Published online Apr 28, 2014. doi: 10.3748/wjg.v20.i16.4692
Revised: February 9, 2014
Accepted: March 5, 2014
Published online: April 28, 2014
Processing time: 118 Days and 22.6 Hours
AIM: To investigate whether Na+-K+-2Cl- cotransporter (NKCC2) is expressed in the mouse distal colonic epithelia and whether it is regulated by vasopressin in the colon.
METHODS: The mRNA expression of NKCC2 in the mouse colonic mucosa was examined by reverse transcription-polymerase chain reaction. NKCC trafficking in the colon stimulated by 1-D-amino(8-D-arginine)-vasopressin (dDAVP) infusion (10 ng/mouse, intraperitoneal injection ) within 15 min, 30 min and 1h was investigated by laser confocal scanning microscopy. Total and membrane NKCC2 expression in the colonic mucosa from control and dDAVP-treated mice was detected by Western blotting. Short circuit current method was performed to determine regulation of NKCC2 by vasopressin in the colon.
RESULTS: NKCC2 was predominantly located in the apical region of the surface of the distal colonic epithelia; by comparison, a large amount of NKCC1 was distributed in the basolateral membrane of the lower crypt epithelia of the mouse distal colon. Short-term treatment with dDAVP, a V2-type receptor-specific vasopressin analog, induced NKCC2 re-distribution, i.e., NKCC2 traffics to the apical membrane after dDAVP stimulation. In contrast, no obvious NKCC1 membrane translocation was observed. Western blotting results confirmed that membrane NKCC2 had significantly higher abundance in the dDAVP-treated mouse colonic mucosa relative to that in the untreated control, which is consistent with our immunostaining data. Moreover, the short-circuit current method combined with a NKCC2 inhibitor demonstrated that NKCC2 was also activated by serosal vasopressin in isolated distal colonic mucosa.
CONCLUSION: Our results provide direct evidence that vasopressin also plays an important role in the colonic epithelia by stimulating NKCC2 trafficking to the apical membrane and inducing NKCC2-mediated ion transport.
Core tip: The Na+-K+-2Cl- cotransporter (NKCC2), which was thought to be only expressed in the apical membrane of the epithelial cells in the thick ascending limb of Henle’s loop, was recently found to be expressed in the colon. However, the role and regulating mechanism of NKCC2 in the gut are still not completely understood. Our results provide direct evidence that vasopressin also plays an important role in the colonic epithelia by stimulating NKCC2 trafficking to the apical membrane and inducing NKCC2-mediated ion transport. The action of vasopressin on NKCC2 in the colon would be recognized to supplement the role of the kidney in modulating whole-body homeostasis and electrolyte balance in physiological or pathophysiologic conditions.
- Citation: Xue H, Zhang ZJ, Li XS, Sun HM, Kang Q, Wu B, Wang YX, Zou WJ, Zhou DS. Localization and vasopressin regulation of the Na+-K+-2Cl- cotransporter in the distal colonic epithelium. World J Gastroenterol 2014; 20(16): 4692-4701
- URL: https://www.wjgnet.com/1007-9327/full/v20/i16/4692.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i16.4692
The bumetanide-sensitive Na+-K+-2Cl- cotransporters (NKCC) mediate the electroneutral movement of 1 Na+, 1 K+ and 2 Cl- ions across cell membranes[1,2]. Two isoforms, NKCC1 and NKCC2, are currently known and are encoded by different genes. NKCC1 (SLC12A2) is widely distributed in the basolateral membrane of secretory epithelial cells[3]. In contrast, NKCC2 (SLC12A1) is primarily localized to the apical membrane of the epithelial cells of the thick ascending limb of Henle’s loop (TALH), where it mediates the apical entry of Na+, K+ (or NH4+) and Cl-[4]. NKCC2 has been thought to be a kidney-specific isoform. However, there is growing evidence for extra-renal expression, including expression in the pancreas and the gastrointestinal tract of rats and humans, where it might mediate Cl- absorption[5-8]. The NKCC2 protein expression and location as well as its mechanism of regulation in the mouse distal colon are not understood.
Vasopressin (also termed antidiuretic hormone) increases NaCl absorption in the TALH[9,10] and Na and water absorption in cortical collecting duct cells[11]. Several reports have verified that vasopressin has both long-term and short-term effects on NKCC2 expression and function in TALH cells. In the short term, the vasopressin-dependent phosphorylation of NKCC2 is associated with vesicular trafficking of the transporter to the luminal membrane[12,13]. Long-term vasopressin exposure in the TALH or in collecting duct principal cells increases the expression of NKCC2[14] and aquaporin 2[15], respectively. The colon could also be a target for vasopressin[16] because vasopressin stimulates NaCl and water absorption in in vitro preparations of mouse, rat, and human colons[17-21]. However, little is known about the mechanism of ion transport that is induced by vasopressin in the colon. Specifically, the identity of the protein that mediates NaCl absorption in the colon and whether NKCC2 is involved in this process are currently unclear.
Previous studies have focused on the regulation of NKCC2 by vasopressin in the kidney. How vasopressin regulates colonic NKCC2 is unknown. The present study addresses this issue by showing the NKCC2 expression and spatial distribution in the mouse colonic epithelia. We investigated the NKCC2 redistribution and trafficking in the colonic epithelia following short-term exposure to vasopressin. We also explored whether NKCC2 is involved in the ion transport induced by vasopressin using the short circuit current method in isolated colonic mucosa. Our results show that the effects of vasopressin on colonic NKCC2 are similar to those described for the kidney. The significance of this finding for colonic epithelial physiology is discussed.
Male C57BL/6 mice weighing 20-25 g (Laboratory Animal Services Center, Capital Medical University, Beijing, China) were fed a normal diet with free access to water. The protocol was approved by the Animal Care and Use Committee of Chinese Capital Medical University. On the day of the experiments, 10 ng of dDAVP (a vasopressin analogue; Sigma) per animal[12] or saline (as a control) was administered by intraperitoneal injection. To reduce the level of endogenous vasopressin, the animals were water-loaded by offering them a 5% dextrose/1% ethanol solution overnight. The water load was assessed by measuring the solution intake. The mice were killed by cervical dislocation. The distal colon was removed by opening the abdominal cavity 15 min, 30 min and 1 h after drug application. Intestinal segments were briefly rinsed with ice-cold PBS. Frozen sections (5 μm) were cut on a cryostat (Leica, CM3050S), mounted on glass slides and stored at -20 °C. Tissues were cut into 2-μm-thick rings, fixed in 2% paraformaldehyde in PBS at pH = 7.4 for 1 h at room temperature and then rinsed with PBS and cryoprotected in 30% sucrose overnight. The approach of the tissue preparation and arrays was completely based on a previously described method[22].
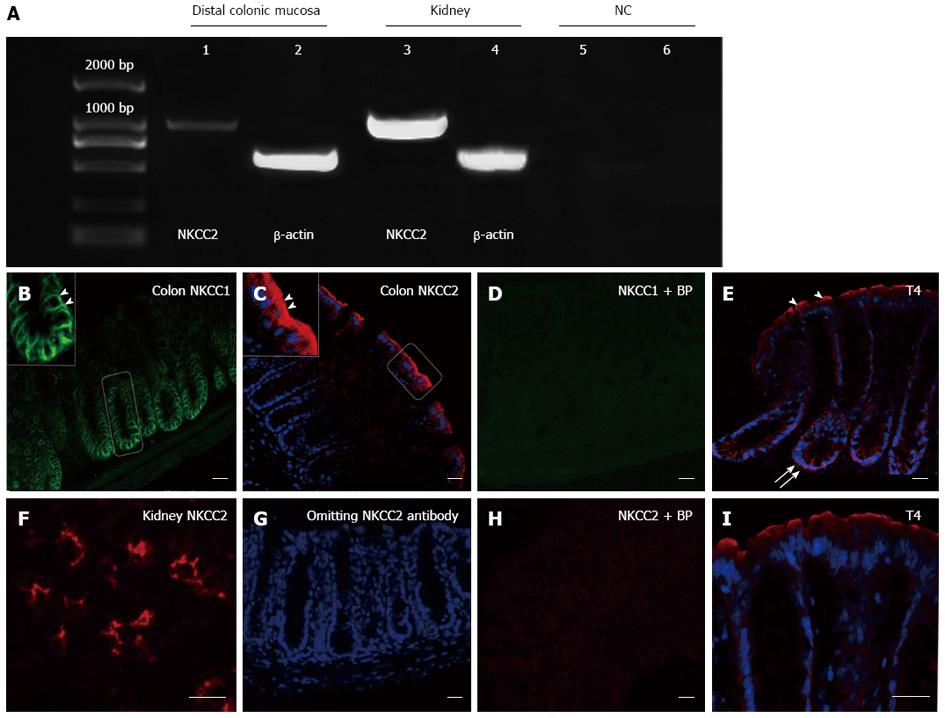
Intestinal tissues were fixed in 2% (w/v) paraformaldehyde-PBS for 1 h at 25 °C. Following fixation, the tissues were cryoprotected in 30% sucrose overnight in the cold, embedded in Tissue-Tek O.C.T medium and frozen in liquid nitrogen. The sections were rehydrated in PBS and incubated for 2 h in a blocking solution (BS) consisting of PBS, 10% goat serum or donkey serum and 0.1% Triton-X (pH = 7.4). Then, the sections were incubated with a primary antibody overnight at 4 °C. After washing with PBS, the sections were incubated with the corresponding secondary antibody for 1 h at 25 °C. The primary and secondary antibodies used in this study are summarized in Table 1. Immunostaining controls were performed by omitting the primary antibody or by using nonspecific IgG. The NKCC2 antibodies were purchased from two different commercial sources. NKCC1 and NKCC2 antibodies were preadsorbed with their corresponding control peptides (Santa Cruz sc-21547P 10 μg per 1 μg NKCC1 antibody; Fitzgerald 33R-6671, 5 μg per 1 μg NKCC2 antibody) to determine the specificity of the antibodies. The specimens were then examined using a fluorescence microscope (Nikon 80i, Japan) or a confocal laser scanning microscope (Leica TCS SP5 MP, Germany).
| Antibody | Host species | Dilution | Source/Catalog No. |
| NKCC2 | Rabbit | 1:100 | Santa cruz/sc-133823 |
| NKCC2 | Rabbit | 1:150 | Fitzgerald/70R-3806 |
| NKCC1 | Goat | 1:100 | Santa cruz/sc-21574 |
| T4 | Mouse | 1:200 | Developmental Studies Hybridoma Bank |
| Rabbit IgG | Donkey (Texas red) | 1:400 | Abcam |
| Goat IgG | Donkey (Alexa 488) | 1:200 | Invitrogen |
| Mouse IgG | Goat (cy3) | 1:200 | Invitrogen |
| Rabbit IgG | Goat (HRP) | 1:2000 | Santa cruz |
Immunolabeled sections were examined using a confocal laser scanning microscope. Confocal images were converted to 2-channel (red and green) mode by subtracting DAPI (blue). The confocal images were analyzed using Image J software. Areas of NKCC2 labeling were highlighted in white using the Image J. Data from 4 to 12 selected areas were averaged in each image; 6 images were randomly selected for analysis from each measurement group of one animal (n = 6 images; n’ = 4-12 selected areas), and data were collected from three animals (n = 3). The method described above was based on the recently published work from the laboratory of Prof. Ameen[22,23]. Statistical analysis was performed using GraphPad Prism software. Differences among groups were determined using one-way ANOVA and the Tukey’s post hoc method of multiple comparisons. The level of significance was set at P < 0.05.
The distal colon was cut longitudinally along the mesenteric border. The serosa, muscularis and submucosa were stripped away with fine forceps to prepare the mucosa sample. The stripped mucosa was mounted in a modified Ussing chamber in a tissue holder (Easy Mount Chamber; Physiologic Instruments, San Diego, CA) with an aperture surface area of 0.3 cm2, and the sample was bathed bilaterally in Krebs-Henseleit solution (KHS).
The short-circuit current was measured in vitro in the Ussing chambers. The transepithelial PD was then clamped at 0 mV, and the short-circuit current (ISC) was recorded with a VCC MC6 voltage-current clamp amplifier (Physiologic Instruments, San Diego, CA). The transepithelial resistance (TR) (Ωcm2) was measured by altering the membrane potential in a stepwise fashion (-0.1 mV) and applying the Ohmic relationship.
Krebs-Henseleit solution (KHS) (mmol/L): NaCl, 117; KCl, 4.7; MgCl2, 1.2; KH2PO4, 1.2; NaHCO3, 24.8; CaCl2, 2.5; and glucose, 11.1. The solution was bubbled with 95% O2 to 5% CO2 to maintain a pH value of 7.4. The dDAVP, bumetanide, indomethacin, tetraethylammonium (TEA), tetrodotoxin (TTX), vasopressin and amiloride were purchased from Sigma (St Louis, MO, United States). Stock solutions of all the above chemicals were dissolved in DMSO. The final DMSO concentrations never exceeded 0.1% (v/v). Preliminary experiments indicated that the vehicle did not alter any baseline electrophysiological parameters.
RNA from the stripped mucosa and renal medulla was harvested using the Trizol RNA purification system (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. The following primers were used for the PCR of NKCC2: forward 5’-TTGAGATTGGCGTGGTCATA-3’ and reverse 5’-AAGCATGTCAGCCAGCTTTT-3’. The following primers were used for β-actin, an internal control: forward 5’-TGT TTG AGA CCT TCA ACA CC-3’ and reverse 5’-CAG TAA TCT CCT TCT GCA TCC-3’. The amplification products and a DNA size marker (DNA maker C; SBS Genetech, Beijing, China) were separated by electrophoresis on a 1.5% agarose gel in 0.5 TRIS-borate-EDTA buffer containing ethidium bromide. The bands of the amplification products were viewed using ultraviolet light, and the images were taken using a GelDoc2000 (Bio-Rad Laboratories, Hercules, CA, United States).
The serosa, muscularis and submucosa were stripped away with fine forceps to prepare the mucosa sample. The protocol was based on the manufacturer’s instructions (Boogo Company, Shanghai W002). The preparation was homogenized in ice-cold tissue protein extraction reagent A, and then sonicated until the sample was completely dissolved. After freezing in liquid nitrogen and thawing at 25 °C three times, the samples were centrifuged at 5000 g at 4 °C for 10 min, and the supernatants were carefully collected. Then, the sample was centrifuged at 14000 g at 4 °C for 30 min to precipitate the membrane fragments. Protein extraction reagent B was added to the precipitates to centrifuge at 14000 g at 4 °C for 10 min. The supernatants were thoroughly removed, and then protein extraction reagent C was added.
Tissue was homogenized in 300 μL of cold lysis buffer, and the total tissue homogenates were sonicated until they were completely dissolved. The sample was then centrifuged at 12000 rpm for 30 min at 4 °C. The protein concentration of the samples was measured using a Bradford assay kit.
Total NKCC2 proteins (20 μg) or membrane NKCC2 proteins (40 μg) were separated by 8% SDS/PAGE, and the separated proteins were electroblotted onto a PVDF membrane (Millipore), which was then washed for 10 min with TBST and immersed in blocking buffer containing 5% non-fat dry milk in TBST for 1 h at 25 °C. The blot was washed with TBST and then incubated with a polyclonal primary antibody against NKCC2 (Santa Cruz sc-133823) overnight at 4 °C. After washing in TBST, the blot was incubated with a secondary antibody against rabbit IgG (Santa Cruz) for 1 h at 25 °C. The blot was finally washed with TBST, and the protein bands were visualized with a chemiluminescence system (ECL Plus, Applygen Technologies Inc.). The resulting image was analyzed using Total Lab Quant software.
Data are expressed as mean ± SD; statistical significance between two individual measurement groups was determined using an unpaired t test. Differences among groups were determined using one-way ANOVA and the Tukey’s post hoc method of multiple comparisons. The level of significance was set at P < 0.05.
We first sought to investigate the expression of NKCC2 and determine the location of NKCC in the mouse colonic epithelia. Reverse transcription-polymerase chain reaction and immunostaining were performed. As shown in Figure 1A, a band of the expected size (1161 bp) was amplified in both the colonic mucosa and the kidney. As a negative control, samples lacking amplified products were obtained by using non-reverse-transcribed RNA from the mouse colonic mucosa or by omitting cDNA. Immunofluorescence staining revealed a high level of NKCC1 in the lower crypt of the colonic epithelia, which is the predominant site of secretion[24]. In the crypt regions, NKCC1 was mainly localized to the basolateral membrane (Figure 1B), consistent with previous observations in mammalian intestines[25]. In contrast, the NKCC2 immunoreactivity at the colonic surface epithelia was observed predominantly in the apical membrane (arrowhead, Figure 1C), consistent with observations in rat and human colonic tissues[6,7]. The spatial distributions of NKCC1 and NKCC2 differed in the mouse colon. NKCC2 was located in the apical membrane of the mouse colonic surface epithelia, whereas NKCC1 was found in the lower crypt epithelia of the mouse distal colon. Preadsorption of NKCC1or NKCC2 antibodies with the corresponding control peptides abolished the immunoreactivity (Figure 1D and H), suggesting that both antibodies were specific. The NKCC2 antibody was also tested in the mouse kidney as a positive control. Greater NKCC2 abundance was detected in the apical regions of cells lining the thick ascending limb segment of the mouse nephron (Figure 1F). No staining was observed when the NKCC2 primary antibody was omitted (Figure 1G). T4 is a monoclonal antibody that is generated against a fusion protein encompassing the carboxy terminus (S760-S1212) of human NKCC[26]. The antibody is known to recognize both the apical NKCC2 and basolateral NKCC1 isoforms of the Na-K-2Cl cotransporter[26]. As expected, T4 immunoreactivity was observed in the apical membrane, further supporting the NKCC2 localization data (Figure 1E and I).
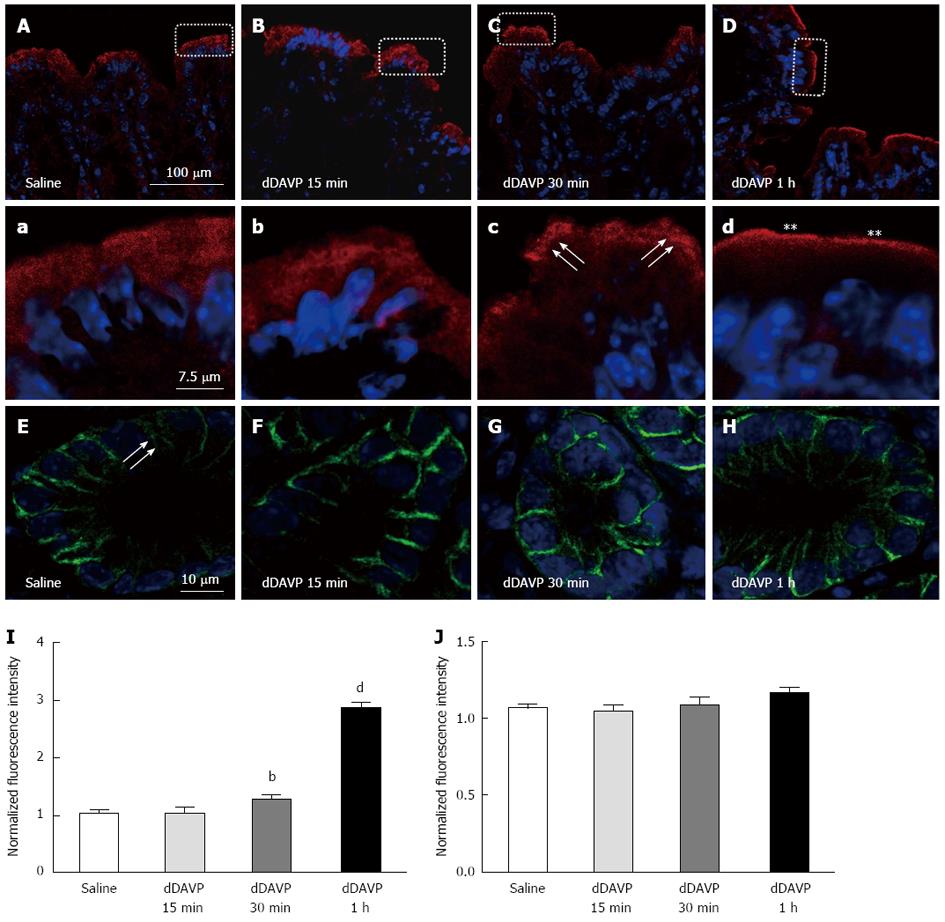
NKCC2 has been demonstrated to be modulated by vasopressin in the TAL[12,13]; thus, we next investigated whether the apically expressed NKCC2 was regulated by short-term treatment with vasopressin. Short-term vasopressin treatment has been reported to induce NKCC2 apical vesicular trafficking in medullary TAL cells[12]. Therefore, we investigated whether vasopressin could cause a redistribution of NKCC2 in the colonic epithelia. In this study, we used a V2-type receptor-specific vasopressin analogue, dDAVP, to examine its effect on NKCC2 trafficking. Higher magnification images of unstimulated colon sections indicated that NKCC2 immunoreactivity was also present in the intracellular compartments (Figure 2A). dDAVP (10 ng per animal, intraperitoneal injection) was administered to adult mice. At 30 min post-stimulation, more intense staining for NKCC2 was detected in the apical region of surface enterocytes, as shown in Figure 2C. Notably, at 1 h post-stimulation, the vasopressin-induced membrane recruitment of NKCC2 resulted in labeling intensities that were higher in the apical membrane than the 30 min post-stimulation intensities (Figure 2D, d). This observation was confirmed by densitometry: the NKCC2 fluorescence intensity on the apical membrane of enterocytes ranged from values that were approximately 1.5-fold to 3-fold higher than that in the untreated mouse (normalized to the apical membrane of the surface cells in untreated mice, n = 3 mice, n = 6 images, n’ = 4-12 selected area P < 0.001, Figure 2I). The dramatic recruitment of NKCC2 to the apical membrane seemed to occur for at least 30 min because no significant differences could be detected between the 15 min post-stimulation and unstimulated states (Figure 2B). At the same time, we also determined whether NKCC1 was redistributed in response to vasopressin. In the control condition, the NKCC1 labeling was predominantly found at the basal and lateral membranes, and there was a partial punctate vesicular-like intracellular pattern in the colonic crypt base (Figure 2E arrow). After 15 min, 30 min and 1 h of stimulation, the NKCC1 localization and labeling intensity in the basolateral membrane did not change significantly, suggesting that vasopressin might mainly affect NKCC2 (Figure 2F, G, H and J).
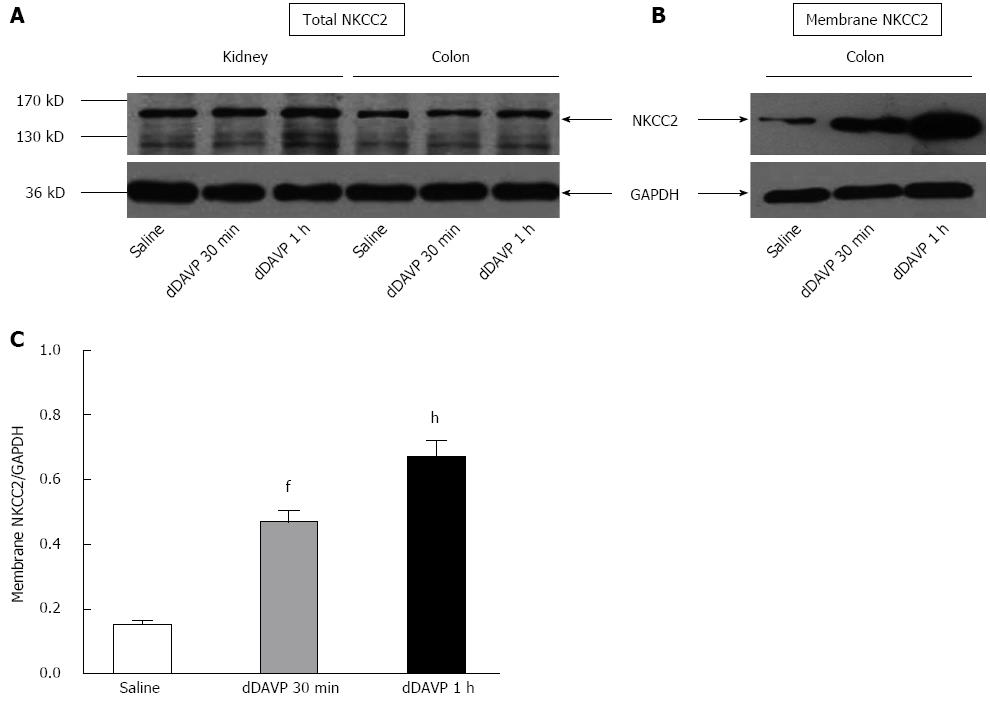
Immunoblot analysis of whole cell homogenates of the mouse colonic mucosa showed the strong signals for the NKCC2 at 140 KD, which were also found in the kidney; this finding also confirmed the NKCC2 expression in the colon. Western blotting showed no obvious change in the overall NKCC2 abundance, similar to its behavior in the kidney (Figure 3A); this finding indicated that vasopressin induced redistribution of NKCC2 from intracellular vesicles to the apical membrane without affecting the overall level of NKCC2 expression. To further substantiate this finding, protein samples that were enriched for plasma membranes were also analyzed. An equal amount of protein (40 μg) was loaded and we found that dDAVP caused a significant increase in the NKCC2 membrane abundance with time by comparison with unstimulated samples (Figure 3B). Densitometry analysis confirmed the significantly higher abundance in the dDAVP-treated mouse colonic mucosa relative to that in the untreated control, which is consistent with our immunostaining data (Figure 3C, n = 4 mice, P < 0.01).
Because NKCC2, an absorptive isoform of NKCC, was expressed in the apical region of the colonic epithelia and regulated by short-term treatment with vasopressin, we investigated whether the apical NKCC2 was responsible for the observed vasopressin-induced electrolyte transport. To avoid complications due to the effects of vasopressin on the microcirculation and motility in vivo, isolated mucosa was used for these in vitro experiments. Based on the parameters reported in the previous study, we used a vasopressin concentration of 5 × 10-8 mol[19]. Before the experiments were performed, the freshly isolated distal colonic segment was pretreated with indomethacin (10 μmol) and TTX (tetrodotoxin, 1 μmol), a neuronal Na+ channel blocker, on the serosal side to suppress endogenous prostaglandin production and the neuronally mediated effects.
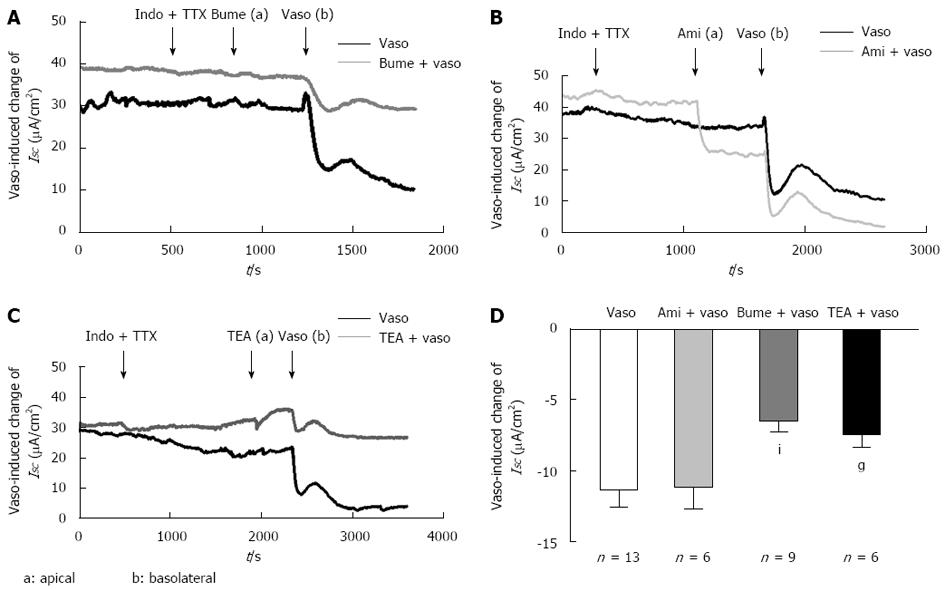
The serosal addition of vasopressin (5 × 10-8 mol) induced an immediate decrease of -11.41 ± 1.12 μA/cm2 (n = 13) in ISC, consistent with the results of the previous study[20]. To determine whether NKCC2 was responsible for the vasopressin-induced decrease in ISC, we pretreated the isolated distal colonic segment with bumetanide (10 μmol), a well-known inhibitor of the NKCC, on the apical side. As shown in Figure 4A and D, apical pretreatment with bumetanide significantly inhibited the vasopressin-induced ISC response by 50% (from -11.41 ± 1.12 μA/cm2 to -6.46 ± 0.76 μA/cm2, n = 9, P < 0.01, Figure 4A), implying that apical NKCC2 was involved in the vasopressin-induced response. Previous studies demonstrated an effect of vasopressin on Na+ absorption in the guinea pig distal colon[27]. Thus, we tested the effect of a blocker of the epithelial Na+ channel, amiloride (10 μmol/L). The results indicated that the change in the vasopressin-induced ISC decrease was not altered by amiloride (from -11.41 ± 1.12 μA/cm2 to -11.18 ± 1.46 μA/cm2n = 6, P > 0.05, Figure 4B and D), indicating that the vasopressin-induced response involved an amiloride-insensitive mechanism. A similar inhibition (33%), from -11.18 ± 1.46 μA/cm2 to -7.61 ± 0.96 μA/cm2, was observed for apical pretreatment with tetraethylammonium (TEA 5 mmol/L) (Figure 4C and D, n = 6, P < 0.05), a putative K+ channel blocker, indicating that the vasopressin-induced downward deflection of ISC was related to K+ transport.
Previous studies have demonstrated that NKCC2 was expressed in the apical membrane of the colon and played an important role in Cl- absorption[6,7]. The present study not only extended these results regarding the cellular location and function of NKCC2 but also demonstrated that NKCC2 in the mouse colon is regulated by vasopressin. Our results confirmed the NKCC2 expression in the mouse distal colon and found a differential spatial distribution of NKCC1 and NKCC2. The absorptive isoform, NKCC2, was mainly located in the apical membrane of surface epithelia, whereas the secretive isoform, NKCC1, was located in the basolateral membrane of the lower crypt epithelia. The differential location pattern of NKCC1 and NKCC2 indicates their distinct roles, which are related to the functions of each cell population. The localization of NKCC2 corresponds to a possible functional role in mediating apical Na+-K+-2Cl- absorption in the colonic epithelia. The location of NKCC1 was consistent with previous observations showing the basolateral localization of NKCC1 in the secretory epithelia, supporting its role in chloride secretion[28,29]. The immunofluorescence results showed that NKCC2 was only expressed in apical membrane of the epithelial cells in the colonic surface epithelium and was not expressed in the crypt epithelium. NKCC1 was only expressed in the basolateral membrane of the crypt epithelium and was not expressed in the surface epithelium. The results showed that the two isoforms were not located in the same cells or the same region.
Recently, the mechanism by which vasopressin regulates NKCC2 by inducing cellular redistribution in the kidney has been studied in more detail[30,31]. Furthermore, the functional activity of NKCC2 depends on the transporter density at the apical region of the surface cells, which is regulated by endocytic and exocytic trafficking[31,32]. Whether NKCC2 expression in the colon is regulated by vasopressin was unclear; our immunolocalization studies showed that a portion of NKCC2 was localized to intracellular vesicles in the unstimulated colonic epithelia. The increase in membrane-bound NKCC2 following short-term vasopressin treatment indicated a change in intracellular trafficking because the total NKCC2 expression did not increase. Moreover, the 0.5 h and 1 h intervals that were examined were shorter than the time (4-6 h) required for new protein synthesis. The approximately 1.5- and 3-fold increase in the NKCC2 fluorescence intensity after the 30 min and 1 h stimulation, respectively, in the apical membrane may reflect the onset of NKCC2 membrane recruitment. NKCC2 trafficking was not observed after a 15 min stimulation, possibly because vasopressin required a longer time to become effective after the intraperitoneal injection. To further elucidate the process of NKCC2 trafficking, we compared the NKCC2 protein expression level in the plasma membrane in the control and stimulation conditions. Consistent with laser scanning confocal microscope (LSCM) observation, the abundance of NKCC2 was indeed greater after vasopressin stimulation. Previous studies have shown that in TAL cells, trafficking to the apical membrane is the mechanism of stimulating NKCC2 activity. This finding indicated that vasopressin could activate apical NKCC2 by stimulating transporter trafficking to the plasma membrane. In contrast to NKCC2, the distribution of NKCC1 did not change obviously after vasopressin stimulation. Whether vasopressin has an effect on NKCC1 in the colon remains to be investigated.
It was reported that vasopressin induced an immediate decrease in the short-circuit current (ISC)[17,18]. However, whether the apical NKCC2 is involved in the vasopressin-induced electrolyte transport in the distal colon remains unclear. Previous studies reported that vasopressin could enhance the amiloride-insensitive Cl--dependent Na+ absorption in the normal rat distal colon[17]. Another report indicated that vasopressin inhibited the electrogenic amiloride-sensitive Na+ absorption in the guinea pig distal colon[23]. In our study, apical pretreatment with amiloride, an epithelial sodium channel (ENaC) blocker, failed to affect the serosal vasopressin-induced ISC decrease, indicating that amiloride-sensitive Na+ absorption cannot be involved in the ISC decrease. Apical pretreatment with bumetanide blunted the vasopressin-induced ISC decrease by approximately 50%, suggesting that the effect depends at least partially on NKCC2. In other words, apical NKCC2 stimulation by vasopressin contributed to the electrolyte transport in the mouse distal colonic epithelia. Interestingly, the apical addition of TEA (5 mmol/L), a putative inhibitor of K+ channel blocker, inhibited the vasopressin-induced ISC decrease by 33%, indicating that an apical K+ channel was involved in this response and that the current was partially mediated by K+ secretion. Moreover, we also examined the effect of vasopressin in conjunction with basolateral pretreatment with bumetanide. The basolateral pretreatment with bumetanide also inhibited the response by approximately 50% (Preliminary data). A similar blocking effect was observed for the pretreatment with basolateral bumetanide, also supporting that vasopressin mainly affects the NKCC2 cotransporter, which is predominantly located in the apical region of the distal colonic epithelia during this process. The possible explanations are that bumetanide is fat soluble and could cross the basolateral membrane to affect the apical NKCC2. Moreover, previous reports demonstrated that NKCC2 is more sensitive to inhibition by bumetanide than NKCC1[33,34]. This finding, together with the finding that no NKCC1 redistribution was observed after stimulation by vasopressin, again suggests that vasopressin might mainly affect NKCC2. Taken together, our results suggest that NKCC2 is involved in the vasopressin-induced ISC decrease in the mouse distal colonic epithelia.
Clearly, the kidney is not the only target organ through which vasopressin regulates the electrolyte balance of the whole body. The gastrointestinal tract is also a potent target of vasopressin because all vasopressin receptor subtypes are expressed throughout the gut in humans[35], and these receptors could be activated by either circulating or local vasopressin. In the present study, we focused on the effects of vasopressin on apical NKCC2 in the mouse colon and found that vasopressin may induce NKCC2 trafficking and activate apical NKCC2 in the mouse distal colon in vivo. These effects could enhance the capacity of the distal colonic epithelia to reabsorb NaCl and water in response to short-term hormonal stimulation. These findings suggest a possible mechanism through which vasopressin regulates apical NKCC2 activity in the colon, and this mechanism resembles its mechanism of action in the kidney. Collectively, the colon and kidney are predicted to work synergistically in the electrolyte absorption regulated by vasopressin. Consequently, the action of vasopressin on NKCC2 in the colon would be recognized to supplement the role in modulating whole-body homeostasis and electrolyte balance under physiological or pathophysiological conditions.
We are grateful to Prof. Zhu JX for kindly providing the Ussing chamber and valuable suggestions for the experiments.
The Na+-K+-2Cl- cotransporter (NKCC2), which was thought to be expressed only in the apical membrane of the epithelial cells in the thick ascending limb of Henle’s loop, was recently found to be expressed in the rat and human distal colon also. However, the role and regulating mechanism of NKCC2 in the gut are still not completely understood.
Previous studies have focused on the regulation of NKCC2 by vasopressin in the kidney. How vasopressin regulates colonic NKCC2 is unknown. The present study addresses this issue by showing the NKCC2 expression and spatial distribution in the mouse colonic epithelia. The results show that the effects of vasopressin on colonic NKCC2 are similar to those described for the kidney.
Several reports have verified that vasopressin has both long-term and short-term effects on NKCC2 expression and function in thick ascending limb of Henle’s loop cells. The colon could also be a target for vasopressin because vasopressin stimulates NaCl and water absorption in in vitro preparations of mouse, rat, and human colons. However, little is known about the mechanism of ion transport that is induced by vasopressin in the colon. Specifically, the identity of the protein that mediates NaCl absorption in the colon and whether NKCC2 is involved in this process are currently unclear. The present study provide direct evidence that vasopressin also plays an important role in the distal colonic epithelia by stimulating trafficking of NKCC2 to the apical membrane and inducing NKCC2-mediated ion transport at the apical region of the colonic epithelia.
The action of vasopressin on NKCC2 in the colon would be recognized to supplement the role of the kidney in modulating whole-body homeostasis and electrolyte balance under physiological or pathophysiologic conditions.
Endocytic trafficking involves the cellular internalization and sorting of extracellular molecules, plasma membrane proteins and lipids. Endocytosis is required for a vast number of functions, including nutrient uptake, cell adhesion and migration, receptor signaling, pathogen entry and cell polarity. Exocytic trafficking is the durable, energy-consuming process by which a cell directs the contents of secretory vesicles out of the cell membrane and into the extracellular space.
The authors have conducted experiments using immunofluorescence, Ussing chamber, reverse transcriptase polymerase chain reaction, and Western blotting techniques to demonstrate, for the first time in mice, that vasopressin plays a role in the trafficking of NKCC2 to the apical membrane of distal colonic epithelia. The results are interesting and may suggest a mechanism through which vasopressin regulates apical NKCC2 activity in the colon.
P- Reviewers: Bouley R, Mentzelopoulos SD, Nonoguchi H S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Flatman PW. Regulation of Na-K-2Cl cotransport by phosphorylation and protein-protein interactions. Biochim Biophys Acta. 2002;1566:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Haas M, Forbush B. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Matthews JB, Hassan I, Meng S, Archer SY, Hrnjez BJ, Hodin RA. Na-K-2Cl cotransporter gene expression and function during enterocyte differentiation. Modulation of Cl- secretory capacity by butyrate. J Clin Invest. 1998;101:2072-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Obermüller N, Kunchaparty S, Ellison DH, Bachmann S. Expression of the Na-K-2Cl cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J Clin Invest. 1996;98:635-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Alshahrani S, Alvarez-Leefmans FJ, Di Fulvio M. Expression of the Slc12a1 gene in pancreatic β-cells: molecular characterization and in silico analysis. Cell Physiol Biochem. 2012;30:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Xue H, Liu S, Ji T, Ren W, Zhang XH, Zheng LF, Wood JD, Zhu JX. Expression of NKCC2 in the rat gastrointestinal tract. Neurogastroenterol Motil. 2009;21:1068-1e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Zhu JX, Xue H, Ji T, Xing Y. Cellular localization of NKCC2 and its possible role in the Cl- absorption in the rat and human distal colonic epithelia. Transl Res. 2011;158:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Ji T, Liu S, Zheng LF, Wang Q, Dou ZF, Zhang Y, Zhu JX. Cellular distribution of NKCC2 in the gastric mucosa and its response to short-term osmotic shock. Cell Tissue Res. 2012;348:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol. 1984;246:F745-F756. [PubMed] |
| 10. | Hebert SC, Friedman PA, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle: I. ADH increases transcellular conductance pathways. J Membr Biol. 1984;80:201-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol. 2009;297:F1411-F1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Giménez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem. 2003;278:26946-26951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Welker P, Böhlick A, Mutig K, Salanova M, Kahl T, Schlüter H, Blottner D, Ponce-Coria J, Gamba G, Bachmann S. Renal Na+-K+-Cl- cotransporter activity and vasopressin-induced trafficking are lipid raft-dependent. Am J Physiol Renal Physiol. 2008;295:F789-F802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle’s loop. Am J Physiol. 1999;276:F96-F103. [PubMed] |
| 15. | Cristià E, Amat C, Naftalin RJ, Moretó M. Role of vasopressin in rat distal colon function. J Physiol. 2007;578:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol. 1997;272:F3-12. [PubMed] |
| 17. | Aulsebrook KA. Effect of vasopressin on sodium transfer by rat colon in vitro. Endocrinology. 1961;68:1063-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Bridges RJ, Nell G, Rummel W. Influence of vasopressin and calcium on electrolyte transport across isolated colonic mucosa of the rat. J Physiol. 1983;338:463-475. [PubMed] |
| 19. | Bridges RJ, Rummel W, Wollenberg P. Effects of vasopressin on electrolyte transport across isolated colon from normal and dexamethasone-treated rats. J Physiol. 1984;355:11-23. [PubMed] |
| 20. | Grady GF, Duhamel RC, Moore EW. Active transport of sodium by human colon in vitro. Gastroenterology. 1970;59:583-588. [PubMed] |
| 21. | Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G82-G98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Jakab RL, Collaco AM, Ameen NA. Lubiprostone targets prostanoid signaling and promotes ion transporter trafficking, mucus exocytosis, and contractility. Dig Dis Sci. 2012;57:2826-2845. [PubMed] |
| 23. | Kravtsov DV, Caputo C, Collaco A, Hoekstra N, Egan ME, Mooseker MS, Ameen NA. Myosin Ia is required for CFTR brush border membrane trafficking and ion transport in the mouse small intestine. Traffic. 2012;13:1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Greger R, Bleich M, Leipziger J, Ecke D, Mall M, Kunzelmann K. Regulation of ion transport in colonic crypts. Am J Physiol. 1997;12:62-66. |
| 25. | Lytle C, Xu JC, Biemesderfer D, Forbush B. Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol. 1995;269:C1496-C1505. [PubMed] |
| 26. | Sato Y, Hanai H, Nogaki A, Hirasawa K, Kaneko E, Hayashi H, Suzuki Y. Role of the vasopressin V(1) receptor in regulating the epithelial functions of the guinea pig distal colon. Am J Physiol. 1999;277:G819-G828. [PubMed] |
| 27. | Bouyer PG, Tang X, Weber CR, Shen L, Turner JR, Matthews JB. Capsaicin induces NKCC1 internalization and inhibits chloride secretion in colonic epithelial cells independently of TRPV1. Am J Physiol Gastrointest Liver Physiol. 2013;304:G142-G156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Del Castillo IC, Fedor-Chaiken M, Song JC, Starlinger V, Yoo J, Matlin KS, Matthews JB. Dynamic regulation of Na(+)-K(+)-2Cl(-) cotransporter surface expression by PKC-{epsilon} in Cl(-)--secretory epithelia. Am J Physiol Cell Physiol. 2005;289:C1332-C1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Zaarour N, Demaretz S, Defontaine N, Zhu Y, Laghmani K. Multiple evolutionarily conserved Di-leucine like motifs in the carboxyl terminus control the anterograde trafficking of NKCC2. J Biol Chem. 2012;287:42642-42653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Carmosino M, Rizzo F, Procino G, Zolla L, Timperio AM, Basco D, Barbieri C, Torretta S, Svelto M. Identification of moesin as NKCC2-interacting protein and analysis of its functional role in the NKCC2 apical trafficking. Biol Cell. 2012;104:658-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ares GR, Ortiz PA. Dynamin2, clathrin, and lipid rafts mediate endocytosis of the apical Na/K/2Cl cotransporter NKCC2 in thick ascending limbs. J Biol Chem. 2012;287:37824-37834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na(+)-K(+)-2Cl(-) cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem. 2009;284:24965-24971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211-276. [PubMed] |
| 34. | Isenring P, Jacoby SC, Payne JA, Forbush B. Comparison of Na-K-Cl cotransporters. NKCC1, NKCC2, and the HEK cell Na-L-Cl cotransporter. J Biol Chem. 1998;273:11295-11301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Monstein HJ, Truedsson M, Ryberg A, Ohlsson B. Vasopressin receptor mRNA expression in the human gastrointestinal tract. Eur Surg Res. 2008;40:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |