Published online Jul 7, 2014. doi: 10.3748/wjg.v20.i25.8260
Revised: March 4, 2014
Accepted: April 5, 2014
Published online: July 7, 2014
Processing time: 182 Days and 22.4 Hours
AIM: To investigate the gastric muscle injury caused by endoplasmic reticulum (ER) stress in rats with diabetic gastroparesis.
METHODS: Forty rats were randomly divided into two groups: a control group and a diabetic group. Diabetes was induced by intraperitoneal injection of 60 mg/kg of streptozotocin. Gastric emptying was determined at the 4th and 12th week. The ultrastructural changes in gastric smooth muscle cells (SMCs) were investigated by transmission electron microscopy. TdT-mediated dUTP nick end labeling (TUNEL) assay was performed to assess apoptosis of SMCs. Expression of the ER stress marker, glucose-regulated protein 78 (GRP78), and the ER-specific apoptosis mediator, caspase-12 protein, was determined by immunohistochemistry.
RESULTS: Gastric emptying was significantly lower in the diabetic rats than in the control rats at the 12th wk (40.71% ± 2.50%, control rats vs 54.65% ± 5.22%, diabetic rats; P < 0.05). Swollen and distended ER with an irregular shape was observed in gastric SMCs in diabetic rats. Apoptosis of gastric SMCs increased in the diabetic rats in addition to increased expression of GRP78 and caspase-12 proteins.
CONCLUSION: ER stress and ER stress-mediated apoptosis are activated in gastric SMCs in diabetic rats with gastroparesis.
Core tip: Endoplasmic reticulum stress and/or ER stress-induced apoptosis in the etiology of diabetic gastroparesis (DGP) remain unclear. This study focuses on the muscle injury caused by ER stress in rats with DGP. We found that apoptosis of gastric smooth muscle cells (SMCs) increased in diabetic rats in addition to increased expression of the ER stress marker, glucose-regulated protein 78, and the ER-specific apoptosis mediator, caspase-12. This is the first study to demonstrate that ER stress and ER stress-induced apoptosis are activated in gastric SMCs in diabetic rats.
- Citation: Chen X, Fu XS, Li CP, Zhao HX. ER stress and ER stress-induced apoptosis are activated in gastric SMCs in diabetic rats. World J Gastroenterol 2014; 20(25): 8260-8267
- URL: https://www.wjgnet.com/1007-9327/full/v20/i25/8260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i25.8260
Diabetes mellitus, especially in patients with refractory symptoms, is associated with a high prevalence of gastrointestinal motility disorders. Gastroparesis is one of the most common and well-established complications of diabetes and is characterized by delayed gastric emptying without mechanical obstruction of the stomach[1]. Gastroparesis affects approximately 20%-58% of the diabetic population, particularly those individuals with long-standing, poorly controlled diabetes[2,3]. Symptoms associated with delayed gastric emptying include nausea, vomiting, bloating, postprandial abdominal pain and early satiety. In addition, other clinical consequences of diabetic gastroparesis (DGP) include alterations in drug absorption and poor glycemic control[4,5]. Therapies including correction of hyperglycemia, prokinetic drugs and alteration in dietary pattern may be helpful in controlling or relieving symptoms of gastroparesis[6]. However, there are still a number of patients who suffer from poorly controlled and long-term dyspeptic symptoms. Therefore, it is of interest to explore new strategies aimed at improving the prognosis of this disease.
The pathophysiology of DGP is complex and remains to be elucidated, and is traditionally considered to be the result of vagal nerve impairment due to systemic autonomic neuropathy in diabetes[7]. However, research has shown that there are no morphologic abnormalities in the myenteric plexus of the stomach or in the abdominal vagus nerves[8]. The interstitial cells of Cajal (ICC), which play a central role in gastrointestinal motility, are located in the greater curvature at the junction between the proximal and distal stomach, and serve as the gastric pacemaker and mediate inputs from enteric motor nerves to the smooth muscle. Recent research has demonstrated that loss or damage of ICC networks is closely associated with gastric motor dysfunction[9-11]. Considerable attention has been paid to the role of gastrointestinal hormones in DGP[12-15], but these studies reached different conclusions.
Endoplasmic reticulum (ER) stress-induced apoptosis has been implicated in the development of multiple diseases. Increasing evidence has demonstrated that ER stress and/or ER stress-induced apoptosis have an important role in the pathogenesis of diabetes mellitus and its complications including diabetic cardiomyopathy, atherogenesis and retinopathy[16-18]. The ER is an important organelle that is required for cell survival, normal cellular function and is highly sensitive to alterations in its homeostasis. Disruption of homeostasis leads to the accumulation of unfolded proteins which are toxic to cells, and by disturbing cellular function, results in a state known as ER stress. In resting cells, all ER stress receptors are maintained in an inactive state through their association with the ER chaperone, glucose-regulated protein (GRP) 78. GRP78 is an ER lumen protein whose expression is induced during ER stress and triggers the ER stress response, cumulatively called the unfolded protein response (UPR) which protects cells against environmental stressors. However, if the influence of ER stress becomes serious, the UPR is unable to restore normal cellular function and signaling switches from pro-survival to pro-apoptotic, procaspase-12 is released and the apoptotic response is initiated. The released procaspase-12 is subsequently cleaved to its active caspase-12 form which has been proposed as a key mediator in the initiation of ER stress-induced apoptosis[19,20].
To date, no studies have demonstrated the role of ER stress or ER stress-induced apoptosis in the etiology of DGP. In the present study, we established a rat model of DGP. We focused on the ER changes in gastric smooth muscle cells (SMCs) in rats with DGP and investigated the apoptosis of SMCs. Furthermore, in order to explore the possible role of ER stress-induced apoptosis in the development of DGP, the expression of GRP78 and caspase-12 in gastric SMCs was also examined.
Healthy Wistar rats weighing 250-300 g were used in this study. The breeders were originally obtained from Luzhou Medical College Laboratories. All animal experiments were conducted according to the guidelines of the Local Animal Use and Care Committee of Luzhou and executed according to the National Animal Welfare Law of China. The animals were housed individually in cages and allowed to acclimate to the animal facilities 1 wk prior to experimentation.
All animals were starved for 12 h before experimentation, but were allowed free access to water. Forty rats were randomly allocated to two groups: a control group (n = 20) and a diabetic group (n = 20). Diabetes was induced by intraperitoneal injection of 60 mg/kg of streptozotocin (STZ; Sigma-Aldrich, MO, United States) dissolved in sodium citrate buffer. The control group received citrate buffer only (0.1 mol/L; 2 mL/kg). Blood glucose levels were measured 72 h after injection of STZ or citrate buffer. Animals were starved, but had access to drinking water for 6 h before blood glucose measurement. Plasma glucose concentrations ≥ 16.9 mmol/L were considered diabetic in these experiments.
Fasting blood glucose concentration in each rat was measured on the 3rd d and at the 4th, 8th and 12th wk, respectively, after injection of STZ or citrate buffer. The weight of each rat was measured at the first, 4th, 8th and 12th wk.
Gastric emptying was assessed after overnight fasting, but with free access to water. Methylcellulose at a concentration of 1.5% was dispersed in water at 80 °C under continuous stirring. The solution was allowed to cool to 37 °C, and then methylene blue, which was used as a nonabsorbable marker, was added to a final concentration of 1 mg/mL. A volume of 0.4 mL of methylene blue solution was given orally into the stomach through a feeding tube. Rats were returned to their cages without food or water and killed 30 min later. The stomach was clamped at the pylorus and the gastroesophageal junction and removed. It was then cut open and the gastric contents were rinsed in 4 mL saline solution. The rinsing solution was collected and centrifuged at 3500 rpm for 15 min. The supernatant was determined at a wavelength of 640 nm using a spectrophotometer. Gastric retention was calculated based on the amount of residual methylene blue using the following formula: gastric residual methylene blue = OD value of the determined tube/OD value of the standard tube × 100%.
Samples were processed for histological and immunohistochemical examinations at the 12th wk after injection of STZ or citrate buffer. A portion of the gastric biopsy was fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemistry and TdT-mediated dUTP nick end labeling (TUNEL) assay, respectively. Sections of the gastric smooth muscle were also fixed in 2.5% glutaraldehyde in Sorensen’s phosphate buffer, stored at 4 °C and subsequently processed for routine transmission electron microscopy (TEM).
Apoptotic cells were detected by TUNEL assay (Roche Diagnostics, Bromma, Sweden). Tissue sections from gastric biopsy were dewaxed and rehydrated, then incubated for 30 min at 37 °C with a proteinase working solution. The slides were placed in a plastic jar containing 200 mL of 0.1 mol/L citrate buffer (pH 6.0) and 350 W microwave irradiation was applied for 5 min. The slides were rinsed twice with PBS and incubated with a solution composed of the enzyme terminal deoxynucleotidyl transferase and nucleotide mixture (label solution) in a humidified box in the dark for 60 min at 37 °C. For the negative control, only 50 μL label solution was added. After incubation, the slides were rinsed 3 times with PBS. Then 50 μL converter-POD was added to the slides. Diaminobenzidine was used as the substrate for peroxidase, which yielded the characteristic brown color for nuclei. The sections were then washed, counterstained with hematoxylin, dehydrated, and sealed. Negative controls were included for each test. All cell counts were performed using a × 40 magnification objective lens. The apoptotic rate was determined as the average percentage of positive cells in ten different fields which were randomly chosen.
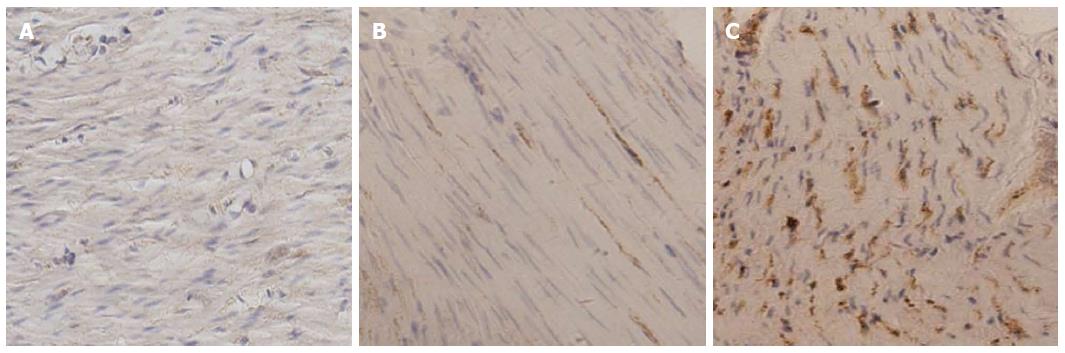
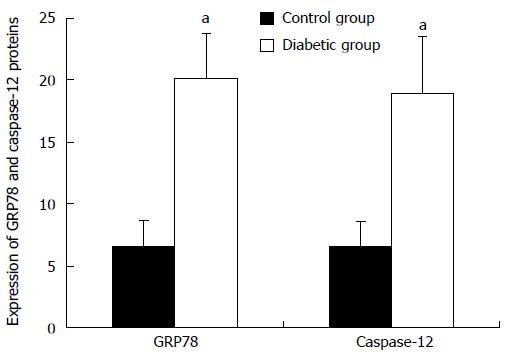
Immunohistochemistry (IHC) was used to determine the expression of GRP78 and caspase-12 proteins. Five-micrometer-thick paraffin-embedded sections were dewaxed in xylene, followed by a graded series of ethanol. Antigen retrieval was performed in Tris-EDTA (pH = 6.0) buffer using a microwave for 12 min. The tissues were washed with PBS (pH = 7.4) for 10 min and 0.3%H2O2 for 30 min in the dark. Washing with distilled water was followed by a blocking step using a blocking solution for 30 min. The sections were then incubated with a 1:100 dilution of rabbit anti-rat GRP78 or caspase-12 antibody (Bioworld Technology Inc., United States) overnight at 4 °C. Then, PBS washing (3 times) was followed by successive incubation with a biotinylated secondary antibody (Biosynthesis Biotechnology CO., China) for 30 min and a horseradish peroxidase-avidin complex for 30 min. After washing 3 times with PBS, the tissue was visualized by reacting in a solution containing diaminobenzidine. The sections were then washed, counterstained with hematoxylin, dehydrated, and sealed. PBS replaced the primary antibodies for the negative control in the IHC procedures. The slides were examined under a light microscope (Olympus, Tokyo, Japan) and ten different images of each sample were randomly captured using a color video camera. The integrated optical density (IOD) value of each image was measured using IPP6.0 software. Semiquantitative results of GRP78 and caspase-12 proteins were calculated by the average of IOD values.
The data are presented as mean ± SD. The Student’s t-test was used to compare the results between the two groups. A P value less than 0.05 was considered statistically significant. The tests were performed using the statistical software package SPSS.
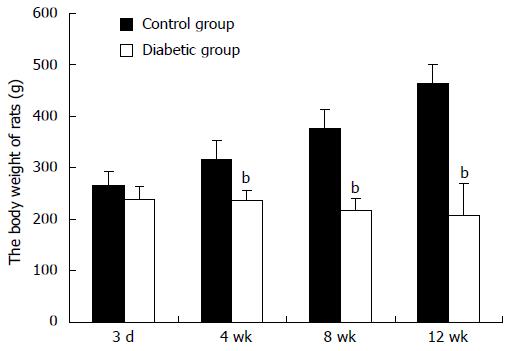
Five rats were excluded from the study due to death resulting from complications of diabetes. Fasting blood glucose concentration consistently reached ≥ 16.9 mmol/L 3 d after injection of STZ. Diabetic rats were severely hyperglycemic, whereas the age/sex matched control rats were normoglycemic (P < 0.01). Body weight was not different between the diabetes group and control group during the first week. After 4 wk, body weight in diabetic rats significantly decreased compared with rats in the control group, and this reduction continued to the end of the experiment (Figure 1).
We determined gastric emptying at the 4th wk, however, no significant difference was noted between the two groups (39.51% ± 1.90%, n = 20 control vs 40.71% ± 1.74%, n = 15 diabetes; P > 0.05). Gastric emptying was significantly lower in the diabetic rats than in the control rats at the 12th week. The residue of gastric pigment was enhanced in diabetic animals compared with controls (40.71% ± 2.50%, n = 20 control vs 54.65% ± 5.22%, n = 15 diabetes; P < 0.05). These alterations in gastric emptying marked the presence of gastroparesis in diabetic rats.
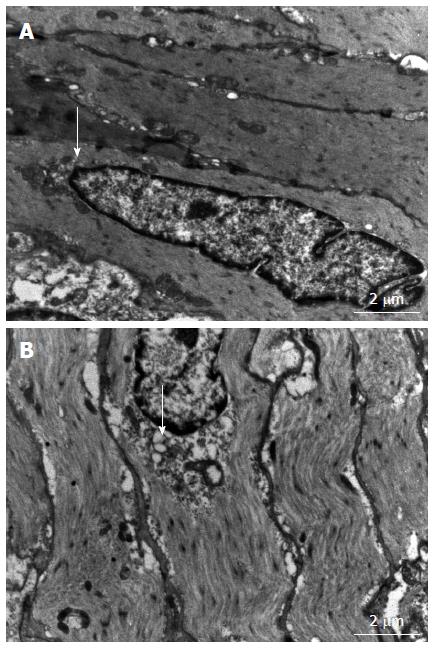
Ultrastructural changes, especially in the ER of gastric SMCs, were investigated using TEM. As shown in Figure 2, gastric SMCs in diabetic rats exhibited swollen and distended ER with an irregular shape. Other ultrastructural abnormalities included swollen, degenerated mitochondria with a loss of cristae. No abnormalities were observed in the control rats.
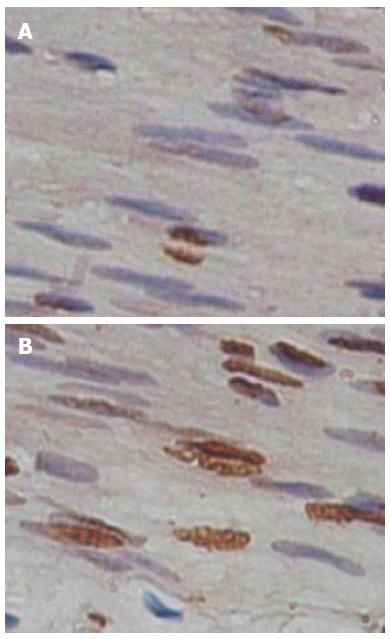
Excess apoptosis is the underlying cause of cell loss in diabetes mellitus. However, whether apoptosis of gastric SMCs increases in diabetic animals is unclear. In this study, apoptosis of gastric SMCs was examined by TUNEL assay (Figure 3). Compared with control animals, the apoptotic rate of gastric SMCs in diabetic rats was significantly higher (13.42% ± 2.74%, n = 15 diabetes vs 2.21% ± 0.63%, n = 20 control; P < 0.05).
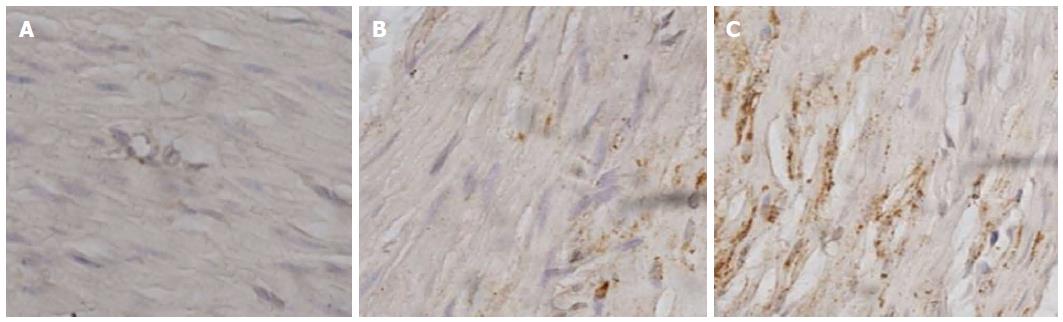
The role of ER stress-mediated apoptosis in the pathophysiology of diabetes and its complications has been demonstrated recently. However, whether ER stress is involved in the apoptosis of gastric SMCs in DGP has not previously been investigated. GRP78 is a key marker of ER stress, and caspase-12, which is located at the ER, is activated by excess ER stress and results in cell death in the absence of the cytochrome c-dependent pathway[21,22]. In the present study, immunohistochemistry was performed to detect the expression and distribution of GRP78 and caspase-12 proteins in gastric SMCs. Immunohistochemistry showed that the positive signal of GRP78 and caspase-12 protein was intense in the cytoplasm (Figures 4 and 5). It was noted that GRP78 protein expression in diabetic rats was significantly higher than that in control rats, indicating that the ER stress response was observed in diabetic gastric SMCs (Figure 6). Caspase-12 protein expression was also increased in diabetic rats compared with controls (Figure 6), suggesting that apoptosis of SMCs in diabetic rats is at least partly induced by ER stress.
Delayed gastric emptying can be due to muscular, neural, or humoral abnormalities[23,24]. Damage to the vagus nerve and humoral abnormalities dominate the causes of DGP[25]. Little attention has been paid to the muscular effect in the development of delayed gastric emptying. The stomach is a hollow organ composed primarily of muscle. Food emptied from the stomach is the result of a combination of relaxation of the muscle and the pressure generated by the muscle that pushes the food into the small intestine. Abnormal changes in smooth muscle have previously been investigated in DGP. Studies on DGP have shown that gastroparesis is associated with abnormal contractility alterations, impaired noncholinergic relaxation and spontaneous rhythmic motility in gastric smooth muscle[6,26]. Histological examination of gastric biopsies obtained from patients with DGP revealed degeneration and increased fibrosis in the muscle layers[27,28]. Another study showed that the ultrastructural changes in gastric smooth muscle of diabetic patients included lipofuscin and thickened basal lamina[29]. In the present study, we primarily focused on gastric smooth muscle in rats with DGP, and the ultrastructural changes in gastric SMCs were investigated by TEM. The results showed swollen, distended ER and mitochondrial lesions in gastric SMCs of diabetic rats. These results further demonstrate injury of gastric SMCs in diabetic rats.
A body of evidence is emerging to show that apoptotic cell death occurs in multiple target organs in diabetes, and thus leads to organ dysfunction. Microvascular damage is considered to be related to complications of diabetes, and vascular endothelial cell apoptosis has been the focus of research in deciphering molecular mechanisms of microangiopathy[30]. Diabetes increases the number of apoptotic myocardial cells, which have an important role in diabetic cardiomyopathy onset and progression[31]. Apoptosis participates in the loss of renal glomerular parenchymal cells and the course of diabetic nephropathy[32]. Hyperglycemia also induces apoptotic changes in dorsal root ganglion neurons, Schwann cells, and peripheral neuronal apoptosis is thought to be involved in diabetic retinopathy[33,34]. In the present report, we attempted to investigate apoptosis of gastric SMCs in a diabetic rat model of gastroparesis. This has not previously been reported. The results obtained from this study demonstrate that increased apoptosis occurs in diabetic gastric SMCs. Increased apoptosis of gastric SMCs can reduce the number of gastric SMCs, and this smooth muscle cell loss suggestive of gastric muscle injury is consistent with the diagnosis of gastroparesis with documented delayed gastric emptying and most importantly, indicates that gastric SMC apoptosis may be involved in the pathogenesis of DGP.
Apoptotic cell death is usually a response to the cell microenvironment. Glucose is one of the microenvironmental factors which may induce apoptosis. A previous study showed that apoptosis observed in diabetic rats was not caused by STZ per se and further demonstrated that apoptosis is directly related to high levels of glucose[35]. Theoretically, hyperglycemia causes abnormalities in calcium homeostasis. Furthermore, it promotes excessive production and release of reactive oxygen species, which induce oxidative stress and lead to abnormal gene expression, faulty signal transduction and ER stress. It is well established that prolonged ER stress can lead to cell apoptosis. Accumulating evidence suggests that ER stress and/or ER stress-induced apoptosis play a role in the pathogenesis of diabetes and its complications[18,36]. Moreover, abnormal morphological changes including dilated and swollen ER were observed in gastric SMCs of diabetic rats, which suggest that ER stress is induced in diabetic rats. This structural injury may be comes along with functional changes. In the present research, we further explored whether ER stress associated with apoptosis in gastric SMCs of diabetic rats.
The ER provides a unique oxidizing compartment for the folding of membrane and secretory proteins. It is extremely sensitive to a variety of different stimuli, and signals are transduced from the ER to the cytoplasm and the nucleus, eventually resulting in adaptation for survival or induction of apoptosis. In response, “unfolded protein response” (UPR) genes are induced, increasing the capacity to fold proteins[18]. The UPR is regulated by three ER transmembrane receptors which mediate signal transduction: inositol requiring ER-to-nucleus signal kinase 1, activating transcription factor 6, and double-stranded RNA-activated kinase (PKR)-like ER kinase (PERK). On accumulation of unfolded proteins, ER resident chaperones, such as GRP78, are up-regulated. GRP78 dissociates from the three receptors, which leads to their activation and triggers the UPR. A previous study established that induction of GRP78 is a marker of ER stress[21]. The present study showed, for the first time, that expression of GRP78 protein was increased in gastric SMCs in diabetic rats, further suggesting that ER stress was induced in gastric SMCs of diabetic animals. However, if stress continues and restoring response fails, the apoptotic response is consequently triggered. Most proapoptotic signals ultimately lead to caspase activation. Of these, caspase-12 is believed to play a central role in the initiation of ER stress-induced cell death in the mouse model, and is essential for the mitochondrial death pathway to take place[37,38]. Nakagawa et al[20] proposed that caspase-12 mediates an ER-specific apoptosis pathway. Research also demonstrated that mice deficient in caspase-12 were resistant to ER stress-induced apoptosis. The result of the present study showed, for the first time, that the expression of caspase-12 protein is increased in diabetic gastric SMCs, which is consistent with the increased number of apoptotic cells, thus suggesting that ER stress-induced apoptosis occurs in gastric SMCs of diabetic rats with gastroparesis.
In conclusion, this study provides a new insight into the mechanisms required for the development of DGP. The gastric muscle injury caused by ER stress in rats with DGP was explored in the present study. Although the precise mechanism of action of gastric SMCs has not yet been elucidated, the results presented here demonstrate that apoptosis of gastric SMCs was increased in rats with DGP. These results further support the hypothesis that ER stress was enhanced in gastric SMCs of diabetic rats and apoptosis of gastric SMCs in diabetic rats was, at least partly, induced by ER stress, which suggests that ER stress and/or ER stress-induced apoptosis are likely to participate in the development of DGP. However, the signal transduction of ER stress was not explored in this research. It would be interesting to determine what induces ER stress in diabetic gastric SMCs, thus further studies are needed to better characterize the role of ER stress and ER stress-induced apoptosis in the development of DGP.
Gastroparesis is a well-established complication of diabetes mellitus and is characterized by delayed gastric emptying without mechanical obstruction of the stomach. Despite many years of intensive research, the pathophysiology of diabetic gastroparesis (DGP) remains to be elucidated. Previous studies have demonstrated that endoplasmic reticulum (ER) stress and/or ER stress-induced apoptosis have an important role in the pathogenesis of diabetes mellitus and its complications. The possible role of ER stress and/or ER stress-induced apoptosis in the etiology of DGP remains elusive. This study highlighted the muscle injury caused by ER stress in rats with DGP.
A body of evidence is emerging to show that apoptotic cell death occurs in multiple target organs in diabetes and thus leads to corresponding organ dysfunction. It is well established that prolonged ER stress can lead to cell apoptosis. ER stress-induced apoptosis has been implicated in the development of multiple diseases. An increasing number of studies have demonstrated that ER stress and/or ER stress-induced apoptosis have an important role in the pathogenesis of diabetes mellitus and its complications.
This is the first study to investigate the apoptosis of gastric smooth muscle cells (SMCs) in a diabetic rat model of gastroparesis. The results demonstrate that apoptotic cell death was increased in diabetic gastric SMCs, which is consistent with the increased expression of the ER stress marker, GRP78, and the ER-specific apoptosis mediator, caspase-12.
The results of this study suggest that the ER stress response and ER stress mediated-apoptosis are activated in gastric smooth muscle injury in diabetic rats with gastroparesis, which may provide further information for understanding muscle injury in diabetic gastroparesis.
Gastroparesis, also known as delayed gastric emptying, is a medical condition consisting of paresis (partial paralysis) of the stomach, resulting in food remaining in the stomach for a longer time than normal. It often occurs in patients with type 1 diabetes or type 2 diabetes.
Novelty can be shown in this paper. Overall, the research has been done scientifically.
P- Reviewers: Jiang N, Yang PC S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang DN
| 1. | Waseem S, Moshiree B, Draganov PV. Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15:25-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 2. | Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, Shearman DJ. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 273] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Abid S, Rizvi A, Jahan F, Rabbani F, Islam N, Khan MH, Masood R, Jafri W. Poor glycaemic control is the major factor associated with increased frequency of gastrointestinal symptoms in patients with diabetes mellitus. J Pak Med Assoc. 2007;57:345-349. [PubMed] |
| 5. | Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Jenkinson KM, Reid JJ. Effect of diabetes on relaxations to non-adrenergic, non-cholinergic nerve stimulation in longitudinal muscle of the rat gastric fundus. Br J Pharmacol. 1995;116:1551-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Tougas G, Hunt RH, Fitzpatrick D, Upton AR. Evidence of impaired afferent vagal function in patients with diabetes gastroparesis. Pacing Clin Electrophysiol. 1992;15:1597-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Yoshida MM, Schuffler MD, Sumi SM. There are no morphologic abnormalities of the gastric wall or abdominal vagus in patients with diabetic gastroparesis. Gastroenterology. 1988;94:907-914. [PubMed] |
| 9. | Wang XY, Huizinga JD, Diamond J, Liu LW. Loss of intramuscular and submuscular interstitial cells of Cajal and associated enteric nerves is related to decreased gastric emptying in streptozotocin-induced diabetes. Neurogastroenterol Motil. 2009;21:1095-1e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 248] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, Hishida A. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | McCallum RW, Cynshi O. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis - a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. 2007;26:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Russo A, Stevens JE, Giles N, Krause G, O’Donovan DG, Horowitz M, Jones KL. Effect of the motilin agonist KC 11458 on gastric emptying in diabetic gastroparesis. Aliment Pharmacol Ther. 2004;20:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | James AN, Ryan JP, Crowell MD, Parkman HP. Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G612-G619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Qiu WC, Wang ZG, Wang WG, Yan J, Zheng Q. Gastric motor effects of ghrelin and growth hormone releasing peptide 6 in diabetic mice with gastroparesis. World J Gastroenterol. 2008;14:1419-1424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Luoma PV. Elimination of endoplasmic reticulum stress and cardiovascular, type 2 diabetic, and other metabolic diseases. Ann Med. 2013;45:194-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Oshitari T, Hata N, Yamamoto S. Endoplasmic reticulum stress and diabetic retinopathy. Vasc Health Risk Manag. 2008;4:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 892] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 19. | Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935-13940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 647] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 20. | Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2547] [Cited by in RCA: 2611] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 21. | Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 800] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 22. | Momoi T. Caspases involved in ER stress-mediated cell death. J Chem Neuroanat. 2004;28:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Hasler WL. Gastroparesis: pathogenesis, diagnosis and management. Nat Rev Gastroenterol Hepatol. 2011;8:438-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Chen CY, Fujimiya M, Laviano A, Chang FY, Lin HC, Lee SD. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. J Chin Med Assoc. 2010;73:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Rodrigues ML, Motta ME. Mechanisms and factors associated with gastrointestinal symptoms in patients with diabetes mellitus. J Pediatr (Rio J). 2012;88:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Soulié ML, Cros G, Serrano JJ, Bali JP. Impairment of contractile response to carbachol and muscarinic receptor coupling in gastric antral smooth muscle cells isolated from diabetic streptozotocin-treated rats and db/db mice. Mol Cell Biochem. 1992;109:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Pasricha PJ, Pehlivanov ND, Gomez G, Vittal H, Lurken MS, Farrugia G. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Ejskjaer NT, Bradley JL, Buxton-Thomas MS, Edmonds ME, Howard ER, Purewal T, Thomas PK, Watkins PJ. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Faussone-Pellegrini MS, Grover M, Pasricha PJ, Bernard CE, Lurken MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL. Ultrastructural differences between diabetic and idiopathic gastroparesis. J Cell Mol Med. 2012;16:1573-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Wu QD, Wang JH, Fennessy F, Redmond HP, Bouchier-Hayes D. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. Am J Physiol. 1999;277:C1229-C1238. [PubMed] |
| 31. | Falcão-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev. 2012;17:325-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 32. | Singh DK, Winocour P, Farrington K. Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008;4:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 312] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Asnaghi V, Gerhardinger C, Hoehn T, Adeboje A, Lorenzi M. A role for the polyol pathway in the early neuroretinal apoptosis and glial changes induced by diabetes in the rat. Diabetes. 2003;52:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 509] [Article Influence: 22.1] [Reference Citation Analysis (36)] |
| 36. | Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 643] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 37. | Di Sano F, Ferraro E, Tufi R, Achsel T, Piacentini M, Cecconi F. Endoplasmic reticulum stress induces apoptosis by an apoptosome-dependent but caspase 12-independent mechanism. J Biol Chem. 2006;281:2693-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 379] [Article Influence: 18.0] [Reference Citation Analysis (0)] |