Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4830
Revised: February 9, 2014
Accepted: March 5, 2014
Published online: April 28, 2014
Processing time: 178 Days and 21.7 Hours
Arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome (OMIM 208085) is an autosomal recessive disorder that is caused by mutations in 2 interacting genes VPS33B and VIPAS39. Mutations in VPS33B gene account for most cases of ARC. As low or normal gamma-glutamyl transpeptidase (GGT) activity has been described in all patients with ARC syndrome identified so far, ARC syndrome is a possible diagnosis for low GGT cholestasis. Here we describe a Chinese patient with neonatal cholestasis and a high GGT level in three consecutive tests. She had other typical manifestations of ARC syndrome, including arthrogryposis multiplex congenita, renal involvement and ichthyosis. Genetic study of the VPS33B gene further confirmed the diagnosis by identification of compound heterozygosity of two known disease-causing mutations, c.403+2T > A and c.1509-1510insG. The mechanism of high GGT in this patient is unclear. Nevertheless, this case indicates that ARC syndrome cannot be excluded from the differential diagnosis of neonatal cholestasis even if high GGT activity is found.
Core tip: Neonatal cholestasis with low or normal gamma glutamyl transpeptidase (GGT) activity was regarded as a characteristic feature of arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome. Here we describe a patient who presented with neonatal cholestasis and high GGT activities. She had all other typical clinical manifestations of ARC syndrome. The diagnosis was finally confirmed by the presence of compound heterozygosity of two known VPS33B disease-causing mutations. Our case indicates that ARC syndrome cannot be excluded in neonatal cholestasis even with unexpected high GGT activity.
- Citation: Wang JS, Zhao J, Li LT. ARC syndrome with high GGT cholestasis caused by VPS33B mutations. World J Gastroenterol 2014; 20(16): 4830-4834
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4830.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4830
Arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome (OMIM 208085) is an autosomal recessive disorder that typically presents with neonatal cholestasis, renal tubular dysfunction and arthrogryposis multiplex congenita[1]. Mutations in 2 interacting genes VPS33B and VIPAS39 have been identified. Mutations in VPS33B gene account for most cases of ARC[2-5].
As low or normal gamma-glutamyl transpeptidase (GGT) activity has been described in all patients with ARC syndrome identified so far, ARC syndrome is one of the differential diagnosis for low GGT cholestasis[6,7]. Recently we diagnosed a case with ARC syndrome caused by VPS33B mutations, but an unexpectedly high GGT level was noticed.
The proband is a female patient, the second child of a non-consanguineous han couple. Oligohydramnios, ascites and enhanced echo of the kidneys of the fetus were demonstrated by ultrasound in the 7th mo of pregnancy. She was born in good condition with a birth weight of 3400 g by a cesarean section at 39 wk of gestation, due to breech presentation. Her weight dropped to 2900 g by day 8 while on mixed formula and breast feeding. Hearing screening tests performed on day 7 yielded no definitive results.
Jaundice was first noticed on day 3 after birth and resolved spontaneously on day 10. It recurred from day 14 after birth and dark urine and light yellow colored stools were noticed thereafter. The investigations at the local hospital revealed mild cholestasis so the child was transferred to a children’s hospital in Beijing at 21 d of age, when her body weight was 2900 g. She received blood transfusion because of anemia with a hemoglobin level of 71 g/L at 28 d of age, and was then referred to a hepatology centre at age 30 days for investigations of the cause of her cholestasis.
Family history revealed that the mother was healthy and the father had polycystic kidney disease. The mother’s first pregnancy produced a full-term girl weighing 3000 g, delivered by cesarean section for breech presentation and IIº contaminated amniotic fluid. Enhanced echoes of the fetal kidneys were demonstrated by ultrasound at the 4th mo of pregnancy and oligohydramnios and ascites at the 7th mo of pregnancy. The limbs and skin of this elder sibling looked similar to those of the proband. Jaundice persisted from birth and stool color became lighter after 20 d of age. Laboratory tests at 1 mo of age revealed persistent positive glucose and protein in the urine and moderate anemia. Liver function tests were listed in Table 1. Ultrasound of the abdomen revealed polycystic kidneys, but CT scan of the brain was normal. This first baby died at 8 mo from infection, anorexia, jaundice and poor weight gain.
| Age (d) | TBIL | DBIL | ALT | AST | ALP | GGT | TBA | TP | ALB |
| (mmol/L) | (mmol/L) | (U/L) | (U/L) | (U/L) | (U/L) | (mmol/L) | (g/L) | (g/L) | |
| The proband | |||||||||
| 17 | 80.5 | 28.1 | 12 | 22 | 694 | 216 | 27.2 | 50.3 | 31.2 |
| 21 | 55.9 | 27.5 | 13 | 22 | 486 | 150 | 34.2 | 49.0 | 24.0 |
| 26 | 107.5 | 36.5 | 40 | 29 | 558 | 202 | 90.7 | NA | NA |
| The past elder sister | |||||||||
| 30 | 263.4 | 122 | 22 | NA | 576 | 48 | NA | 48.9 | 29.0 |
| 32 | 334.7 | 210.5 | 16 | 30 | 454 | 43 | NA | 46.4 | 30.2 |
| 34 | 271.3 | 162.9 | 17 | 40 | 419 | 37 | NA | 57.1 | 31.5 |
| Reference range | 5-21 | 0-6 | 0-40 | 0-40 | 0-500 | 3-50 | 0-10 | 60-85 | 35-55 |
On examination of the proband, obvious arthrogryposis multiplex, exfoliative skin (ichthyosis), mild jaundice and simian lines on the right palm were seen. A weak response to surrounding stimulus and no response to sound were noted. Liver was palpable 2 centimeters below the costal edge with normal texture. The spleen was not palpable.
Laboratory investigations of the proband showed mildly elevated conjugated bilirubin, raised alkaline phosphatase, elevated GGT and total bile acids, hypoalbuminemia but normal ALT and AST (Table 1). Proteinuria and glucosuria were present. There was a mildly elevated lactate level. The following results were normal or unremarkable: blood urea, creatinine, electrolytes, free T4 and thyroid stimulating hormone, ammonia, α1-antitrypsin level, blood tandem mass spectrometry (MS/MS) study of amino acid and carnitine profile, serology for hepatitis A to E, blood immunoglobulin M antibodies to toxoplasma, rubella, cytomegalovirus, herpes simplex virus, Epstein-Barr virus, blood cytomegalovirus DNA and chromosome G bands.
The proband’s previous X-ray revealed pneumonia. Cardiac ECHO revealed patent foramen ovale. Abdominal ultrasound showed normally sized kidneys with multiple cysts of various sizes in both kidneys with the largest in the left kidney of 0.8 cm × 0.6 cm, and the largest in the right kidney, 0.6 cm × 0.5 cm. Granular high-echo spots in the medulla of kidneys were revealed. Ultrasound of the hip showed no sign of dislocation. Brain CT scan showed symmetric, bilateral hypodense white matter of the cerebral hemispheres with a CT number of about 14 HU on the Hounsfield scale. CT also revealed swollen bilateral frontal and temporal lobes, narrowed bilateral lateral and third ventricles, and basal ganglia of heterogeneous density.
From the clinical manifestations and previously performed investigations, the diagnosis of ARC syndrome was suspected. In view of the bad prognosis, the parents were not willing to allow the child to undergo any further tests. However they consented to genetic analysis to help with future prenatal diagnosis. Ursodeoxycholic acid and fat-soluble vitamins were prescribed and follow-up was made over the telephone or by email. Her ichthyosis got much better with olive oil massage after bathing and the patient attained her birth weight at 67 d of age. Facial eczema developed from about 5 mo of age and she died at 7.5 mo of age.
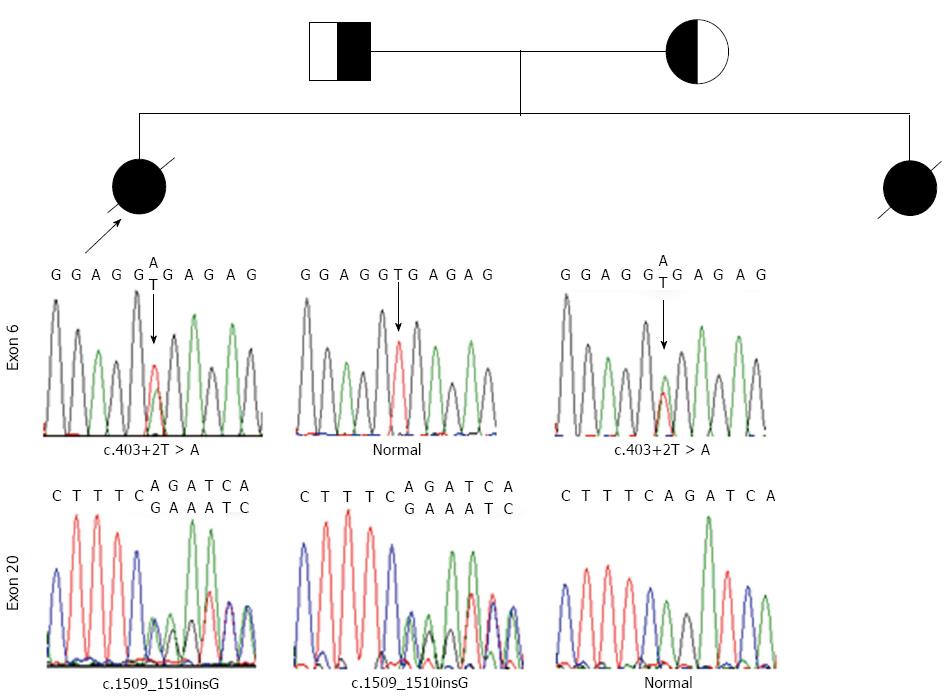
The study protocol conforms to the ethical guidelines of the Declaration of Helsinki of 1975. With the approval by the Ethics Committee on human research of the Children’s Hospital of Fudan University and informed consent of the parents, 1 mL of whole blood was drawn from the proband and her parents. DNA was extracted routinely and all the coding exons together with adjacent intronic sequence of the VPS33B gene were amplified and sequenced according to Gissen P et al with modifications (detailed primers, PCR and sequencing condition are available upon request)[2,3].
In the proband, compound heterozygosity for c.403+2T > A and c.1509-1510insG mutations was revealed. The parents were found to be heterozygous (Figure 1).
ARC syndrome is known to be caused by VPS33B and VIPAS39 mutations and has been reported to occur in many ethnic groups[3,8]. Normal or low GGT is one of the characteristics of neonatal cholestasis in ARC syndrome and it was listed as one of the four diagnostic features of the syndrome (arthrogryposis, renal tubular dysfunction and cholestasis with a low GGT activity)[3]. By reviewing the literature to date, none of the cases reported manifested cholestasis with significantly high GGT. Therefore, the proband case here is the first report that ARC syndrome could present as neonatal cholestasis with significantly high GGT activities.
The patients had three major diagnostic features: arthrogryposis multiplex congenita, renal involvement and cholestasis. The genetic study of VPS33B gene of the proband further confirmed the diagnosis by identification of two mutations previously reported in the East Asians[8]. c.1509-1510insG is a frame-shift mutation. c.403+2T > A mutation disrupts the original donor site following new donor site creation and therefore, a 16 bp intronic sequence that contains a stop codon is inserted into the mRNA sequence and results in a truncated VPS33B protein[9]. Based on this, a diagnosis of ARC syndrome caused by VPS33B mutations in the proband case could be confirmed.
An interesting finding is the parallel increase in serum levels of GGT and total bile acids in the proband. Her elder sister demonstrated a typical neonatal cholestasis with low GGT, who should have same genetic background of VPS33B, indicating that the high GGT in the proband could not be explained by the specific mutations.
The normal reference range of GGT is age dependent[7]. It could be quite high in newborns and then decreased to adult range. In Mainland China, because of the lack of age specific data, 50 U/L is widely used as the upper normal limit regardless of age. 93 U/L is defined as the upper normal limit of GGT in infants less than 6 mo of age in National Taiwan University Hospital[6]. One feature of this proband is that she went to see a doctor much earlier than her elder sister, so the elevation of GGT might be explained as an age-related evolution of normal GGT activity. The limitation of this case report is the lack of follow-up of her liver function test beyond neonatal stage. As a result, we do not know whether the elevated GGT activity would reduce as age advances. However, all cases with available data on GGT activity reported until now had GGT activity labeled normal or with a peak level no more than 83 U/L (Table 2). Her elder sister’s GGT activity tested at 30 days of age was also below 50 U/L (Table 1). However, the proband had a GGT activity over 200 U/L at 26 days of age, making it unlikely that it can be fully explained by the specific age.
| Ref. | GGT level |
| Di Rocco et al[10] (1995) | Normal (3 patients) |
| Franceschini et al[11] (1997) | Normal (3 patients) |
| Papadia et al[12] (1996) | Normal (1 patient) |
| Coleman et al[13] (1997) | Normal (2 patients, 60-70 U/L) |
| Abdullah et al[14] (2000) | Normal (3 patients) |
| Denecke et al[15] (2000) | Normal (2 patients), mildly elevated (1 patient 78 U/L) |
| Eastham et al[16] (2001) | Normal (4 patients) |
| Howells et al[17] (2002) | Normal (1 patient) |
| Gissen et al[2] (2004) | Normal (29 patients) |
| Abu-Sa'Da et al[18] (2005) | Normal (2 patients) |
| Choi et al[19] (2005) | Normal (1 patient) |
| Tekin et al[20] (2005) | Normal (2 patients) |
| Bull et al[21] (2006) | Normal (1 patient) |
| Gissen et al[3] (2006) | Normal (9 patients) |
| Taha et al[22] (2007) | Normal (1 patient) |
| Hershkovitz et al[23] (2008) | Normal (2 patients, 35-83 U/L) |
| Arhan et al[24] (2009) | Normal (1 patient) |
| Jang et al[8] (2009) | Normal (6 patients) |
| Kim et al[25] (2010) | Normal (10 patients) |
This case shows that the presence of high GGT activity cannot exclude ARC if the diagnosis is strongly suspected due to the presence of other cardinal features such as ichthyosis, arthrogryposis, agranular platelets, failure to thrive, and renal tubular acidosis. It indicates that ARC syndrome should be considered as a diagnostic possibility in various populations and a cholestasis with significantly high GGT activity, especially in the early stage after birth, should not exclude the diagnosis.
We thank Prof. Ying Kit Leung for the revision and editing of the manuscript, thank Dr. P. Gissen for his technique advice on VPS33B gene sequencing. We also thank the proband and her parents.
Arthrogryposis multiplex congenita and ichthyosis were found in a cholestatic infant with high gamma-glutamyl transpeptidase (GGT) activity.
Arthrogryposis multiplex congenita, renal dysfunction and cholestasis.
Biliary atresia, progressive familial intrahepatic cholestasis, citrin deficiency, idiopathic neonatal cholestasis, etc., should be considered.
Genetic study revealed compound heterozygote with known disease-causing mutations in VPS33B.
Multiple cysts in kidneys and patent foramen ovale were revealed by ultrasound.
Ursodeoxycholic acid and fat-soluble vitamins were prescribed.
Cholestasis of arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome has never been associated with significantly high GGT activities.
ARC refers to arthrogryposis multiplex congenita, renal dysfunction and cholestasis.
ARC syndrome should not be excluded from the list of differential diagnoses in a cholestatic infant with high GGT activity, especially in the first months after birth.
This article indicated that ARC syndrome cannot be excluded from the differential diagnosis of neonatal cholestasis based on serum levels of GGT activity.
P- Reviewers: Al Mehaidib A, Giovannoni I, Marin JJG, Richter B S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Horslen SP, Quarrell OW, Tanner MS. Liver histology in the arthrogryposis multiplex congenita, renal dysfunction, and cholestasis (ARC) syndrome: report of three new cases and review. J Med Genet. 1994;31:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, McKiernan PJ, Klomp LW, Morris AA, Wraith JE. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet. 2004;36:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Gissen P, Tee L, Johnson CA, Genin E, Caliebe A, Chitayat D, Clericuzio C, Denecke J, Di Rocco M, Fischler B. Clinical and molecular genetic features of ARC syndrome. Hum Genet. 2006;120:396-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Cullinane AR, Straatman-Iwanowska A, Zaucker A, Wakabayashi Y, Bruce CK, Luo G, Rahman F, Gürakan F, Utine E, Ozkan TB. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet. 2010;42:303-312. [PubMed] |
| 5. | Smith H, Galmes R, Gogolina E, Straatman-Iwanowska A, Reay K, Banushi B, Bruce CK, Cullinane AR, Romero R, Chang R. Associations among genotype, clinical phenotype, and intracellular localization of trafficking proteins in ARC syndrome. Hum Mutat. 2012;33:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Chen HL, Chang PS, Hsu HC, Ni YH, Hsu HY, Lee JH, Jeng YM, Shau WY, Chang MH. FIC1 and BSEP defects in Taiwanese patients with chronic intrahepatic cholestasis with low gamma-glutamyltranspeptidase levels. J Pediatr. 2002;140:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Tse K. Serum gamma glutamyltransferase levels in cholestasis of infancy. HK J Paediatr (new series). 2008;13:4-6. |
| 8. | Jang JY, Kim KM, Kim GH, Yu E, Lee JJ, Park YS, Yoo HW. Clinical characteristics and VPS33B mutations in patients with ARC syndrome. J Pediatr Gastroenterol Nutr. 2009;48:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Kim KM, Kim GH, Park YS, Yoo HW. Aberrant splicing by a mutation, c.403+2T& gt; A, in Korean patients with arthrogryposis-renal-dysfunction-cholestasis syndrome. Pediatr Int. 2011;53:609-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Di Rocco M, Callea F, Pollice B, Faraci M, Campiani F, Borrone C. Arthrogryposis, renal dysfunction and cholestasis syndrome: report of five patients from three Italian families. Eur J Pediatr. 1995;154:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Franceschini P, Barberis L. Arthrogryposis, renal tubular dysfunction, cholestasis, ichthyosis syndrome (ARCI). Eur J Pediatr. 1997;156:78. [PubMed] |
| 12. | Papadia F, Pollice L, Natale B, Bellantuono R, Conti P, Carnevale F. Biliary malformation with renal tubular insufficiency in two male infants: third family report. Clin Genet. 1996;49:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Coleman RA, Van Hove JL, Morris CR, Rhoads JM, Summar ML. Cerebral defects and nephrogenic diabetes insipidus with the ARC syndrome: additional findings or a new syndrome (ARCC-NDI)? Am J Med Genet. 1997;72:335-338. [PubMed] |
| 14. | Abdullah MA, Al-Hasnan Z, Okamoto E, Abomelha AM. Arthrogryposis, renal dysfunction and cholestasis syndrome. Saudi Med J. 2000;21:297-299. [PubMed] |
| 15. | Denecke J, Zimmer KP, Kleta R, Koch HG, Rabe H, August C, Harms E. Arthrogryposis, renal tubular dysfunction, cholestasis (ARC) syndrome: case report and review of the literature. Klin Padiatr. 2000;212:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Eastham KM, McKiernan PJ, Milford DV, Ramani P, Wyllie J, van't Hoff W, Lynch SA, Morris AA. ARC syndrome: an expanding range of phenotypes. Arch Dis Child. 2001;85:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Howells R, Ramaswami U. ARC syndrome: an expanding range of phenotypes. Arch Dis Child. 2002;87:170-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Abu-Sa'da O, Barbar M, Al-Harbi N, Taha D. Arthrogryposis, renal tubular acidosis and cholestasis (ARC) syndrome: two new cases and review. Clin Dysmorphol. 2005;14:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Choi HJ, Lee MW, Choi JH, Moon KC, Koh JK. Ichthyosis associated with ARC syndrome: ARC syndrome is one of the differential diagnoses of ichthyosis. Pediatr Dermatol. 2005;22:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Tekin N, Durmuş-Aydoğdu S, Dinleyici EC, Bör O, Bildirici K, Akşit A. Clinical and pathological aspects of ARC (arthrogryposis, renal dysfunction and cholestasis) syndrome in two siblings. Turk J Pediatr. 2005;47:67-70. [PubMed] |
| 21. | Bull LN, Mahmoodi V, Baker AJ, Jones R, Strautnieks SS, Thompson RJ, Knisely AS. VPS33B mutation with ichthyosis, cholestasis, and renal dysfunction but without arthrogryposis: incomplete ARC syndrome phenotype. J Pediatr. 2006;148:269-271. [PubMed] |
| 22. | Taha D, Khider A, Cullinane AR, Gissen P. A novel VPS33B mutation in an ARC syndrome patient presenting with osteopenia and fractures at birth. Am J Med Genet A. 2007;143A:2835-2837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Hershkovitz D, Mandel H, Ishida-Yamamoto A, Chefetz I, Hino B, Luder A, Indelman M, Bergman R, Sprecher E. Defective lamellar granule secretion in arthrogryposis, renal dysfunction, and cholestasis syndrome caused by a mutation in VPS33B. Arch Dermatol. 2008;144:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Arhan E, Yusufoğlu AM, Sayli TR. Arc syndrome without arthrogryposis, with hip dislocation and renal glomerulocystic appearance: a case report. Eur J Pediatr. 2009;168:995-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Kim SM, Chang HK, Song JW, Koh H, Han SJ; Severance Pediatric Liver Disease Research Group. Agranular platelets as a cardinal feature of ARC syndrome. J Pediatr Hematol Oncol. 2010;32:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |