Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3693
Revised: December 27, 2013
Accepted: January 20, 2014
Published online: April 7, 2014
Processing time: 158 Days and 12.6 Hours
Pancreato-biliary malignancies often present with locally advanced or metastatic disease. Surgery is the mainstay of treatment although less than 20% of tumours are suitable for resection at presentation. Common sites for metastases are liver, lungs, lymph nodes and peritoneal cavity. Metastatic disease carries poor prognosis, with median survival of less than 3 mo. We report two cases where metastases from pancreato-biliary cancers were identified in the colon and anal canal. In both cases specific immunohistochemical staining was utilised in the diagnosis. In the first case, the presenting complaint was obstructive jaundice due to an ampullary tumour for which a pancreato-duodenectomy was carried out. However, the patient re-presented 4 wk later with an atypical anal fissure which was found to be metastatic deposit from the primary ampullary adenocarcinoma. In the second case, the patient presented with obstructive jaundice due to a biliary stricture. Subsequent imaging revealed sigmoid thickening, which was confirmed to be a metastatic deposit. Distal colonic and anorectal metastases from pancreato-biliary cancers are rare and can masquerade as primary colorectal tumours. The key to the diagnosis is the specific immunohistochemical profile of the intestinal lesion biopsies.
Core tip: Pancreato-biliary malignancies often present with locally advanced or metastatic disease. Surgery is the mainstay of treatment although less than 20% are suitable for resection at presentation. Common sites for metastases are liver, lungs, lymph nodes and peritoneal cavity and carry poor prognosis, with median survival of less than 3 mo. Distal colonic and anorectal metastases from pancreato-biliary cancers are rare and can masquerade as primary colorectal tumours. The key to the diagnosis is the specific immunohistochemical profile of the intestinal lesion biopsies.
- Citation: Ejtehadi F, Chatzizacharias NA, Brais RJ, Hall NR, Godfrey EM, Huguet E, Praseedom RK, Jah A. Colonic and anal metastases from pancreato-biliary malignancies. World J Gastroenterol 2014; 20(13): 3693-3697
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3693
Pancreato-biliary malignancies often present with locally advanced or metastatic disease[1,2]. The commonest tumour type is adenocarcinoma followed by rarer varieties such as neuroendocrine and adeno-squamous cell carcinomas[3-5]. Surgery is the mainstay of treatment although less than 20% are deemed resectable at the time of presentation[1,5-8]. Metastatic disease carries a poor prognosis with median survival of less than 3 mo[5-12].
The common sites for metastases are the liver, lungs, lymph nodes and peritoneal cavity[2]. Unusual metastatic sites such as kidney[13], colon[14-17] and skin[18], have also been reported. We report two cases where metastases from pancreato-biliary cancers were identified in the colon and anal canal.
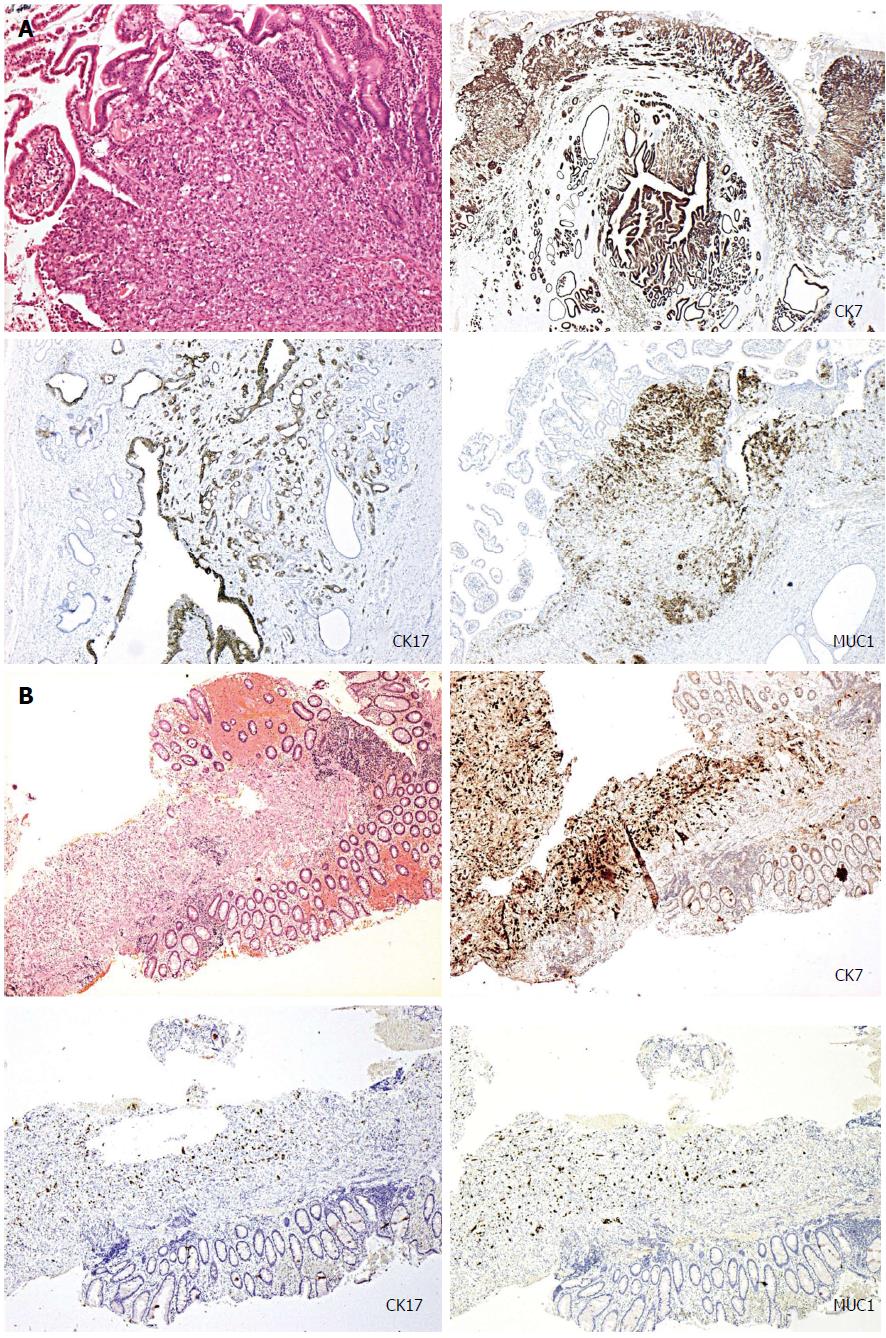
A 79-year-old lady presented with obstructive jaundice. A computed tomography (CT) scan and a subsequent endoscopic ultrasound scan identified a resectable ampullary mass. Staging CT did not reveal any evidence of metastases and the patient underwent a Whipple’s resection following which she made an uncomplicated recovery. The histopathology of the resected specimen confirmed poorly differentiated ampullary adenocarcinoma. The tumour demonstrated a pancreato-bilary immunophenotype being cytokeratin-7/cytokeratin-17/mucin-1 (CK7/CK17/MUC1) positive and cytokeratin-20/homeobox protein CDX2/mucin-2 (CK20/CDX2/MUC2) negative (Figure 1A). Approximately 4 wk after discharge, the patient re-presented with perianal pain exacerbated by defecation. Examination under anaesthesia (EUA) confirmed the presence of an atypical anal fissure, biopsies of which revealed anal mucosa extensively infiltrated by a poorly differentiated carcinoma, demonstrating similar morphological appearance to the original ampullary adenocarcinoma. Subsequent, immunohistochemistry also demonstrated an identical pancreato-biliary phenotype as seen previously (Figure 1B), suggesting that this was a metastatic deposit from the ampullary cancer. The patient underwent two cycles of palliative radiotherapy to the anal canal for control of pain and was discharged with full palliative support. She died approximately 3 wk after discharge.
A 63-year-old lady presented with obstructive jaundice due to a biliary stricture involving the hilum of the bile ducts and extending longitudinally into the distal common bile duct. Endoscopic retrograde cholangiopancreatographic brushings were inconclusive. She underwent further staging with a CT scan and laparoscopy. The laparoscopy showed no evidence of peritoneal disease, however the CT scan revealed thickening of the sigmoid colon. Colonoscopic biopsies confirmed a submucosal infiltrating moderately differentiated adenocarcinoma with no associated mucosal dysplasia. This was not supportive of a colorectal primary and favoured an extrinsic origin. Subsequent immunohistochemical analysis showed strong staining for CK7, with occasional positive staining for CK20; and negative CDX2, CK17 and Estrogen Receptor (ER) immunoreactivity. This profile, although not specific, was consistent with metastatic deposit from a hilar cholagiocarcinoma. She underwent palliative biliary stenting and was referred for palliative chemotherapy.
The colon, rectum and anal canal are rare sites for metastases for any type of malignancy. However, such cases have been reported from primary carcinomas of breast[19-22], lung[23,24], colon[25,26] and prostate[27]. Additionally, a small number of such metastases have been reported to occur in relation to cholangiocarcinomas[14,15] and pancreatic adenocarcinomas[13,16,17]. To the best of our knowledge metastasis to the anal canal from pancreato-biliary malignancies has not been reported before in the literature.
The extrinsic nature of these metastatic deposits may not be apparent in superficial mucosal biopsies. Immunohistochemical studies may be warranted in such cases where there is a suspicion of metastases or if there are unusual features such as adenocarcinoma undermining an intact, non-dysplastic mucosal surface. In the two cases that we have presented (Table 1) the immunohistochemical profiles of the tumours were more consistent with pancreato-bilary origin than lower gastrointestinal tract origin although are not definitive and final diagnosis requires correlation with the clinical and radiological parameters. Ampullary adenocarcinomas can demonstrate either an intestinal (CK20/CDX2/MUC2 positive) or pancreaticobilary immunophenotype (CK7/CK17/MUC1 positive)[28-31], as in case 1. The tumours demonstrating a pancreato-bilary phenotype can also co-express CK20, but this is usually only focal in distribution. On the contrary, the vast majority of colorectal adenocarcinomas demonstrate a typical lower gastrointestinal immunophenotype, being CK20 and CDX2 positive (CDX2 is an intestine-specific nuclear transcription factor, which can be used as a marker for intestinal-type differentiation of colorectal adenocarcinomas)[29,30].
| Demographic information | Mode of presentation | Initial investigations and findings | Site of primary lesion | Site of metastases | Histology of metastasis | Outcome |
| 79, female | Presented with obstructive jaundice | EUS, CT, ERCP, tissue diagnosis obtained after Whipple resection | Ampullary | Anal canal | Adeno carcinoma, PB type. CK7/CK17/MUC1 positive, (negative for K20/CDX2/MUC2) | Palliation |
| 63, female | Presented with obstructive jaundice | Colonoscopy and biopsy, staging CT | Hilum of bile ducts | Sigmoid colon | Microscopy Immunohistochemistry strong CK7 staining, with occasional positive staining for CK20; (negative CDX2, CK17 and ER immunoreactivity) | Palliative percutaneous biliary stenting and chemotherapy |
We acknowledge that we do not have direct corroborative histology of the pancreato-biliary primary in the second case. However, unequivocal evidence of a locally advanced malignant pancreatic mass noted on staging CT. Pancreatic biopsy was not attempted due to poor performance status of the patient. In this case there was sufficient radiological and immunohistochemical evidence to support the diagnosis of metastatic deposit in the large intestine from the primary biliary cancer.
Although pancreato-biliary malignancies commonly lead to metastatic deposits on the peritoneal surfaces, the cases described here did not have any evidence of diffuse peritoneal involvement. There was no evidence of peritoneal nodules, omental infiltration or ascites on any of the CT scans or staging laparoscopy. Therefore we believe that these are focal metastases to the colon and anal canal rather than a part of diffuse peritoneal involvement.
Distal colonic and anorectal metastases are rare. They may present simultaneously with or in isolation from the primary, with symptoms identical to a primary colo-rectal tumour. The key to diagnosis is a high index of suspicion if the clinical picture is atypical coupled with specific immunohistochemical staining. Atypical immunohistochemical pattern that does not fit with a colorectal primary should raise suspicion regarding metastases from an extrinsic source.
Case 1, a 79-year-old lady presented with peri-anal pain approximately 4 wk after discharge following a Whipple's procedure for an ampullary mass; case 2, a 63-year-old lady presented with obstructive jaundice.
Perianal pain, with an atypical anal fissure on examination under anaesthesia; obstructive jaundice, nil else.
Benign anal fissure, anal cancer, metastatic disease; gallstone disease, benign biliary stricture, pancreatic cancer, cholangiocarcinoma, ampullary cancer, metastatic disease from unknown primary.
Nortmal blood indices; liver function tests of obstructive picture.
Not applicable; endoscopic retrograde cholangiopancreatographic showed a proximal biliary stricture and staging computed tomography scan revealed thickening of the sigmoid colon.
Histopathological analysis revealed anal mucosa extensively infiltrated by a poorly differentiated carcinoma, demonstrating similar morphological appearance to the original ampullary adenocarcinoma [cytokeratin-7/cytokeratin-17/mucin-1 (CK7/CK17/MUC1) positive CK20/CDX2/MUC2 negative]; colonoscopic biopsies confirmed a submucosal infiltrating moderately differentiated adenocarcinoma with strong positivity for CK7, occasional positive staining for CK20 and negative CDX2, CK17 and ER immunoreactivity, profile consistent with metastatic deposit from a hilar cholagiocarcinoma.
Palliative chemoradiotherapy; palliative palliative biliary stenting and chemotherapy.
Metastases of pancreatobiliary malignancies to the colon are extremely rare and metastasis to the anal canal has not been reported before in the literature to the best of our knowledge.
Immunohistochemistry is refers to the process of detecting antigens (e.g., proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues.
Symptoms from distal colonic and anorectal metastases may be identical to a primary colorectal tumour and the key to diagnosis is a high index of suspicion if the clinical picture is atypical coupled with specific immunohistochemical staining.
This is a report of two unusual metastases of pancreato-biliary malignancies to the sigmoid colon and anal canal. The cases stress the importance of complete physical examination, including rectodigital examination, and surveillance of the lower gastro-intestinal tract in upper gastro-intestinal and pancreato-biliary malignancies, even though the incidence of such metastasis is rare.
P- Reviewers: Lin JK, Redondo-Cerezo E S- Editor: Song XX L- Editor: A E- Editor: Ma S
| 1. | Patel T. Cholangiocarcinoma. Nat Clin Pract Gastroenterol Hepatol. 2006;3:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 2. | Yeo TP, Hruban RH, Leach SD, Wilentz RE, Sohn TA, Kern SE, Iacobuzio-Donahue CA, Maitra A, Goggins M, Canto MI. Pancreatic cancer. Curr Probl Cancer. 2002;26:176-275. [PubMed] |
| 3. | Carter JT, Grenert JP, Rubenstein L, Stewart L, Way LW. Tumors of the ampulla of vater: histopathologic classification and predictors of survival. J Am Coll Surg. 2008;207:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 4. | Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Postgrad Med J. 2008;84:478-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 5. | Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1-VI9. [PubMed] |
| 6. | David M, Lepage C, Jouve JL, Jooste V, Chauvenet M, Faivre J, Bouvier AM. Management and prognosis of pancreatic cancer over a 30-year period. Br J Cancer. 2009;101:215-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526-532. [PubMed] |
| 8. | Talamini MA, Moesinger RC, Pitt HA, Sohn TA, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg. 1997;225:590-599; discussion 599-600. [PubMed] |
| 9. | Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlburt AA. SEER summary staging manual-2000: Codes and coding instructions. Bethesda, MD: National Cancer Institute 2001; . |
| 11. | Lee JH, Lee KG, Ha TK, Jun YJ, Paik SS, Park HK, Lee KS. Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg. 2011;77:322-329. [PubMed] |
| 12. | Yeh CC, Jeng YM, Ho CM, Hu RH, Chang HP, Tien YW. Survival after pancreaticoduodenectomy for ampullary cancer is not affected by age. World J Surg. 2010;34:2945-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Bellows C, Gage T, Stark M, McCarty C, Haque S. Metastatic pancreatic carcinoma presenting as colon carcinoma. South Med J. 2009;102:748-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Tokodai K, Kawagishi N, Miyagi S, Takeda I, Sato K, Akamatsu Y, Sekiguchi S, Ishida K, Satomi S. Intestinal obstruction caused by colonic metastasis from intrahepatic cholangiocarcinoma 6 years after removal of the primary tumor: report of a case. Surg Today. 2012;42:797-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Fujii K, Goto A, Yoshida Y, Suzuki K, Matunaga Y, Shinomura Y. Education and imaging. Gastrointestinal: Transmural colonic metastasis arising from primary cholangiocarcinoma. J Gastroenterol Hepatol. 2010;25:1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Ogu US, Bloch R, Park G. A rare case of metachronous skip metastasis of pancreatic cancer to the colon. Am Surg. 2012;78:E342-E343. [PubMed] |
| 17. | Fukatsu H, Nagahara Y, Ishiki K, Iwamura M, Hamada F. Pancreatic cancer metastasis to the rectum detected on colonoscopy. Endoscopy. 2009;41 Suppl 2:E167-E168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Hafez H. Cutaneous pancreatic metastasis: a case report and review of literature. Indian J Cancer. 2007;44:111-114. [PubMed] |
| 19. | Bochicchio A, Tartarone A, Ignomirelli O, Latorre G, Cangiano R, Gallucci G, Coccaro M, Feudale E, Aieta M. Anal metastasis from breast cancer: a case report and review of the literature. Future Oncol. 2012;8:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Puglisi M, Varaldo E, Assalino M, Ansaldo G, Torre G, Borgonovo G. Anal metastasis from recurrent breast lobular carcinoma: a case report. World J Gastroenterol. 2009;15:1388-1390. [PubMed] |
| 21. | Efthimiadis C, Kosmidis C, Fotiadis P, Anthimidis G, Vasiliadou K, Mekras A, Ioannidou G, Basdanis G. Breast cancer metastatic to the rectum: a case report. Tech Coloproctol. 2011;15 Suppl 1:S91-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Matsuda I, Matsubara N, Aoyama N, Hamanaka M, Yamagishi D, Kuno T, Tsukamoto K, Yamano T, Noda M, Ikeuchi H. Metastatic lobular carcinoma of the breast masquerading as a primary rectal cancer. World J Surg Oncol. 2012;10:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kawahara K, Akamine S, Takahashi T, Nakamura A, Kusano H, Nakagoe T, Nakazaki T, Ayabe H, Tomita M. Anal metastasis from carcinoma of the lung: report of a case. Surg Today. 1994;24:1101-1103. [PubMed] |
| 24. | Sakai H, Egi H, Hinoi T, Tokunaga M, Kawaguchi Y, Shinomura M, Adachi T, Arihiro K, Ohdan H. Primary lung cancer presenting with metastasis to the colon: a case report. World J Surg Oncol. 2012;10:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Takahashi H, Ikeda M, Takemasa I, Mizushima T, Yamamoto H, Sekimoto M, Doki Y, Mori M. Anal metastasis of colorectal carcinoma origin: implications for diagnosis and treatment strategy. Dis Colon Rectum. 2011;54:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Smiley D, Goldberg RI, Phillips RS, Barkin JS. Anal metastasis from colorectal carcinoma. Am J Gastroenterol. 1988;83:460-462. [PubMed] |
| 27. | Abbas TO, Al-Naimi AR, Yakoob RA, Al-Bozom IA, Alobaidly AM. Prostate cancer metastases to the rectum: a case report. World J Surg Oncol. 2011;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Duval JV, Savas L, Banner BF. Expression of cytokeratins 7 and 20 in carcinomas of the extrahepatic biliary tract, pancreas, and gallbladder. Arch Pathol Lab Med. 2000;124:1196-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Groisman GM, Bernheim J, Halpern M, Brazowsky E, Meir A. Expression of the intestinal marker Cdx2 in secondary adenocarcinomas of the colorectum. Arch Pathol Lab Med. 2005;129:920-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | De Lott LB, Morrison C, Suster S, Cohn DE, Frankel WL. CDX2 is a useful marker of intestinal-type differentiation: a tissue microarray-based study of 629 tumors from various sites. Arch Pathol Lab Med. 2005;129:1100-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Moriya T, Kimura W, Hirai I, Takasu N, Mizutani M. Expression of MUC1 and MUC2 in ampullary cancer. Int J Surg Pathol. 2011;19:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |