Published online Jul 21, 2014. doi: 10.3748/wjg.v20.i27.9128
Revised: February 7, 2014
Accepted: April 8, 2014
Published online: July 21, 2014
Processing time: 223 Days and 12.6 Hours
AIM: To investigate the genetic background of human defensin expression in type 1 and 2 diabetes.
METHODS: Associations between DEFA1/DEFA3 gene copy number polymorphism and diabetes as well as between the promoter polymorphisms of DEFB1 and diabetes were studied. The copy number variation of the DEFA1/DEFA3 genes was determined in 257 diabetic patients (117 patients with type 1 and 140 with type 2 diabetes). The control group consisted of 221 age- and gender-matched healthy blood donors. The cumulative copy numbers of the DEFA1/DEFA3 genes were detected by using quantitative PCR analysis. To evaluate the HNP 1-3 (human neutrophil peptide 1-3 or α-defensin) levels in the circulation, plasma HNP 1-3 concentrations were measured by ELISA. The expression of DEFA1/A3 in peripheral leukocytes of the diabetic patients was measured by quantitative RT PCR analysis. Three SNPs of the human DEFB1 (human defensin β-1) gene: DEFB1 G-20A (rs11362), DEFB1 C-44G (rs1800972) and DEFB1 G-52A (rs1799946) were genotyped by Custom TaqMan® Real Time PCR assay.
RESULTS: Significant differences were observed in HNP1-3 levels between the healthy subjects and both groups of diabetic patients. The mean ± SE was 28.78 ± 4.2 ng/mL in type 1 diabetes, and 29.82 ± 5.36 ng/mL in type 2 diabetes, vs 11.94 ± 2.96 ng/mL in controls; P < 0.01 respectively. There was no significant difference between patients with type 1 and type 2 diabetes in the high plasma concentrations of HNP1-3. The highest concentrations of α-defensin were found in diabetic patients with nephropathy (49.4 ± 4.8 ng/mL), neuropathy (38.7 ± 4.8 ng/mL) or cardiovascular complications (45.6 ± 1.45 ng/L). There was no significant difference in the cumulative copy numbers of DEFA1/DEFA3 genes between controls and patients, or between patients with the two types of diabetes. Comparisons of HNP 1-3 plasma level and DEFA1/A3 copy number of the same patient did not reveal significant relationship between defensin-α levels and the gene copy numbers (r2 = 0.01). Similarly, no positive correlation was observed between the copy numbers and the mRNA expression levels of DEFA1/A3. Regarding the C-44G polymorphism of DEFB1, the GG “protective” genotype was much less frequent (1%-2%) among both groups of patients than among controls (9%).
CONCLUSION: Elevated HNP1-3 levels in diabetes are independent of DEFA1/DEFA3 copy numbers, but GG genotype of C-44G SNP in DEFB1 gene may result in decreased defensin β-1 production.
Core tip: There is growing evidence of the role of innate immunity in diabetes To our knowledge our data provide the first report on a complex investigation of defensin-α and defensin β-1 in type 1 and type 2 diabetes. The main conclusion of our manuscript is, that the elevated HNP1-3 levels in diabetes are independent of the DEFA1/DEFA3 copy numbers, but the GG genotype of C-44G SNP in the DEFB1 gene may result in a decreased level of defensin β-1 production. Our data support the view that both alpha and beta-defensins may have an important role in the pathogenesis of diabetes and diabetic complications.
- Citation: Németh BC, Várkonyi T, Somogyvári F, Lengyel C, Fehértemplomi K, Nyiraty S, Kempler P, Mándi Y. Relevance of α-defensins (HNP1-3) and defensin β-1 in diabetes. World J Gastroenterol 2014; 20(27): 9128-9137
- URL: https://www.wjgnet.com/1007-9327/full/v20/i27/9128.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i27.9128
Defensins are members of small antimicrobial peptides of the innate immune system[1,2]. However, today these peptides are also known as danger signals or ”alarmins” playing important roles in inflammation and immunity[3]. Mammalian defensins are divided into two major families, the α- and β-defensins. Human α-defensins include human neutrophil peptide 1-4 (HNP1-4) and intestinal human defensins (HD-5 and HD-6) produced by Paneth cells. Besides the antimicrobial effects, alpha defensins display chemotactic activity and induce proinflammatory cytokines[4-6]. HNPs increase the binding of low density lipoprotein (LDL) cholesterol to the endothelial surface suggesting that alpha defensins may modulate the development of atherosclerosis[7]. Neutrophil granulocytes are considered to be the primary cellular origin of α-defensins; HNP 1-3 comprise 30%-50% of the granule proteins. HNPs can be released into the extracellular milieu following granulocyte activation as a consequence of degranulation, leakage, cell death, and lysis during inflammation[8]. α-defensins are also involved in the formation of neutrophil extracellular traps[9].
Human β-defensins make up another family of antimicrobial peptides[1,10]. In addition to their antibacterial and antiviral effects, the chemoattractive function of these defensins has been shown to play a role in immunological reactions that protect the host from various pathogens[11]. While the expression of human defensin beta-1 (HBD1) is generally constitutive, the levels of human defensin beta-2 (HBD2) are inducible by proinflammatory cytokines and bacteria[10,12,13]. Human beta defensins are expressed by epithelial cells of the skin, gut, respiratory and urogenital tissues, the pancreas and the kidneys. HBD1 is also constitutively expressed by leukocytes[14-16].
The level of defensin expression varies among individuals, and it has been suggested that this variation is due to genetic differences in the genes encoding defensins. Defensin genes have been mapped to 8p22-p23[17]. Two types of genetic polymorphisms have been identified in genes encoding defensins: copy number polymorphisms and single nucleotide poymorphisms (SNPs). Human defensin beta-1 (HBD-1) is encoded by the gene DEFB1 (OMIM: 602056), in which several SNPs (single nucleotide polymorphisms) have been characterized. Three frequent SNPs at positions G-20A (rs11362), C-44G (rs 1800972) and G-52A (rs1799946) in the 5’-untranslated region (UTR) of DEFB1 were described[18]. The untranslated variants influence HBD-1 expression or function[19].
The cluster of human alpha-defensin genes on chromosome 8 includes the genes DEFA1 (OMIM: 125220) and DEFA3 (OMIM: 604522), which are copy-variables. The genes DEFA1 and DEFA3 differ only in a single base substitution in the coding sequence, corresponding to a single amino acid difference between the peptides encoded[20]. HNP 1-3 differs only in a single N-terminal acid, and the HNP-2 peptide lacks this residue and might be a proteolytic product of the other two peptides because no separate gene has been identified to encode HNP-2[21]. Several copy number polymorphisms form the major source of genetic polymorphism of α-defensin genes DEFA1 and DEFA3, encoding human neutrophil peptides HNP-1, -2 and 3[20,22]. These genes are present in a cluster that is close to but independent from the β-defensin cluster on 8p23. The total DEFA1/DEFA3 copy number has been found to range between 4 and 11 copies per diploid genome with 5 to 9 copies being the most common[22].
To date, little is known about the genetic basis and the functions of α- and β-defensins in diabetes. Infections are frequent in diabetic patients because the antimicrobial function of their immune response is impaired. It has been reported that mRNA levels of rat β-defensin-1 are significantly low in the kidneys, which may explain the high incidence of urinary tract infections in diabetes mellitus[23]. The effects of glucose and insulin on the β-defensin expression have recently been demonstrated[24], but no connection has been found between genetic polymorphisms of the HBD1 gene and diabetes in a Brazilian study on diabetic children[25].
Increased levels of alpha-defensin -1, -2 and -3 have recently been reported in patients with type 1 diabetes with nephropathy and in cardiovascular complications[26,27]. It is tempting to speculate whether copy number polymorphisms and the DEFA1/DEFA3 mRNA in the granulocytes may influence the levels of HNP1-3 in patients with types 1 and 2 diabetes.
The aim of our study was to investigate the genetic background of human defensin-α and human defensin β-1 production in adult patients with type 1 and type 2 diabetes, especially with complications. Therefore, we carried out an association study between DEFA1/DEFA3 copy number polymorphism and diabetes, and between the promoter polymorphisms of DEFB1 and diabetes. We also measured the plasma levels of HNP 1-3 in both types of diabetes, and the mRNA expression of DEFA1/DEFA3 in leukocytes.
257 diabetic patients (122 men and 135 women) were enrolled in our study, which included 117 patients with type 1 and 140 patients with type 2 diabetes. All patients participating in the study were diagnosed according to the ADA criteria: Diagnosis and classification of diabetes mellitus. Diabetes Care 36 (Suppl 1) 2013. S64-S74.
The mean age of type 1 diabetic patients was 40.6 years ± 1.51 years, the mean duration of diabetes was 17.7 ± 1.12 years, and their mean HbA1c was 8.86% ± 0.17%. In type 2 diabetic subjects, the mean age was 58.4 ± 1.27 years, the mean duration of diabetes was 14.5 ± 0.8 years, and the mean HbA1c was 8.03% ± 0.13%.
Seventy-one subjects in the cohort had diabetic nephropathy (32 with type 1 and 39 with type 2 diabetes) defined as an albumin-to-creatinine ratio in a random spot collection being higher than 3.4 mg/mmol, or the protein content being over 300 mg/d in collected urine. Abnormal kidney function was described when the glomerular filtration rate (GFR) was lower than 60 mL/min per 1.73 m2.
One hunred twenty-one patients suffered from retinopathy (47 with type 1 and 68 with type 2 diabetes). This complication was evidenced as the presence of background or proliferative retinopathy, macular edema or diabetes-related blindness, or the administration of retinal photocoagulation therapy. The retinopathy status was checked by color stereo-ophthalmography and fluorescence angiography. Neuropathy was diagnosed in 95 patients (35 with type 1 and 60 with type 2 diabetes). Neuropathy was proven when abnormal peripheral sensory functions or altered lower limb tendon reflexes as well as impaired cardiovascular reflex tests were detected. 54 diabetic patients (14 with type 1 diabetes and 40 with type 2 diabetes) had previously been diagnosed with macrovascular disease including major coronary events, stroke or a transient ischemic attack, peripheral artery disease or amputation. A high number of the patients (182) had controlled hypertension (50 with type 1 and 132 with type 2 diabetes).
The control group consisted of 221 age- and gender-matched healthy blood donors. These control subjects were selected from blood donors at the regional Center of Hungarian National Blood Transfusion Service, Szeged, Hungary. The exclusion criteria for blood donors were diabetes, nephropathy, hypertension, or ischemic heart disease. All cases and controls were of Hungarian ethnic origin and resident in Hungary. Informed consent was obtained from all patients and controls, and the local Ethics Committee gave prior approval to the study. All patients consented to the study and were treated according to the Patient Right Protection Act of our institutions and according to international guidelines.
Blood samples containing EDTA were obtained from patients and controls. Plasma was isolated after the blood was centrifuged at 3000 g for 3 min and stored at -80 °C for further analysis. The HNP1-3 concentrations in plasma were determined by ELISA (Hycult-Biotech HK324, Uden, The Netherlands) according to the instructions of the manufacturer.
Genomic DNA purified from peripheral blood was used. Leukocyte DNA was isolated using the High Pure PCR Template Preparation Kit according to the instructions of the manufacturer (Roche Diagnostic GmbH, Mannheim, Germany). DNA concentrations were measured with a Qubit™ fluorometer (Invitrogen, Carlsbad, CA, United States) according to the instructions of the manufacturer. Genomic DNA was stored at -20 °C until further use.
Genomic DNA purified from peripheral blood was used. Gene copy number determination was carried out as previously described by Linzmeier[20] with slight modifications. BIO-RAD CFX 96 instrument (Bio-Rad, Hercules, CA, United States) was used for quantitation. The reaction volume was 15 μL, containing 3 μL of DNA, 1 μmol L-1 each of the primers, 7.5 μL of reaction buffer (Fermentas Probe/ROX qPCR MasterMix, Fermentas, Lithuania) and 0.6 μL EVAGreen (20 × EVAGreen™ Biotium Inc., Hayward, CA, United States). Forward primer: DEFA1 1 F (5’ TAC CCA CTG CTA ACT CCA TAC 3’), reverse primer: DEFA1 1 R (5’ GAA TGC CCA GAG TCT TCC C 3’); MPO (myeloperoxidase) reference gene primer set MPO 1 F (5’ CCA GCC CAG AAT ATC CTT GG 3’), MPO 1 R (5’ GGT GAT GCC TGT GTT GTC G 3’). PCR conditions were as follows: initial denaturation at 95 °C for 10 min followed by 40 cycles of denaturation (95 °C for 15 s) and extension (54 °C for 1 min).
Quantification was performed by monitoring the emitted fluorescence after each cycle of PCR reaction of genomic DNA samples in order to identify the exact time point at which the log-linear phase could be distinguished from the background (crossing point). The precise amount of DNA added to each reaction mix was based on optical density. Each DNA sample was analyzed in triplicate, in 2 independent experiments.
We collected further 2 mL of venous blood in EDTA tubes from patients with diabetes and controls. Leukocytes from blood were separated by centrifugation at 1200 rpm/min for 15 min. By using the reverse transcription polymerase chain reaction (RT-PCR), we examined the expression of DEFA1/DEFA3 mRNA in 24 patients with diabetes (12 cases of type 1 and 12 cases of type 2 diabetes). Total RNA was extracted with High Pure RNA isolation kit (Roche) according the manufacturer’s instruction. RNA concentration was determined by the A260 value of the sample. Complementary DNA (cDNA) was generated from 1 μg total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) in a final volume of 20 μL. After reverse transcription, amplification was carried out by using Light Cycler Fast Start DNA MasterPLUS SYBR Green I mix (Roche). Samples were loaded into capillary tubes and placed in the fluorescence thermocycler (LightCycler). Initial denaturation at 95 °C for 10 min was followed by 45 cycles of 95 °C for 10 s, annealing at 58 °C for 8 s, and elongation at 72 °C for 12 s. DEFA1/DEFA3 sense, 5’-TCC CAG AAG TGG TTG TTT CC-3’; and antisense, 5’-GCA GAA TGC CCA GAG TCT TC-3’, and for the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) sense, 5′-AAG GTC GGA GTC AAC GGA TTT-3′; antisense, 5′-TGG AAG ATG GTG ATG GGA TTT-3′ primers were used to amplify specific products from cDNA samples. At the end of each run, melting-curve profiles were achieved by cooling the sample to 40 °C for 15 s, and then heating the sample slowly at 0.20 °C/s up to 95 °C with continuous measurement of the fluorescence to confirm the amplification of specific transcripts. Cycle-to-cycle fluorescence emission readings were monitored and analyzed by using LightCycler software (Roche Diagnostics GmbH). All quantifications were normalized to the housekeeping GAPDH gene. Relative gene expression was determined by using the ∆∆Ct method.
Genotyping was performed by means of Custom TaqMan® SNP Genotyping Assays (Applied Biosystems, CA). Fluorogenic minor groove binder probes were used for each case using the dyes 6-carboxyfluorescein (FAM; excitation, 494 nm) and VIC (excitation, 538 nm): beta-defensin-1 polymorphisms DEFB1 G-20A (rs11362) Applied Biosystems code c_11636793_20, DEFB1 C-44G (rs1800972) c_11636794_10 and DEFB1 G-52A (rs1799946) c_11636795_20. Thermal cycling was performed on ABI Prism 7000 sequence-detection PCR systems. The amplification mix contained the following ingredients: 7.5 μL of TaqMan® universal PCR master mix (Applied Biosystems, CA), 0.375 μL of primer-probe mix, 6.375 μL of RNase- and DNase-free water (Sigma), and 0.8 μL of sample DNA, in a total volume of 15 μL per single tube reaction. Assay conditions were 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 95 °Cfor 15 s and 60 °C for 1 min. Each 96-well plate contained 90 samples of an unknown genotype and six reactions with reagents but no DNA. DNase-free water was used as nontemplate control. Initial and postassay analysis was performed by using the Sequence Detection System (SDS) version 2.1 software (Applied Biosystems, CA) as outlined in the TaqMan Allelic Discrimination Guide. Genotypes were determined visually based on the dye-component fluorescent emission data depicted in the X-Y scatter plot of the SDS software. Genotypes were also determined automatically by the signal processing algorithms in the software. Results of each scoring method were saved in two separate output files for later comparison.
Comparisons of plasma concentrations were carried out by the Mann-Whitney test and with two-tailed paired Student test. The level of significance of the genotype frequency of different DEFB1 SNPs was analyzed by using the χ2 test and the Fischer test. Levels P < 0.05 indicated statistical significance. All statistical calculations were performed with the Graph Pad Prism 5.0 statistical program (GraphPad Software, San Diego CA. United States). The genotype frequencies for each polymorphism of DEFB1 were tested for deviation from the Hardy-Weinberg equilibrium by the χ2 test with 1 degree of freedom.
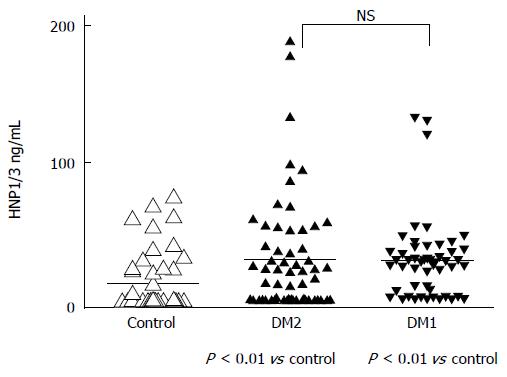
In a pilot study, plasma levels of α-defensin, HNP1-3, in 50 patients with type 1 diabetes and in 60 patients with type 2 diabetes were determined and compared with those of 50 healthy blood donors. There was a high individual variation in the plasma levels of α-defensin, but significant differences were observed between the healthy subjects and both groups of diabetic patients. The mean value ± SE was 28.78 ± 4.2 ng/mL in patients with type 1 diabetes and 29.82 ± 5.36 ng/mL in patients with type 2 diabetes vs 11.94 ± 2.96 ng/mL in controls (P < 0.01 respectively). The difference between the high plasma concentrations of HNP1-3 in patients with type 1 or type 2 diabetes was not significant (Figure 1).
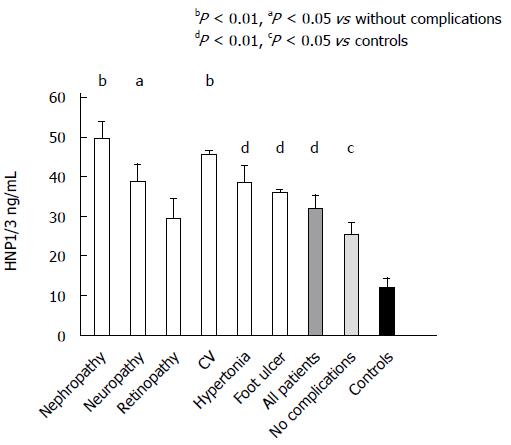
After that, we checked whether the generally high level of HNP1-3 in the peripheral blood of both groups (type 1 and type 2) of diabetic patients was connected to diabetic complications. Nephropathy was diagnosed in 71 patients (32 with type 1 and 39 with type 2 diabetes) and neuropathy in 95 ones (35 with type 1 and 60 with type 2 diabetes). 115 patients suffered from retinopathy (47 with type 1 and 68 with type 2 diabetes), 54 patients had cardiovascular diseases (14 with type 1 diabetes and 40 with type 2 diabetes), and 182 had hypertension (50 with type 1 and 132 with type 2 diabetes). Their data concerning the HNP1-3 levels were compared with those of the 67 patients who did not suffer from these complications, and with the data of the 100 healthy subjects (Figure 2). The highest HNP 1-3 concentrations were found in the diabetic patients with nephropathy (49.4 ± 4.8 ng/mL) and with neuropathy (38.7 ± 4.8 ng/mL) or with cardiovascular complications (45.6 ± 1.4 ng/mL). These concentrations were significantly higher than those in the diabetic patients without complications (25.4 ± 3.5 ng/mL). These data are in accordance with previous observations[26,27] of high levels of HNP 1-3 in type 1 diabetic patients with cardiovascular diseases. In a relatively smaller group of patients (n = 28) with diabetic foot ulcer (20 with type 1 diabetes and 8 with type 2 diabetes), the HNP1-3 plasma levels were 35.9 ± 1.1 ng/mL. These high HNP1-3 levels might be the consequence of the degranulation of recruited neutrophils from the skin frequently following infections. Our results suggest that in diabetic complications such as nephropathy, neuropathy and cardiovascular diseases, the HNP1-3 level in the circulation is elevated independently of the type of diabetes. All diabetic patients, with or without complications, exhibited significantly higher plasma levels of HNP1-3 than the control subjects (Figure 2).
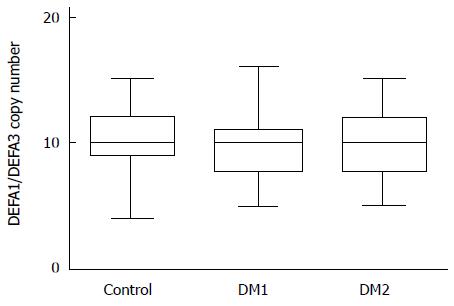
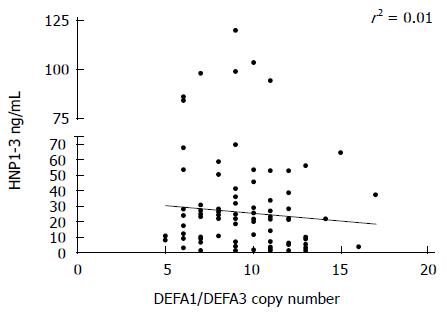
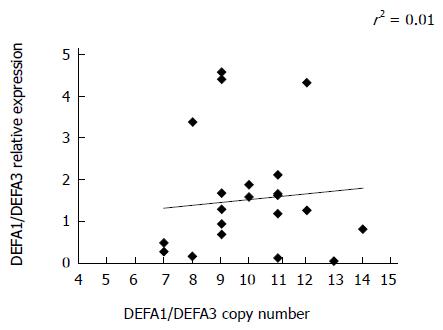
The cumulative copy numbers of DEFA1/DEFA3 were determined by using quantitative PCR analysis. In the control group, 133 DNA samples were used for copy number determination and 100 DNA samples of diabetic patients with type 1 or type 2 diabetes. There was no significant difference in copy number between the controls and the patients or between the patients with the two types of diabetes (Figure 3). In the control group, DEFA1/DEFA3 copy numbers ranged from 4 to 15 per genome with a median number of 10 copies. The median copy number of DEFA1/DEFA3 in the patients with type 1 diabetes was 10 copies per genome (range 5 to 16 copies), and that in the patients with type 2 diabetes was also 10 (range 5 to 15). Comparing the HNP 1-3 plasma level and the DEFA1/A3 copy number of the same patient, no significant correlation was observed between defensin levels and genomic copy numbers (r2 = 0.01; Figure 4.).
The expression of DEFA1/A3 was measured in the peripheral leukocytes of diabetic patients. DEFA1/DEFA3 mRNA was determined in blood samples from 12 patients with type 1 and another 12 with type 2 diabetes, and the relative expressions of DEFA1/DEFA3 were compared with the DEFA1/DEFA3 copy numbers of the same patients (Figure 5). The data indicated that peripheral leukocytes had the ability to transcribe DEFA1/DEFA3 genes (mean ± SE of relative expression 1.5 ± 0.28) and to biosynthetize HNP1-3 peptides. However, no positive correlation was observed between the copy numbers and the expression levels of the human neutrophil peptide 1-3 (Figure 5). The variation in expression levels between individuals did not exhibit a positive correlation with the copy number. Similarly, the expression of specific mRNA in the leukocytes for HNP 1-3 did not parallel the HNP 1-3 plasma levels (data not shown).
The genotypic distributions of DEFB1 G-20A, and DEFB1 G-52A and DEFB1 C-44G polymorphisms are presented in Table 1.
| DEFB1 G-20A | GG | GA | AA | χ2 test1 |
| Patients with diabetes | 82 (32) | 131 (51) | 44 (17) | 0.568 |
| n = 257 | ||||
| Type 1 diabetes | 36 (31) | 60 (51) | 21 (18) | 0.775 |
| n = 117 | ||||
| Type 2 diabetes | 46 (33) | 71 (51) | 23 (16) | 0.573 |
| n = 140 | ||||
| Controls | 62 (31) | 96 (48) | 42 (21) | |
| n = 200 | ||||
| DEFB1 G-52A | GG | GA | AA | χ2 test1 |
| Patients with diabetes | 114 (44) | 104 (40) | 39 (15) | 0.572 |
| n = 257 | ||||
| Type 1 diabetes | 52 (44) | 47 (40) | 18 (17) | 0.702 |
| n = 117 | ||||
| Type 2 diabetes | 62 (44) | 57 (41) | 21 (15) | 0.658 |
| n = 140 | ||||
| Controls | 80 (40) | 84 (42) | 36 (18) | |
| n = 200 | ||||
| DEFB1 C-44G | CC | CG | GG | χ2 test1 |
| Patients with diabetes | 156 (61) + | 95 (37) | 6 (2)2 | 0.002 |
| n = 257 | ||||
| Type 1 diabetes | 70 (60) | 44 (37) | 3 (2.5) | 0.01 |
| n = 117 | ||||
| Type 2 diabetes | 86 (61) | 51 (36) | 3 (2) | 0.003 |
| n = 140 | ||||
| Controls | 90 (45) | 92 (46) | 18 (9) | |
| n = 200 |
The distribution of the DEFB1 G-20A genotypes was in accordance with the Hardy-Weinberg equilibrium both in the control population and in the patients (P = 0.912 and P = 0.795, respectively). There was no significant difference in genotype distribution between the patients overall and the healthy controls. Similarly, no significant differences in genotypes were observed when the patients were grouped according to type 1 and type 2 diabetes.
As concerns the DEFB1 G-52A SNP, distribution of the genotypes was in accordance with the Hardy-Weinberg equilibrium both in the control population and in the patients (P = 0.252 and P = 0.181, respectively). We did not detect any significant difference in genotypes between the patients and the controls, either in type 1 or type 2 diabetes.
The genotypic distribution of DEFB1 C- 44G polymorphism is shown in Table 1.
The distribution of genotypes was in accordance with the Hardy-Weinberg equilibrium among the patients with diabetes (P = 0.151) and also in the control population (P = 0.722). But there was a significant difference in genotype distribution between the patients overall and the healthy controls (χ2 test, P = 0.002). The frequency of the GG genotype was significantly lower in both types of diabetes (2.5% and 2%, respectively) than in the healthy controls (9%) (Fisher test vs control, P = 0.001, OR = 9.136, 95%CI: 3.512-23.82). Conversely, the prevalence of the DEFB1 CC genotype was 61 % in the group of diabetic patients vs 45% in the controls (Fisher test: P = 0.0009, OR = 2.055, 95%CI: 1.248-2.746). When the patients were grouped according to the diabetic complications, there was a lower frequency of the GG genotype among the patients with nephropathy and among those with neuropathy (1.4% and 1%, respectively).
HNP1-3 levels in the circulation were measured, and the copy number variation of DEFA1/A3 genes was determined in diabetic patients. The diabetic patients exhibited overall higher plasma levels of HNP 1-3 (α-defensin) with either type 1 or type 2 form of the disease than the healthy controls. The highest concentrations of HNPs were detected in patients with nephropathic or neuropathic and cardiovascular complications. An essential question arises as to why an increased concentration of plasma α-defensin (HNP1-3) level is associated with type 1 and type 2 diabetes, especially in the event of diabetic complications such as nephropathy, neuropathy or cardiovascular problems. The explanation might be that the elevation in the plasma HNP1-3 level is the consequence of the decreased renal degradation of the peptides in patients with advanced nephropathy[26]. HNP 1-3 promote the accumulation of low density lipoprotein in the vasculature, inhibit fibrinolytic activity on the surface of vascular cells, and accumulate in the intima of atherosclerotic plaques. Therefore, HNP 1-3 may have clinical implications in diabetic patients with hypercholesteremia or vascular dysfunction[7,27].
The association of high levels of HNP1-3 in patients with neuropathy in type 1 and type 2 diabetes is yet to be clarified. The next question is whether there is an increased gene expression responsible for the elevated plasma levels of α-defensins. Our study revealed that there was no correlation between HNP1-3 plasma levels and the copy numbers of DEFA1/A3 genes. The effect of copy number variations in DEFA1/DEFA3 on the disease or even on the plasma concentrations of the peptides remain unclear. Controversal data have been published about the correlation between DEFA1/A3 copy number and the α-defensin peptide concentration. Linzmeier and Ganz[20] have shown that the intracellular HNP1-3 levels in human neutrophils are proportional to the copy numbers of the DEFA1 and DEFA3 genes Copy numbers of DEFA1/A3 may be proportional to the intracellular levels of HNP1-3 in neutrophil granulocytes but possibly not to the circulating HNP1-3 levels. Additionally, a discrepancy between gene copy number and the HNP1-3 protein levels has recently been reported in septic patients[28]. There are several potential explanations of the discrepancy between the gene copy number and the plasma levels. HNP1-3 is stored primarily in the granules of neutrophils and is released into the circulation during the activation of the neutrophils. Moreover, it might also be due to different transcriptional mechanisms modulating these genes or to an increased distance between the regulators of the genes[28,29].
The fact that in our study no significant correlation was observed between the genomic copy number variation of DEFA1/DEFA3 and the mRNA expression levels (Figure 5) suggests that the degranulation rather than the increased gene expression may be responsible for the increased plasma HNP 1-3 levels in diabetes.
Similar observations have been published about the copy number polymorphism and expression level variation of DEFA1 and DEFA3 genes[22]. In that study, the combined expression levels of DEFA1/A3 and the genomic copy number have not been correlated, suggesting the superimposed influence of trans-acting factors.
It is noteworthy that there are several examples of the absence of a correlation between copy number polymorphisms and the relative transcription level[29].
There are no direct data showing that exaggerated degranulation is linked to diabetic complications, or it is higher in DM patients than in controls. However, it has recently been published that pro-inflammatory conditions during hyperglycemia favor NET - neutrophil extracellular traps - formation[30], and HNP 1-3 are also involved in the formation of neutrophil extracellular traps[9]. It is noteworthy that diabetes is associated with low grade, sub-clinical and chronic inflammation characterized by abnormal cytokine production. Therefore, the diabetic microenvironment can induce NET formation, which may result in a basic high HNP-1 concentration in the circulations.
In order to detect whether increased mRNA expression is responsible for elevated defensin levels in diabetic patients, quantitative RT-PCR reactions were performed. Expression of specific mRNA in the leukocytes was observed for HNP 1-3 but not parallel with HNP1-3 plasma levels. The mRNA values between patients and controls were rather equal (mean ± SE of relative expression 1.5 ± 0.28 vs 1.49 ± 0.35, respectively) suggesting that not an increased gene expression may be responsible for increased plasma levels of HNP 1-3. Our findings were in good correlation with the observations of Fang et al[31], that is, α-defensin genes were constitutively transcribed at low level in mature neutrophils, but they were not inducible.
Eosinophils with transcriptionally active α-defensin production have recently been detected in the capillary blood of diabetic patients[32]. Eosinophils but not neutrophils displayed the augmentation of transcriptional activation of α-defensin expression. In our study, the majority of the cells in peripheral venous blood were neutrophils; therefore, our purified DNA and RNA samples were derived mostly from neutrophils.
The present study demonstrated that the distributions of the C-44G genotypes were different between patients with diabetes and healthy controls, whereas the frequency of the GG genotype was significantly higher in the control population. It indicates that the presence of G allele probably leads to strengthened HBD1 antimicrobial activity, which is less frequent in patients with diabetes. The G allele of C-44G SNP generates a putative binding site for nuclear factor κB (NF-κB), and it is very likely to induce an overexpression. The proposed effect of this SNP could partially explain why the GG genotype was considered to be a protective genotype in atopic dermatitis[33] and also in the susceptibility to Candida colonization in diabetic patients[34]. Conversely, in these studies, subjects carrying the CC genotype at the -44 locus site of the gene were at a greater risk of acquiring infection. It has been recently suggested that the C allele of DEFB1 C-44G SNP probably abrogates NF-κB-dependent DEFB1 upregulation[35].
These data are consistent with our present observation that the GG phenotype could also be protective in diabetes, and vice versa, the higher frequency of CC genotype might be connected with lower expression of human defensin β-1. Among the 257 patients with diabetes, only 6 (2%) were GG homozygotes, and 61% of the patients were CC homozygotes, as compared with 45% of CC homozygotes in the control group. Furthermore, the number of GG homozygotes was even lower (1%) among patients with nephropathy and neuropathy. These observations draw the attention to the importance of DEFB1 polymorphisms in diabetes, especially in the cases with nephropathy and neuropathy. Foot ulcerations in diabetic patients are often combined with infections. None of the 28 patients with foot ulcer displayed GG genotype of C- 44G SNP of DEFB1 gene. A high blood glucose level itself can result in low levels of β-defensin[23], and it might be further downregulated in humans as a consequence of C-44G polymorphism. It is noteworthy that insulin is an important factor mediating hBD-1 expression[24].
Taken together, our study demonstrated elevated levels of α-defensin (HNP1-3) in type 1 and type 2 diabetes, which were more pronounced when there were diabetic complications. However, there was no correlation between the circulating HNP1-3 levels and the DEFA1/DEFA3 copy number. Similarly, no correlation was found between the mRNA expression and the copy number variation. Further studies are needed to explore whether the elevated α-defensin levels of the plasma in diabetes are causally linked to this disease and its complications, or they are simply the consequences of the degranulation of neutrophils under pathologic conditions. Whatever the mechanism, the elevated HNP1-3 level might not be genetically determined or at least independent of the copy number variation of the DEFA1/DEFA3 genes. In contrast, the CC genotype of the C-44G SNP of DEFB1 was more frequent in diabetic patients than in healthy controls, which draws the attention to the genetic background of a potentially impaired function of hBD1 (human defensin β-1) in diabetes. These data support the view that both alpha and beta-defensins may have important roles in the pathogenesis of diabetes and diabetic complications. Our results should be regarded as preliminary results, which should be confirmed on a larger series of patients in a future multicenter study.
We thank Mrs.Györgyi Müller for expert technical assistance.
There is a growing evidence of the role of innate immunity in diabetes. Defensins are members of small antimicrobial peptides of the innate immune system. In addition to their antibacterial and antiviral effects, immunologic functions of defensins has been shown to play a role in the homeostasis. To date, little is known about the genetic basis and the functions of α- and β-defensins in diabetes. The aim of this study was to investigate the genetic background of human defensin-α and human defensin β-1 production in adult patients with type1 and type 2 diabetes, especially with complications.
Research Frontiers
Neutrophil granulocytes are considered to be the primary cellular origin of α-defensins; HNP 1-3. HNPs can be released into the extracellular milieu following granulocyte activation as a consequence of degranulation, leakage, cell death, and lysis during inflammation. Human beta defensins are expressed mainly by epithelial cells of the skin, gut, respiratory and urogenital tissues, the pancreas and the kidneys. The level of defensin expression varies among individuals, and it has been suggested that this variation is due to genetic differences in the genes encoding defensins.
These data provide the first report about a complex investigation of defensin-alpha and defensin β-1 in type 1 and type 2 diabetes. Increased levels of alpha-defensin -1, -2 and -3 have recently been reported in patients with type 1 diabetes with nephropathy and in cardiovascular complication. In this study not only HNP1-3 levels in the circulation were measured, but also the copy number variation of DEFA1/A3 genes was determined in diabetic patients, together with the expression of DEFA1/A3 in peripheral leukocytes. Several SNPs (single nucleotide polymorphisms) have been characterized of human DEFB1 (human defensin β-1) gene in previous studies. In this complex study the authors demonstrated that elevated HNP1-3 levels in diabetes are independent of DEFA1/DEFA3 copy numbers, but the GG genotype of C-44G SNP in DEFB1 gene may result in decreased β1-defensin production.
The data support the view, that both alpha and beta-defensins may have an important role in the pathogenesis of diabetes and diabetic complications. The results may contribute to a better understanding of the roles of defensins in the pathomechanism of diabetes and may represent a future possibility toward broadening of the prognostic laboratory markers.
HNP 1-3 are Human Neutrophil Peptides, members of the human α-defensin family. Human defensin β-1 (HBD1) is the member of another family of antimicrobial peptides. Two types of genetic polymorphisms have been identified in genes encoding defensins: copy number polymorphisms and single nucleotide poymorphisms (SNPs). Several copy number polymorphisms form the major source of genetic polymorphism of α-defensin genes DEFA1 and DEFA3, encoding human neutrophil peptides Human defensin beta-1 (HBD-1) is encoded by the gene DEFB1. Three frequent SNPs at positions G-20A (rs11362), C-44G (rs 1800972) and G-52A (rs1799946) in the 5’-untranslated region (UTR) of DEFB1 were described. The untranslated variants influence HBD-1 expression or function.
The current manuscript addresses the role of defensins in patients with type 1 and type 2 diabete. The authors, in addition to the protein levels, correlate absolute mRNA expression to the diabetic patients. The authors explain the biological role/relevance of the defensine increase.
P- Reviewers: Kanda N, Kietzmann T S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2123] [Cited by in RCA: 2125] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 2. | Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, Cen P. Recent advances in the research and development of human defensins. Peptides. 2006;27:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 602] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 4. | Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005;6:35-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Chertov O, Yang D, Howard OM, Oppenheim JJ. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev. 2000;177:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 500] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 7. | Quinn K, Henriques M, Parker T, Slutsky AS, Zhang H. Human neutrophil peptides: a novel potential mediator of inflammatory cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2008;295:H1817-H1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987;55:568-571. [PubMed] |
| 9. | Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 463] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 10. | Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63:1294-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 398] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 11. | Dürr M, Peschel A. Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect Immun. 2002;70:6515-6517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 797] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 13. | De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 358] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 429] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Schnapp D, Reid CJ, Harris A. Localization of expression of human beta defensin-1 in the pancreas and kidney. J Pathol. 1998;186:99-103. [PubMed] |
| 16. | Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology. 2002;106:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Hollox EJ, Barber JC, Brookes AJ, Armour JA. Defensins and the dynamic genome: what we can learn from structural variation at human chromosome band 8p23.1. Genome Res. 2008;18:1686-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Dörk T, Stuhrmann M. Polymorphisms of the human beta-defensin-1 gene. Mol Cell Probes. 1998;12:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Sun CQ, Arnold R, Fernandez-Golarz C, Parrish AB, Almekinder T, He J, Ho SM, Svoboda P, Pohl J, Marshall FF. Human beta-defensin-1, a potential chromosome 8p tumor suppressor: control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res. 2006;66:8542-8549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Linzmeier RM, Ganz T. Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in alpha- and beta-defensin regions at 8p22-p23. Genomics. 2005;86:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 886] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 22. | Aldred PM, Hollox EJ, Armour JA. Copy number polymorphism and expression level variation of the human alpha-defensin genes DEFA1 and DEFA3. Hum Mol Genet. 2005;14:2045-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Froy O, Hananel A, Chapnik N, Madar Z. Differential effect of insulin treatment on decreased levels of beta-defensins and Toll-like receptors in diabetic rats. Mol Immunol. 2007;44:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Barnea M, Madar Z, Froy O. Glucose and insulin are needed for optimal defensin expression in human cell lines. Biochem Biophys Res Commun. 2008;367:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Guimarães RL, Segat L, Rocha CR, Brandão LA, Zanin V, Araujo J, Naslavsky MS, de Lima Filho JL, Crovella S. Functional polymorphisms of DEFB1 gene in type 1 diabetes Brazilian children. Autoimmunity. 2009;42:406-413. [PubMed] |
| 26. | Saraheimo M, Forsblom C, Pettersson-Fernholm K, Flyvbjerg A, Groop PH, Frystyk J. Increased levels of alpha-defensin (-1, -2 and -3) in type 1 diabetic patients with nephropathy. Nephrol Dial Transplant. 2008;23:914-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Joseph G, Tarnow L, Astrup AS, Hansen TK, Parving HH, Flyvbjerg A, Frystyk J. Plasma alpha-defensin is associated with cardiovascular morbidity and mortality in type 1 diabetic patients. J Clin Endocrinol Metab. 2008;93:1470-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Chen Q, Hakimi M, Wu S, Jin Y, Cheng B, Wang H, Xie G, Ganz T, Linzmeier RM, Fang X. Increased genomic copy number of DEFA1/DEFA3 is associated with susceptibility to severe sepsis in Chinese Han population. Anesthesiology. 2010;112:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Henrichsen CN, Chaignat E, Reymond A. Copy number variants, diseases and gene expression. Hum Mol Genet. 2009;18:R1-R8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 30. | Joshi MB, Lad A, Bharath Prasad AS, Balakrishnan A, Ramachandra L, Satyamoorthy K. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 2013;587:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Fang XM, Shu Q, Chen QX, Book M, Sahl HG, Hoeft A, Stuber F. Differential expression of alpha- and beta-defensins in human peripheral blood. Eur J Clin Invest. 2003;33:82-87. [PubMed] [DOI] [Full Text] |
| 32. | Neuwirth A, Dobeš J, Oujezdská J, Ballek O, Benešová M, Sumník Z, Včeláková J, Koloušková S, Obermannová B, Kolář M. Eosinophils from patients with type 1 diabetes mellitus express high level of myeloid alpha-defensins and myeloperoxidase. Cell Immunol. 2012;273:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Prado-Montes de Oca E, García-Vargas A, Lozano-Inocencio R, Gallegos-Arreola MP, Sandoval-Ramírez L, Dávalos-Rodríguez NO, Figuera LE. Association of beta-defensin 1 single nucleotide polymorphisms with atopic dermatitis. Int Arch Allergy Immunol. 2007;142:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. Single-nucleotide polymorphisms (SNPs) in human beta-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J Clin Microbiol. 2003;41:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Prado-Montes de Oca E, Velarde-Félix JS, Ríos-Tostado JJ, Picos-Cárdenas VJ, Figuera LE. SNP 668C (-44) alters a NF-kappaB1 putative binding site in non-coding strand of human beta-defensin 1 (DEFB1) and is associated with lepromatous leprosy. Infect Genet Evol. 2009;9:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |