INTRODUCTION
Hepatitis B virus (HBV) infection is a major public health problem in most countries, with approximately 2 billion people worldwide showing exposure to the virus, nearly 300 million people carrying an HBV chronic infection and with over 1 million deaths per year from HBV-related diseases such as cirrhosis and liver cancer[1-5]. HBV is an enveloped Hepadnavirus with an incomplete double-stranded DNA genome of 3.2 kb[6]. Eight genotypes have been identified so far, with a distinct geographical distribution.
The prevalence of chronic HBV infection is about 5% worldwide, but it differs between regions, 0.1%-2.0% in the United States and western Europe, 2.0%-8.0% in Mediterranean countries and Japan, and 8.0%-20.0% in Southeast Asia and Sub-Saharan regions[1,7].
Risk factors for HBV infection include transfusion of unscreened blood, sexual promiscuity, sharing or re-using syringes among injection drug users, tattooing, working or residing in a health-care setting, living in a correctional facility, renal dialysis, and long-term household or intimate non-sexual contact with an hepatitis B surface antigen (HBsAg)-positive individual.
The majority of HBsAg-positive individuals acquire HBV infection at birth or in the first decade of life in highly endemic areas, whereas in countries with a low endemicity level HBV transmission mostly occurs in adulthood due to unprotected sexual contact or sharing syringes with HBsAg carriers[1,7-9]. HBV genotypes have a distinct geographical distribution: genotype A is prevalent in northwestern Europe and the United States, genotypes B and C in Asia, genotype D in the Mediterranean basin, Middle East, and India, genotype E in west Africa, genotype F in South and Central America, genotype G in the United States and France and genotype H in Mexico and South America[10].
In Italy, HBV genotype D was responsible for nearly 95% of the cases of acute and chronic hepatitis for decades[11]. In the last two decades, however, Italy has became a land of immigration, mainly from Africa, Eastern Europe and Eastern Asia and HBV genotypes other than D have been introduced and found to be responsible for nearly 40% of cases of acute hepatitis[12,13].
The epidemiology of HBV has largely changed in Italy over the last 50 years, with a substantial, progressive reduction in the endemicity levels. The main reasons for this change are due to the improvement in socio-economic conditions associated with a better standard of hygiene, a reduction in family size, the educational and media campaigns against human immunodeficiency virus (HIV) infection and, finally, the mass vaccination campaign against HBV started in 1991 for all newborn babies and all 12-year-old children[14].
Hepatitis delta virus (HDV), first discovered in Italy some 30 years ago, is a small defective RNA virus which requires the helper function of HBV for its replication and transmission to anti-HDV-negative individuals[15]. HDV infection can be transmitted either simultaneously with HBV infection (co-infection) or to people who are already chronic HBV carriers (super-infection)[16]. It has been estimated that 15-20 million people worldwide have a chronic HDV infection, with substantial geographical differences[17,18]. The highest prevalences are found in the Mediterranean basin, the Middle East, central and northern Asia, western and central Africa, the Amazon basin (Brazil, Peru, Venezuela, and Colombia), the Pacific islands[19] and Vietnam[20]. HDV infection can be acquired at birth from positive mothers, or sexually transmitted or through a parenteral route[21].
As a consequence of the decrease in the HBV endemicity, the spread of HDV infection has dramatically decreased in Italy in the last 15 years[22,23]. However, due to the high rate of progression to chronicity of HDV infection acquired as a superinfection, in Italy about 7% of patients with HBsAg-positive chronic hepatitis are still coinfected with HDV.
Changes in HBV and HDV epidemiology, documented in Italy by several seroepidemiological surveys both in cohorts of hospitalized patients and in the general population by independent groups of investigators, by the national surveillance program for acute viral hepatitis of the National Institute of Health (Sistema Epidemiologico Integrato Epatite Virale Acuta-SEIEVA) and by the annual reports of the National Institute of Statistics (ISTAT), are extensively analyzed in this review article. The SEIEVA national surveillance program, coordinated by the Istituto Superiore di Sanità, was set up in 1985[24]; nearly 60% of cases of acute viral hepatitis occurring in Italy are notified to this surveillance system through the local public health units[25]. ISTAT, the National Institute of Statistics for Italy, analyzes for the Italian Government all data registered in Italy, including all diseases, and publishes an official report every year.
ACUTE HEPATITIS B
The clinical presentation of acute hepatitis B varies according to the age at acquisition: in newborns and young children it is usually asymptomatic and progresses to chronicity in most cases; in the adult population it is usually symptomatic, but is self-limiting with HBsAg seroclearance in most cases and with a fulminant course in less than 1%. Fulminant hepatitis is characterized by a mortality rate around 70% and requires orthotopic liver transplantation in most cases[1,7,26].
Before the surveillance SEIEVA program on acute viral hepatitis started in 1985, the only available epidemiological data consisted of the yearly incidence of acute viral hepatitis (AVH) of different etiologies registered all together by ISTAT under one single entry. The introduction of serum aminotransferase determination for the diagnosis of acute hepatitis in the 1960s was followed by a substantial increase in the number of cases notified of acute hepatitis of different etiologies, which rapidly reached an incidence of 98 per 100000 inhabitants per year. A slow decline in this rate was observed starting from 1970, but a marked reduction in this incidence was recorded from 1985 to 1987, from 40/100000 to 20/100000 inhabitants[14].
The first attempt to define AVH by etiology was made by Giusti et al[27] in Naples in 1980, who found a 60% prevalence of acute HBV among hospitalized patients with acute hepatitis of different etiologies. This prevalence was 45% in Genoa between 1974 and 1979[28].
Data on the incidence of acute hepatitis B in the general population are available only from 1985, after the start of the SEIEVA program of the Italian National Institute of Health.
Before showing and interpreting the changes in the incidence of acute HBV and the changing impact of different risk factors for its acquisition, we should comment on two important events occurring in Italy between 1985 and 1991 that affected the spread of HBV infection: the educational campaign against HIV infection and the introduction of the national program of mass vaccination against HBV infection. The national educational campaign against HIV infection, which started in 1985 in Italy and strongly advised the use of condoms for unsafe sexual intercourse and the avoidance of sharing syringes for intravenous drug use (IDU), certainly contributed to the reduction in the spread of HBV[29]. The selective HBV vaccination program that started in 1983 on a regional basis and targeted people at increased risk of infection because of their lifestyle (i.e., intravenous drug users, male homosexuals, individuals with multiple sexual partners, healthcare workers, household contacts of HBsAg carriers and newborn babies born to HBsAg-carrier mothers)[16] had little or no success in reducing HBV endemicity in Italy. This induced the Italian government to rule in 1991 on mandatory universal vaccination on a national scale for infants and 12-year-old adolescents (for the latter restricted to the first 12 years of the application of the law). At the same time, HBV vaccination continued to be offered free of charge to people at increased risk of infection[30,31]. The overall coverage rate has always been around 95%[32] and nearly all Italian people under 34 years have now been vaccinated against HBV infection.
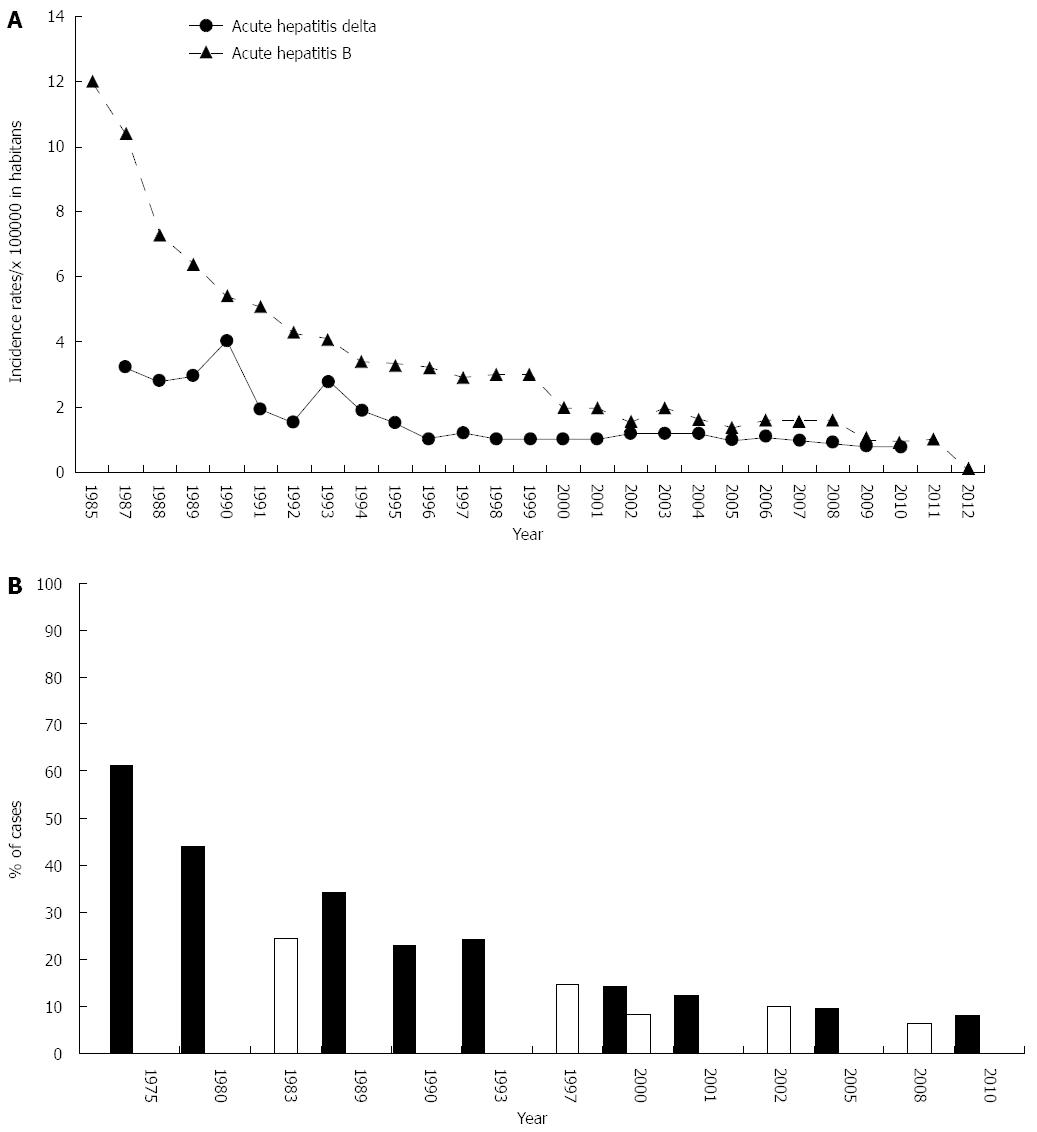
All this contributed to the downward trend in the incidence of acute HBV, particularly in the age class 15-24[33] as demonstrated by the SEIEVA surveillance program. The incidence of acute HBV gradually decreased from 12 cases per 100000 inhabitants in 1985 to 0.9 in 2012. This reduction, however, was already evident before the introduction of the HBV mass vaccination program, which stresses the great impact of the improvement in socio-economic and hygiene conditions. In fact, this incidence decreased from 10.4 cases per 100000 inhabitants in 1987 to 5.4 per 100000 in 1990[34] (Figure 1A). The yearly incidence of acute hepatitis B was also analyzed by age and three age classes, 0-14, 15-24 and 25 or more, were considered. In 1985 the incidence rate was 6 cases per 100000 inhabitants in the age class 0-14, 41 cases in the age class 15-24 and 7 in the aged 25 or more. A steady decline was observed in subsequent years in all age classes: 1, 17 and 4 cases per 100000 inhabitants, respectively, in 1990; 1, 6 and 3 cases, respectively, in 1995; 0.1, 2 and 2 cases, respectively, in the year 2000; 0, 0.5 and 1.8 cases, respectively, in 2005 and 0, 0.5 and 1.2 cases per 100000 inhabitants, respectively, in 2011. In addition, males predominated in all age classes and over time. Thus, the national HBV mass vaccination program, covering at present the Italian population aged 0-33 years, strongly contributed to the impressive reduction in the incidence of acute hepatitis B in Italy in recent years. The decrease was observed both in the north and in the south of the country, i.e., an acute HBV incidence of 1.03/100000 inhabitants in northern and central Italy and 0.23/100000 in the south of the country and the islands of Sardinia and Sicily, with a moderately higher incidence still evident in males[35].
Figure 1 Epidemiological data on hepatitis B virus and hepatitis delta virus infection in Italy from 1975 to 2012.
A: Incidence of acute hepatitis B and acute hepatitis delta; B: Prevalence of chronic hepatitis B among patients with chronic hepatitis of different etiologies (black); Prevalence of chronic hepatitis delta among hepatitis B surface antigen chronic carriers (white).
Before HBsAg screening during pregnancy or at the time of delivery and active/passive immunoprophylaxis of newborn babies of HBsAg-positive mothers became mandatory, vertical transmission of HBV was one of the main routes of HBV transmission in Italy. Sexual transmission of HBV infection also had an important role, due to the infrequent use of condoms in unsafe sexual intercourse[33-36]. Parenteral transmission of the virus was also frequent in household contact with an HBsAg chronic carrier, in the use of improperly sterilized medical and surgical instruments, blood transfusions, IDU, and for men shaving at a barber’s shop.
After 1991, the epidemiological impact of risk factors showed a tendency to change in Italy, and the most important risk factors in the 1990s were IDU, cosmetic treatment with percutaneous exposure (piercing, tattooing, manicure, pedicure, and acupuncture) dental therapy and promiscuous sexual activity[37-40].
By 2012 the percentage of persons with acute HBV reporting IDU had decreased dramatically, a beneficial effect of the HBV mass vaccination which to date covers nearly all the Italian population aged 0-33 years, and a lesser decrease was observed for household contact with an HBsAg carrier[32-38].
Besides these beneficial effects, HBV mass vaccination of the younger population has generated new clinical and virological problems. Acute HBV occurs almost exclusively in non-vaccinated subjects aged over 33 years with a high probability of severe clinical presentation and an increased risk of fulminant hepatitis, clinical conditions frequently requiring early treatment with a nucleos(t)side analogue[32]. In addition, recent studies have shown antiviral resistance mutations or viral mutants in the antigenic “a” determinant of HBsAg in patients with acute hepatitis B[13,41,42], the clinical impact of which requires further investigation.
ACUTE HEPATITIS DELTA
Acute hepatitis delta may be due to an HDV infection in HBsAg chronic carriers (superinfection) or to a simultaneous HBV/HDV infection (coinfection). Nearly 95% of patients with acute HBV/HDV co-infection show a self-limiting course of the illness, but there is a high risk of a fulminant course (3%-4% of cases), whereas in most HBsAg chronic carriers HDV superinfection becomes chronic[43-45].
Due to the biological link of HDV to HBV, characterized by the necessary helper function of HBV for HDV replication, the epidemiology of HDV infection closely mimics that of HBV infection and, consequently, the changes over time in the HBV epidemiology are mirrored by concurrent variations in the HDV epidemiology. Two sera collections from patients with acute hepatitis B obtained in Naples in 1972 and 1974 were retrospectively evaluated, and for the first time the IgM to anti-HDV was discovered and detected in more than 90% of cases. Most of the anti-HDV IgM-positive patients were also hepatitis B c Antigen (HBcAg) IgM-positive, suggesting an epidemic of HBV/HDV coinfection in Naples in those years[46].
The epidemiological data on acute HDV collected from 1974 to 1986 are scanty, since the SEIEVA project evaluated the yearly incidence of acute HDV infection only from 1987. The incidence of primary HDV infection in the general population progressively declined from 3.2/100000 inhabitants in 1987 to 0.2 in 2010. In this period, however, three peaks of HDV epidemics were registered, in 1990 and 1993 in the age classes 14-25 and 25 or more and in 1997 only in the age class 15-24, due to the spread of HDV infection among intravenous drug users[36] (Figure 1A). IDU was recognized as the main risk factor in these periods, but also household contact with an HBsAg chronic carrier was considered to have contributed. Apart from these three peaks, the incidence of acute HDV infection declined over time from 1987 to 2010, paralleling the trend observed for acute HBV infection[47].
In 1992-1994, IDU, household contact with an HBsAg chronic carrier, promiscuous sexual activity, unsafe sexual intercourse, cosmetic treatment with percutaneous exposure and dental therapy were considered the risk factors responsible for the high epidemiological impact, whereas in 2008-2010, the risk factors most frequently reported by the patients were dental therapy, cosmetic treatment with percutaneous exposure and promiscuous sexual activity. The marked decrease between the periods 1992-1994 and 2008-2010 in the percentage of patients with acute HDV infection reporting IDU, household contact with an HBsAg carrier and unsafe sexual practices was most probably an effect of the mass vaccination which had covered all Italian citizens aged 0 to 31 years by 2010[48].
TRENDS IN HBV AND HDV RISK FACTORS IN ITALY
The epidemiological impact of the risk factors for the acquisition of HBV infection has changed substantially over the last 5 decades. During the seventies and eighties, perinatal transmission from infected mothers to their newborn babies, household contact with an HBV chronic carrier, the use of improperly sterilized medical and surgical instruments, blood transfusions and IDU were identified as the main risk factors[33,36-40]. The general improvement in hygiene standards and living conditions, the reduction in the mean size of families, the use of disposable syringes instead of non-disposable glass syringes, screening of pregnant women to detect circulating HBsAg and the prophylaxis procedures for their newborn babies, universal vaccination against HBV infection introduced in 1991 and the national media campaigns against HIV infection are considered the main reasons for such an impressive reduction. In the nineties, the most important risk factors were IDU (stated by 24.9% of patients with acute hepatitis B), cosmetic treatment with percutaneous exposure (i.e., piercing, tattooing, manicure/pedicure, and shaving at a barber’s shop, as well as acupuncture, stated by 29.9% of patients), dental therapy (27.9%) and promiscuous sexual activity (27.1%). More recently, from the year 2000 to the present, cosmetic treatment with percutaneous exposure (stated by 32.4% of patients with acute hepatitis B), dental therapy (stated by 34%) and promiscuous sexual activity (by 27.2%) have been recognized as the risk factors with a high epidemiological impact[36]. Also for the acquisition of HDV infection the epidemiological impact of risk factors has changed substantially over time: the most important risk factors were IDU, promiscuous sexual activity, cosmetic treatment with percutaneous exposure and dental therapy in 1992-1994, whereas in 2008-2010 only cosmetic treatment with percutaneous exposure, promiscuous sexual activity and dental therapy played a prominent role in the spread of HDV[36].
CHRONIC HEPATITIS B
Chronic hepatitis B is a dynamic process that is usually schematically divided into five phases, not necessarily sequential: an “immune tolerant” phase characterized by HBeAg positivity, high levels of HBV replication, normal or low aminotransferase levels, mild or no liver necroinflammation and no or slow progression of liver fibrosis; an “immune reactive phase” still characterized by HBeAg positivity, but with a lower level of HBV replication and increased or fluctuating aminotransferase levels, moderate or severe liver necroinflammation and a more rapid progression of fibrosis; an “inactive HBV carrier state” characterized by very low or undetectable serum HBV DNA levels and low or normal aminotransferase levels and in most cases detectable seroconversion to anti-HBe; a phase called “HBeAg-negative chronic hepatitis B” characterized by a pattern of fluctuating HBV DNA and aminotransferase levels and by histological features of active hepatitis, linked to an e-minus HBV variant with nucleotide substitutions in the pre-core and/or the basal core promoter regions that is unable to express HBeAg or expresses it at low levels[2,49-52]. A possible fifth phase is “occult HBV infection” characterized by the loss of HBsAg and low-level HBV replication detectable in the liver and in some cases in the blood. The clinical impact of “occult HBV infection” is still unclear and needs further investigation[53-55].
At the end of the 1970s, HBV endemicity in Italy was intermediate, with an estimated prevalence of HBsAg chronic carriers in the general population just over 3%[56,57] and with an increasing gradient from the north to the south of the country, where this prevalence reached 5%[58,59]. Enclaves with high levels of HBV endemicity were also described, with prevalences of HBsAg chronic carriers above 10% in Afragola[60], a town near Naples, and in two cities in northern Italy, Bergamo and Brescia[56-61]. At that time there was a large proportion of HBeAg-positive cases among HBsAg chronic carriers, reaching 40% in some studies[62]. IDU and living in a large household with an HBsAg chronic carrier were identified as the major risk factors for developing chronic HBV infection. The most active routes of HBV transmission inside the family, associated with a high rate of progression to chronicity of HBV infection, were the transmission from mothers to newborn babies at the time of delivery and the spread among siblings in large families[63].
New sero-epidemiological investigations were carried out in the late 1980s and important changes were observed. An impressive reduction in the HBsAg-positive prevalences in children and teenagers was documented in several Italian regions between 1987 and 1989. This decline was well documented in the city of Naples, where in a primary school the HBsAg-positive prevalence in children aged 7-12 years decreased from 2.2% observed in 1980 to 0.8% in 1988[64]. This decline was associated with a considerable improvement in socio-economic conditions, a decrease in the family size, and with the introduction of disposable syringes instead of re-usable glass syringes. The decline in HBsAg chronic carriage was confirmed in studies performed in the second half of the 1990s in pregnant women at delivery[65], in young males at their enrolment for mandatory military service and in the general population[66]. At present, nearly 1% of subjects in the open population are HBsAg positive.
Other studies investigated the prevalences of HBsAg-positive cases among patients with chronic hepatitis of different etiologies observed in Italian hospitals as inpatients or outpatients. The HBsAg-positive prevalences were 61% in 1975[67], 44% in 1980, 34% in 1989[68] and 12.2% in 2001[69-71] (Figure 1B).
In the last two decades Italy has become a country of immigration prevalently from African, eastern European and Asiatic countries, geographical areas of high or moderate endemicity levels for HBV infection. At present, nearly five million immigrants live in Italy, most of whom have not received HBV vaccination and 250000 of whom are estimated to be HBsAg positive with an HBeAg-positive prevalence of almost 30%[72]. The continuous increase in the number of immigrants not vaccinated for HBV could impair the favorable results obtained in the Italian population in the last three decades.
CHRONIC HEPATITIS DELTA
Nearly 90% of patients with chronic hepatitis delta acquire HDV by superinfection. Chronic HDV infection is frequently associated with an active chronic hepatitis that leads to cirrhosis in almost 70% of patients in 5-10 years. The incidence of cirrhosis is estimated as three times higher in patients with HBV/HDV chronic coinfection than those with chronic HBV monoinfection, with a higher risk of early decompensation and the development of hepatocellular carcinoma[44,73,74].
Multicenter epidemiological studies carried out in Italy in the 1980s showed a high prevalence of anti-HDV positivity in HBsAg chronic carriers, 24.6% in 1981[75] and 23.4% in 1987[23] (Figure 1B), and the spread of HDV infection in intravenous drug users[16]. In the 1987 study, the prevalence of anti-HDV positivity in HBsAg chronic carriers was higher in southern than in northern Italy (26.6% vs 19.1%) and increased with the increase in severity of liver disease, from 3.8% in asymptomatic carriers to 42.5% in patients with cirrhosis. This prevalence was higher in the south than in the north of the country also in 1992 both in patients with chronic active hepatitis and in those with cirrhosis[23]. The HDV endemicity dramatically declined in the 1990s (Figure 1), with an anti-HDV-positive prevalence of 14% in 1992 and 8.3% in 1997. In those years, the greatest reduction in the percentage of anti-HDV-positive cases was found in HBsAg chronic carriers aged 30 to 50, whereas it remained almost unchanged in subjects over 50, and as occurred in 1987, the highest prevalence of anti-HDV-positive cases was found in patients with cirrhosis[48]. However, the hope that hepatitis delta was a vanishing disease in Italy proved futile, as in 2000-2001 the prevalence of anti-HDV positivity was 9.7%[22], a prevalence confirmed in a subsequent study[18] (Figure 1).
Although reduced to a low level of endemicity in the Italian population[28], a new increase in HDV infection cannot be excluded, since Italy has become a country of immigration prevalently from sub-Saharan Africa and Eastern Europe, where several countries have a high level of HDV endemicity[72]. Studies on HDV epidemiology in immigrants are few and inconclusive, but it has been estimated that nearly 6% of the 5 million immigrants living in Italy, most of whom are not vaccinated against HBV infection, are HBsAg positive. The Italian Healthcare Authorities should carefully consider an urgent HBV mass vaccination campaign for the immigrant population.
CONCLUSION
The epidemiology of HBV and HDV infection has decreased in Italy over the last 50 years. The main contribution to the downward trend in the incidence of acute HBV and acute HDV infections, particularly in the age class 15-24 years, and of their consequent chronic sequelae can be attributed to the improvement in socio-economic and hygiene conditions, the educational and media campaigns against HIV infection and HBV mass vaccination introduced in 1991[14]. As a consequence, acute HBV infection now occurs mostly in non-vaccinated subjects aged over 33, who frequently show a severe clinical course.
The epidemiological impact of risk factors for the acquisition of HBV infection has changed over the last five decades in Italy. Due to the introduction of mass passive/active immunization of newborn babies, vertical transmission is no longer a frequent route of transmission of HBV infection. Also the role of IDU has been more recently impaired by the gradual extension of the HBV vaccination to older subjects. Besides HBV mass vaccination, the impressive reduction in the size of families, particularly in southern Italy and the main islands, has reduced HBV transmission through household contact with an HBsAg chronic carrier. Other risk factors with a dramatic reduction in their epidemiological impact are the use of improperly sterilized medical and surgical instruments, blood transfusions, the use of glass syringes and for men shaving at a barber’s shop. Sexual transmission of HBV infection plays a major role in HBV transmission nowadays, due to the infrequent use of condoms by the Italian population in unsafe sexual activity, whereas a more marginal role is played by cosmetic treatment with percutaneous exposure (piercing, tattooing, manicure, pedicure, and acupuncture) and dental treatment[35].
At present, due to the improved epidemiological and socio-economic conditions, the incidence rate of acute hepatitis B in Italy is less than 1 per 100000 inhabitants, higher in males than in females and in northern-central regions than in the south, most cases being non-vaccinated individuals aged over 33 years with unsafe sexual habits[32]. Also the prevalence of HBV chronic carriers in the general population has undergone a steady decrease and is at present less than 1%.
Although several studies have provided important information on HBV and HDV epidemiology in the last 5 decades in Italy, our knowledge is still limited. The SEIEVA surveillance system, which is one of the most informative surveillance systems in the world, covers about 70% of the Italian territory at present and, therefore, the possibility that some relevant local information may be unaccounted for cannot be excluded. In addition, no study has been carried out on the prevalence of HBV and HDV chronic infections in the general Italian population and the information available is from multicenter studies on inpatients and outpatients observed at clinical centers of the National Healthcare System, the last study dating back to 2001.
Finally, limited information is at present available on the immigrant population permanently living in Italy, who constitute about 8.5% of the Italian population and frequently come from countries with high or intermediate endemicity of HBV and or HDV infection. The importance of screening the immigrant population is also underscored by a recent multicenter study of the Italian Association of Infectious and Tropical Diseases (SIMIT)[72], which enrolled 3760 HBsAg-positive subjects observed at 72 units of infectious diseases in Italy, of whom 24.8% were immigrants. The possibility that the increasing rate of immigration from geographical areas with high levels of endemicity of both HBV and HDV infection will bring about a deterioration in the current epidemiological conditions in the native Italian population should induce the National Healthcare Authorities to extend the national HBV vaccination program to the immigrant population.