was collected and recorded at initiation and each subsequent follow up visit. Disease activity was assessed by physician global assessment (PGA) at the time of the visit and categorized as inactive (remission), mild, moderate, or severe disease.
All patient management decisions were determined by each practitioner rather than a standardized protocol. Variations in practice approach among practitioners included thiopurine dosage, decisions about continuing thiopurines, timing or need to obtain 6MP metabolite levels, need for additional medications, and frequency of follow up visits.
Patients were included in this analysis if clinical remission by PGA had been achieved within 70-365 d from thiopurine initiation. This visit was considered time zero for this analysis. We restricted this analysis to patients achieving clinical remission between 70-365 d in order to isolate the effect of thiopurine monotherapy. Seventy days was chosen a priori with the expectation that the therapeutic effect of thiopurines would not have been achieved prior to 10 wk after initiation. Patients not achieving remission by 365 d after thiopurine initiation were considered non-responders and excluded.
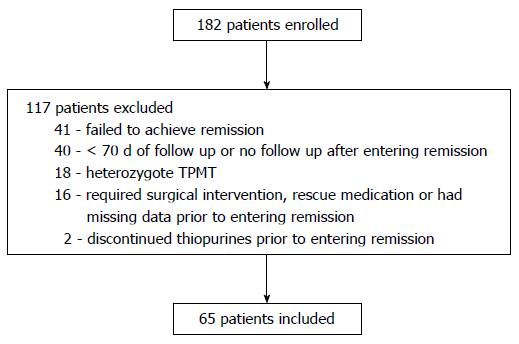
Additional exclusion criteria for the primary analysis were: inadequate follow up data (< 70 d of follow up after thiopurine initiation or no follow up visit after achieving remission), current or previous treatment with infliximab, methotrexate (MTX), cyclosporine, or tacrolimus, heterozygote or homozygote mutant TPMT status, or patients who required surgical intervention prior to entering remission.
After entering remission, each subsequent visit was evaluated for maintenance of SFR or treatment failure. In our primary analysis (analysis 1), treatment failure was defined as a change in PGA from remission to mild, moderate or severe; disease relapse recorded between visits; a need for rescue therapy [MTX, infliximab, cyclosporine, tacrolimus, corticosteroids (CS)]; thiopurine discontinuation; or hospitalization/surgical intervention after entering remission. Patients treated with CS upon entering remission who remained on CS for an additional 30 d were also considered treatment failures.
A secondary outcome considered a change in PGA from remission to moderate or severe disease (but not mild) as a treatment failure (analysis 2) in addition to the previously defined criteria for treatment failure in the primary analysis.
Exploratory analyses included patients with normal or heterozygote TPMT status that met inclusion criteria defined above. Primary and secondary outcomes for this combined cohort were performed using the above definitions for treatment failure to determine if TPMT status impacted maintenance of remission.
Thiopurine dosage in mg/kg per day was determined at registry enrollment and upon entering remission and was expressed as dose of 6MP. For patients treated with AZA, a conversion factor of dose in mg/2.07[21] was used to express medication dose as a 6MP equivalent. Dosing and decisions to obtain 6MP metabolites levels was determined by each practitioner and varied according to their clinical practice.
Kaplan Meier survival curves evaluating maintenance of SFR were performed for each analysis described above. A comparison of demographic data for included and excluded patients was performed using chi-square testing for discrete variables and student t-test for continuous variables.
One hundred eighty-two patients were enrolled in the PIBDNet cohort. Sixty-five patients met inclusion criteria for this analysis. Reasons for exclusion are shown in Figure 1. Demographic data including age, gender, disease duration, disease location, and disease activity at enrollment by PGA was similar for included and excluded patients (Table 1).
| Included (n = 65) | Excluded (n = 117) | P value | |
| Age (yr) | 12.98 ± 2.8 | 13.4 ± 2.9 | NS |
| Gender | |||
| Male | 39 (60.0) | 65 (55.6) | NS |
| Female | 26 (40.0) | 52 (44.4) | |
| Disease duration | |||
| < 12 mo | |||
| > 12 mo | 59 (90.7) | 100 (85.5) | NS |
| 6 (9.2) | 17 (14.5) | ||
| Race | |||
| Caucasian | 52 (80.0) | 102 (87.2) | NS |
| Disease location | |||
| Upper | 37 (56.9) | 79 (67.5) | NS |
| Ileal | 49 (75.4) | 91 (77.9) | NS |
| Colonic | 54 (83.1) | 97 (82.9) | NS |
| Thiopurine | |||
| 6MP | 42 (64.6) | 87 (77.0) | NS |
| AZA | 23 (35.4) | 26 (23.0) | |
| PGA | |||
| Remission | 7 (10.8) | 5 (4.4) | NS |
| Mild | 22 (33.8) | 41 (36.3) | |
| Moderate | 31 (47.7) | 58 (51.3) | |
| Severe | 5 (7.7) | 9 (8.0) | |
| Mean initiation dosage | |||
| (mg/kg) | 0.89 ± 0.31 | 1.06 ± 0.37 | 0.002 |
| 6TG level | 258 ± 147 | 231 ± 161 | NS |
| (pmole/8 × 108 RBC) | (n = 31) | (n = 55) |
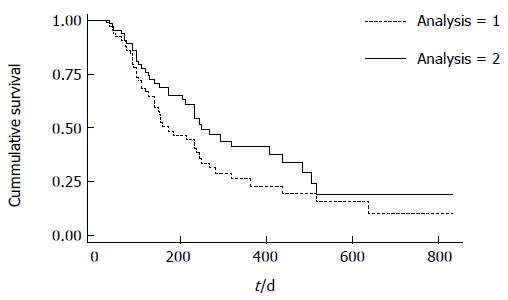
For the primary outcome, 47% of patients remained in SFR 6 mo after achieving remission (Figure 2-analysis 1). By 12 mo, 23% of patients remained in SFR. Forty-five of the 65 patients achieved remission between 70-180 d after thiopurine initiation. For this cohort, 51% and 28% maintained remission at 6 and 12 mo.
Patients could have multiple reasons for treatment failure. Thirty-eight patients had a change in PGA from remission to active disease. Fourteen patients required steroid therapy. Sixteen patients were lost to follow up within 12 mo of study entry.
For the secondary outcome in which a PGA of mild was not considered a reason for treatment failure, we found that 65 % and 42 % of patients remained in SFR at 6 and 12 mo (Figure 2-analysis 2). For the 45 patients achieving remission between 70-180 d, 67% and 47% maintained remission for this secondary outcome at 6 and 12 mo.
The exploratory analysis included the 65 patients with normal TPMT status and 12 additional patients with heterozygote TPMT status (total n = 77) and found similar results: 45% and 26% of patients remained in SFR for the primary outcome at 6 and 12 mo; 61% and 42% of patients remained in SFR for the secondary outcome at 6 and 12 mo.
The mean dose in mg/kg per day (mean ± SD) at thiopurine initiation for the 65 included patients was 0.89 ± 0.31 mg/kg per day compared to 1.06 ± 0.38 mg/kg per day for the 117 excluded patients (P = 0.002). The mean dose upon entering remission for the 65 included patients was 1.09 ± 0.33 mg/kg per day.
In total, 47% (86/182) of patients had metabolite levels obtained. Thirty-one of the 65 included patients had 6TG levels [pmole/8 × 108 red blood cells (RBC)] obtained with an mean level of 258 pmole/8 × 108 RBC ± 147 [median 238 (range: 25-746)]. Of the 117 patients excluded from study entry, 55 patients had 6TG levels with a mean of 231 pmole/8 × 108 RBC ± 161 [median 195 (range: 29-965)]. This difference between included and excluded patients was not significant (P = 0.2).
Thiopurines are commonly used therapies for adult and pediatric patients with CD, having been shown to be effective for maintaining remission and providing a steroid sparing effect. Our multi-center prospective study evaluating patients from diverse centers throughout the United States found that 47% and 23% of patients maintained SFR at 6 and 12 mo; 65% and 42 % of patients remained steroid free with a PGA of either remission or mild disease at 6 and 12 mo respectively.
The effectiveness of thiopurines in maintaining remission in previous pediatric studies in CD has been mixed. The RCT evaluating children with moderate to severe CD found greater than 90% of patients treated with steroid therapy and 6MP at diagnosis maintained disease remission through 18 mo[7]. Maintenance of remission in our study was comparatively reduced. Despite differences in study design (RCT vs prospective cohort study), methods of assessing disease activity (Harvey-Bradshaw index vs PGA), dosing variation and definition of treatment failures between the studies, the differences in study outcomes warrant further consideration.
Our findings expand upon a growing body of literature suggesting that the effectiveness of thiopurines in routine clinical practice for pediatric CD is lower than might have been predicted from the initial RCT. A single-center, retrospective study found 60% and 40% of pediatric patients with CD to maintain SFR at 6 and 12 mo. Another small pediatric study evaluating patients with CD or UC treated with thiopurines found 30% SFR at 6 mo[14,15].
The 2008 Cochrane review evaluating the maintenance of disease remission in adult patients with CD found thiopurines to be effective in maintaining remission with higher doses of AZA appearing more effective than lower dosages[22]. The SONIC trial found that steroid free clinical remission was achieved in only 30% and 24% at 26 and 52 wk respectively for patients receiving thiopurine monotherapy[16]. Furthermore, a trial of 63 patients aged 15-65 years found that 42% of patients receiving thiopurines vs 7% receiving placebo remained in remission 15 mo after thiopurine initiation[5].
For our study, variation in practitioner practice patterns could have impacted outcomes. The mean dose at thiopurine initiation for included patients was less than recommended dosing (< 1.0 mg/kg per day) suggesting the relative decreased SFR for this cohort could relate to inadequate dosing rather than medication ineffectiveness. The use of quality improvement methods in pediatric IBD to impact clinical outcomes by delivering more consistent care, including with regard to thiopurine dosing, has been previously described[18]. Metabolite measurement for this cohort was also variable with < 50% of included patients having metabolites obtained. This failure to optimize thiopurine dosing may also have contributed to the reduced maintenance of remission[23].
Additional limitations of our study common to all studies utilizing registry data include patients lost to follow-up and inability to account for missing data. Furthermore, the impact of medication adherence upon outcomes was not captured and therefore could not be evaluated. Previous studies evaluating adherence in pediatric IBD have found that nearly 40% of thiopurine doses may be missed[24,25]. The use of PGA as our study outcome also allowed for variation in practitioner assessment of clinical remission vs active disease. Finally, the number of patients who met inclusion criteria was somewhat limited.
However, despite the limitations described, it is recognized that variable thiopurine dosing, metabolite measurement by practitioners, and imperfect adherence by patients is a clinical reality. Thus, we believe that our results reflect clinical effectiveness of thiopurines in actual practice. A more structured approach to care, including more consistent thiopurine dosing and potentially metabolite testing may improve clinical outcomes.
In conclusion, the use of thiopurines by practicing clinicians was effective for some pediatric patients with CD in maintaining clinical remission. However, treatment failure within 12 mo of achieving remission was common for this cohort of patients. A more consistent approach to thiopurine dosing and metabolite measurement may improve clinical outcomes.
We are thankful and appreciative of each of the practitioners who contributed data to the PIBDNet cohort study[19].
Thiopurines to maintain remission for pediatric Crohn’s disease (CD) have had variable effectiveness. This manuscript describes the prospective observational outcomes for a cohort of patients in maintaining remission with thiopurines.
The effectiveness of thiopurines in maintaining remission in pediatric CD may be less than might have been predicted by the results of the initial randomized control study. This real world cohort found variable approaches to the use of thiopurines among practitioners regarding dosing and metabolite measurement.
The results of this study are more similar to subsequent pediatric studies and to more recent adult studies evaluating the use of thiopurines in the treatment of CD. The variable approach to dosing and metabolite measurement could have impacted in the poorer outcomes that were found.
The study suggests thiopurines may be less effective in maintaining remission for pediatric CD than previously described. The impact of optimization of the thiopurines through more standardized dosing and metabolite measurement could be further explored.
The term thiopurines refers to the medications mercaptopurine and azathiopurine that are commonly used therapies for treatment of pediatric CD.
This multi-center, prospective observational study suggests the real world use of thiopurines may be less effective in maintaining remission for pediatric Crohn’s disease than might have been predicted by the initial randomized controlled trial.
P- Reviewers: Keljo D, Sorrentino D S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
| 1. | Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423-439. [PubMed] |
| 2. | Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14 Suppl 2:S9-S11. [PubMed] |
| 3. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 4. | Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn‘s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981-987. [PubMed] |
| 5. | Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37:674-678. [PubMed] |
| 6. | Pearson DC, May GR, Fick G, Sutherland LR. Azathioprine for maintaining remission of Crohn’s disease. Cochrane Database Syst Rev. 2000;CD000067. [PubMed] |
| 7. | Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology. 2000;119:895-902. [PubMed] |
| 8. | Saibeni S, Virgilio T, D’Incà R, Spina L, Bortoli A, Paccagnella M, Peli M, Sablich R, Meucci G, Colombo E. The use of thiopurines for the treatment of inflammatory bowel diseases in clinical practice. Dig Liver Dis. 2008;40:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Reinisch W, Panés J, Lémann M, Schreiber S, Feagan B, Schmidt S, Sturniolo GC, Mikhailova T, Alexeeva O, Sanna L. A multicenter, randomized, double-blind trial of everolimus versus azathioprine and placebo to maintain steroid-induced remission in patients with moderate-to-severe active Crohn’s disease. Am J Gastroenterol. 2008;103:2284-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Mantzaris GJ, Christidou A, Sfakianakis M, Roussos A, Koilakou S, Petraki K, Polyzou P. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis. 2009;15:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Jaspers GJ, Verkade HJ, Escher JC, de Ridder L, Taminiau JA, Rings EH. Azathioprine maintains first remission in newly diagnosed pediatric Crohn’s disease. Inflamm Bowel Dis. 2006;12:831-836. [PubMed] |
| 12. | Punati J, Markowitz J, Lerer T, Hyams J, Kugathasan S, Griffiths A, Otley A, Rosh J, Pfefferkorn M, Mack D. Effect of early immunomodulator use in moderate to severe pediatric Crohn disease. Inflamm Bowel Dis. 2008;14:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Lund JL, Cook SF, Allen JK, Carroll CF, Kappelman MD. Patterns of 6-mercaptopurine and azathioprine maintenance therapy among a cohort of commercially insured individuals diagnosed with Crohn’s disease in the United States. Clin Epidemiol. 2013;5:501-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Riello L, Talbotec C, Garnier-Lengliné H, Pigneur B, Svahn J, Canioni D, Goulet O, Schmitz J, Ruemmele FM. Tolerance and efficacy of azathioprine in pediatric Crohn’s disease. Inflamm Bowel Dis. 2011;17:2138-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Goodhand JR, Tshuma N, Rao A, Kotta S, Wahed M, Croft NM, Sanderson IR, Epstein J, Rampton DS. Do children with IBD really respond better than adults to thiopurines? J Pediatr Gastroenterol Nutr. 2011;52:702-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2377] [Article Influence: 158.5] [Reference Citation Analysis (1)] |
| 17. | Wennberg JE. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325:961-964. [PubMed] |
| 18. | Kappelman MD, Bousvaros A, Hyams J, Markowitz J, Pfefferkorn M, Kugathasan S, Rosh J, Otley A, Mack D, Griffiths A. Intercenter variation in initial management of children with Crohn’s disease. Inflamm Bowel Dis. 2007;13:890-895. [PubMed] |
| 19. | Colletti RB, Baldassano RN, Milov DE, Margolis PA, Bousvaros A, Crandall WV, Crissinger KD, D’Amico MA, Day AS, Denson LA. Variation in care in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2009;49:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Crandall WV, Margolis PA, Kappelman MD, King EC, Pratt JM, Boyle BM, Duffy LF, Grunow JE, Kim SC, Leibowitz I. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics. 2012;129:e1030-e1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705-713. [PubMed] |
| 22. | Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009;CD000067. [PubMed] |
| 23. | Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731-743. [PubMed] |
| 24. | Hommel KA, Greenley RN, Maddux MH, Gray WN, Mackner LM. Self-management in pediatric inflammatory bowel disease: A clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2013;57:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:589-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |