Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3628
Revised: February 10, 2014
Accepted: March 7, 2014
Published online: April 7, 2014
Processing time: 90 Days and 17.9 Hours
AIM: To explore the feasibility and oncologic outcomes of segmental jejunal resection on the left side of the mesenteric vessels in patients with tumors of the angle of Treitz using data from a single center.
METHODS: Thirteen patients with tumors of the angle of Treitz who underwent surgery at our institution were prospectively followed. A segmental jejunal resection on the left side of the mesenteric vessels was performed in all patients. Formalin-fixed and paraffin-embedded tumor samples were examined. The primary end point of this analysis was disease-free survival.
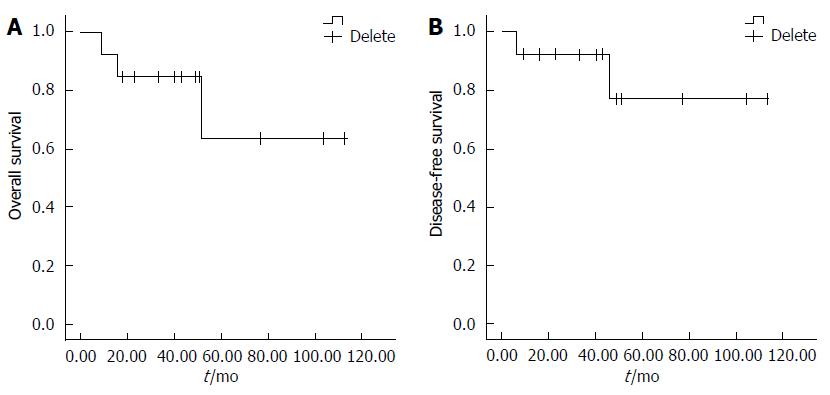
RESULTS: In this study, there were 8 males and 5 females (mean age, 50.1 years; range, 36-74 years). The mean tumor size was 8.1 cm (range, 3.2-15 cm). Histologic examination showed 11 gastrointestinal stromal tumors (GISTs) and 2 adenocarcinomas. Five of the GIST patients presented with potential low risk, and 6 presented with intermediate and high risk, according to the National Institutes of Health criteria. One potentially high-risk patient showed tumor progression at 46 mo and died 52 mo after surgery. One patient with locally advanced adenocarcinoma received neoadjuvant chemotherapy and adjuvant radiotherapy, but the disease progressed, and the patient died 9 mo after surgery. One GIST patient without progression died 16 mo after surgery because of a postoperative intestinal obstruction. The median overall survival rate was 84.6 mo, and the median disease-free survival rate was 94.5 mo.
CONCLUSION: The overall survival of patients with tumors of the angle of Treitz was encouraging even when the tumor size was relatively large. A segmental resection on the left side of the mesenteric vessels is considered to be a reliable and curative option for tumors of the angle of Treitz.
Core tip: This single-center study investigated a type of rare tumor originating from the angel of Treitz. The symptoms, diagnosis, surgical procedure, histology, and prognosis were evaluated. Although the tumors tended to be large, segmental jejunal resection on the left side of the mesenteric vessels was the treatment of choice.
- Citation: Xie YB, Liu H, Cui L, Xing GS, Yang L, Sun YM, Bai XF, Zhao DB, Wang CF, Tian YT. Tumors of the angle of Treitz: A single-center experience. World J Gastroenterol 2014; 20(13): 3628-3634
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3628
It is known that tumors of the small intestine, which account for less than 5% of digestive tract tumors[1], rarely occur. Tumors occurring in the angle of Treitz are even rarer. As a result, tumors of the angle of Treitz have never been reported on a large scale. Most published studies are case reports[2-5]. Most cases occur sporadically, but some may occur in the context of a familial syndrome (i.e., Peutz-Jeghers syndrome or Crohn’s disease)[6]. These patients usually present with abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, or vomiting caused by an obstruction[7]. A tumor of the angle of Treitz may also be an incidental finding during clinical examination.
Although segmental resection is the treatment of choice, this is not apparent from the literature. Moreover, data from our center suggest that most tumors of the angle of Treitz are gastrointestinal stromal tumors (GISTs), and only a few are adenocarcinomas. In this study, we report a series of patients with tumors of the angle of Treitz to clarify the diagnosis and treatment method.
This study examined 13 patients (8 men and 5 women; mean age, 50.1 years; range, 36-74 years). All patients in this study underwent surgical resection of tumors of the angle of Treitz (Table 1). Formalin-fixed and paraffin-embedded tumor samples were examined. The pathological categories for the 13 patients were GIST (11 cases) and adenocarcinoma (2 cases). For the patients with GISTs, immunohistochemical evaluations of CD117, CD34, S-100, smooth muscle actin (SMA), desmin, and Ki67 were performed in all cases (Table 2). Assessments of the maximal tumor size, histologic growth pattern, and mitotic count in 50 high-power fields (HPFs, for GISTs) were independently performed by two senior pathologists. The malignant potential of the GISTs was estimated based on tumor size, mitotic count, rupture, and tumor location, according to the criteria published in 2008 by the National Institutes of Health (NIH)[8]. The survival data were obtained either by reviewing the clinical records or at follow-up. In addition, preoperative and postoperative therapies with tyrosine kinase inhibitors or chemotherapy were assessed.
| Patient | Age (y) | Gender | Size (cm) | Presentation | Pathology | Surgical procedure | Lymph nodepositive/total | Adjuvant therapy | Site of progression | Cause of death |
| 1 | 41 | Male | 15 | Health examination | GIST | Segmentectomy | 0/0 | Gleevec 3 yr | ||
| 2 | 74 | Male | 4 | Health examination | GIST | Segmentectomy | 0/0 | |||
| 3 | 59 | Male | 5 | Health examination | GIST | Segmentectomy | 0/11 | |||
| 4 | 36 | Male | 5 | Health examination | GIST | Segmentectomy | 0/0 | |||
| 5 | 66 | Female | 7.5 | Pain | GIST | Segmentectomy | 0/0 | Obstruction | ||
| 6 | 51 | Female | 6.7 | Health examination | GIST | Segmentectomy | 0/0 | Gleevec 1 yr | ||
| 7 | 61 | Male | 13 | Health examination | GIST | Segmentectomy | 0/0 | Liver | Liver metastasis | |
| 8 | 36 | Male | 4.5 | Bleeding | GIST | Segmentectomy | 0/2 | |||
| 9 | 58 | Female | 3.2 | Pain | GIST | Segmentectomy | 0/0 | |||
| 10 | 40 | Male | 14 | Pain | GIST | Multi-visceral | 0/7 | Gleevec 3 yr | ||
| 11 | 60 | Male | 8.5 | Bleeding, pain, anemia | GIST | Segmentectomy | 0/1 | |||
| 12 | 62 | Female | 3.3 | Pain, vomiting | Cancer | Segmentectomy | 0/9 | |||
| 13 | 46 | Female | 15 | Vomiting, pain, anemia | Cancer | Segmentectomy | 2/7 | Radiotherapy | Local | Tumor progression |
| Patient | CD117 | CD34 | Desmin | SMA | S-100 | Ki67 | Mitotic count (/50HPF) | Other malignancy | NIH risk scale |
| 1 | +++ | - | - | - | - | 2%+ | 3 | No | High |
| 2 | ++ | ++ | - | - | + | < 5% | < 2 | No | Low |
| 3 | +++ | + | - | - | - | 2% | < 5 | No | Low |
| 4 | +++ | ++ | - | ++ | + | 2%+ | 1 | No | Low |
| 5 | +++ | - | - | - | + | < 2% | 1 | No | Medium |
| 6 | + | ++ | - | - | + | < 2% | 3 | No | Medium |
| 7 | + | - | - | + | - | 5% | 1 | Colon cancer | High |
| 8 | +++ | +++ | - | - | - | < 5% | 1 | No | Low |
| 9 | +++ | + | - | ++ | + | < 2% | 1 | Gastric cancer | Low |
| 10 | +++ | - | - | - | - | 15% | > 10 | No | High |
| 11 | +++ | +++ | + | - | 5% | 2 | Intestinal GIST 16 years ago | Medium |
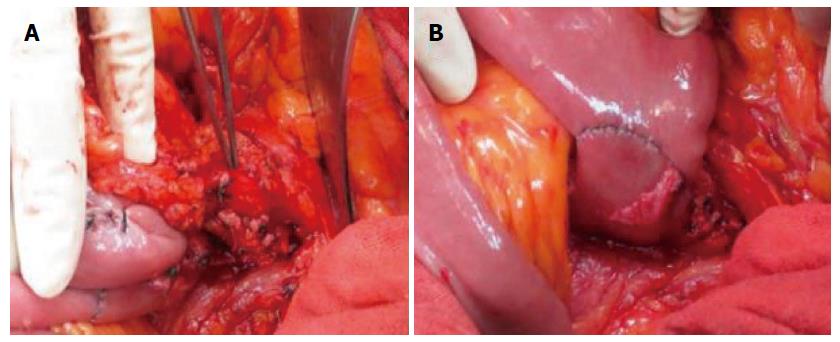
The surgical procedure included segmental jejunal resection and multivisceral resection. The decision regarding which of the two procedures to use was made intraoperatively based on tumor size and site. Segmental jejunal resection was performed on the left side of the mesenteric blood vessels (Figure 1). When the tumor infiltrated into adjacent organs, multivisceral resection was performed.
All statistical analyses were performed with the software program IBM SPSS Statistics 21 (IBM Corp., Armonk, NY, United States). The disease-free survival and overall survival were estimated using the Kaplan-Meier method. The nonparametric log-rank test was used to compare the groups.
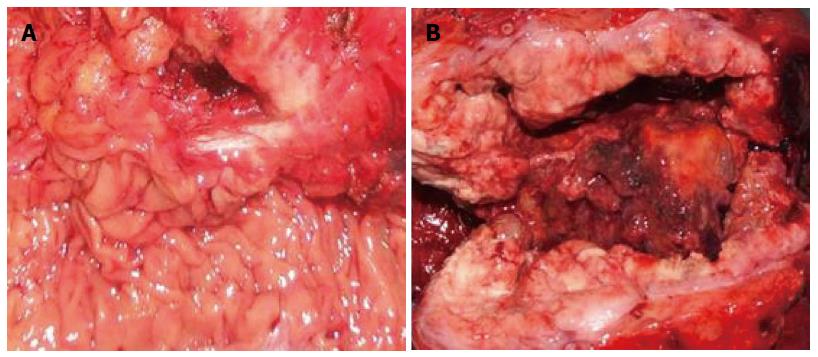
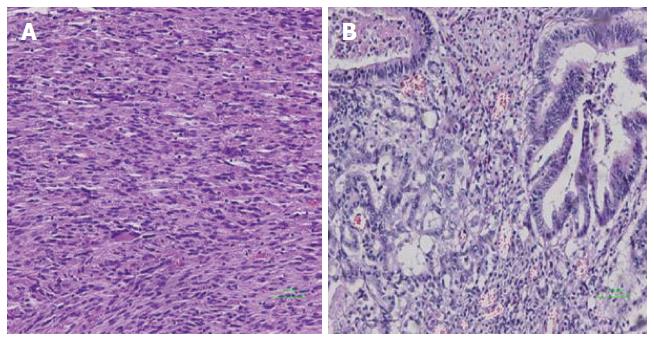
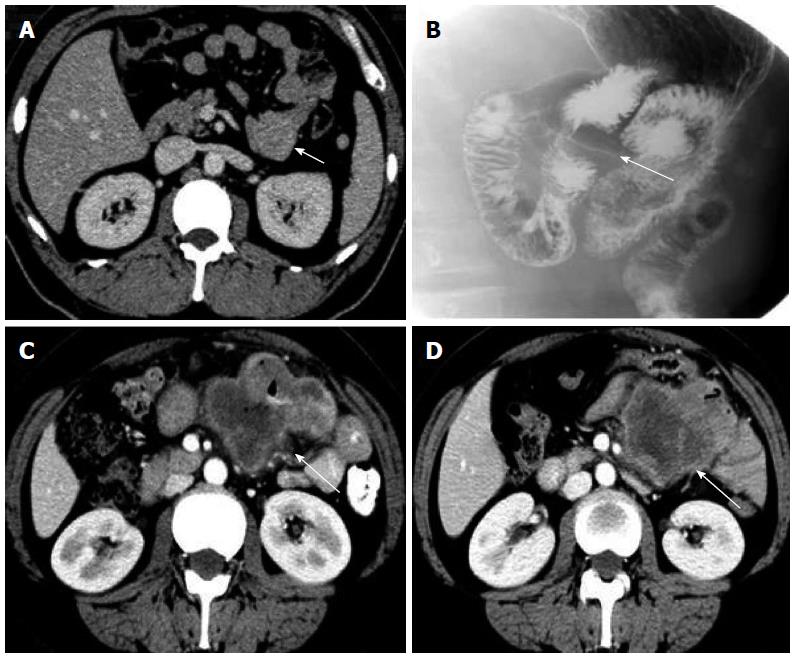
Table 1 summarizes the clinicopathologic characteristics of the 13 patients with tumors of the angle of Treitz. Mean patient age was 50.1 years, and there was no significant correlation between age and tumor recurrence (95%CI: 0.928-1.121; P = 0.686). Mean tumor size was 8.1 cm (range, 3.2-15 cm). There was no significant correlation between tumor size and tumor recurrence (95%CI: 0.613-5.309; P = 0.284). Six patients were diagnosed without symptoms in clinical examinations. Six of the remaining patients presented with abdominal pain: 2 presented with vomiting; 2 presented with anemia; and 2 presented with gastrointestinal bleeding. Both of the patients with adenocarcinoma presented with vomiting, whereas none of the GIST patients had such symptoms. The GISTs tended to be sharply demarcated without infiltrative growth, which was in contrast to the adenocarcinomas (Figure 2). Based on tumor histology, 11 tumors were diagnosed as GISTs with spindle-cell differentiation, and the other 2 as adenocarcinomas (Figure 3). The immunohistochemical analysis showed that all GISTs expressed CD117. Five tumors expressed S-100, 8 tumors expressed CD34, and 5 tumors expressed SMA. No desmin-positive tumors were identified. The mitotic rate per 50 HPFs ranged from 1 to more than 10 (median, 2 per 50 HPFs). The potential risk of malignancy (according to NIH criteria) was rated as low in 5 cases, medium in 3 cases, and high in 3 cases. All 13 patients underwent computed tomography (CT) examination. The CT results indicated that 5 tumors originated from the angle of Treitz, and 8 tumors were detected in the left upper abdomen (Figure 4). Only one patient was diagnosed using traditional endoscopy.
Survival data for all patients were obtained. One of the two patients with adenocarcinoma had tumor progression and died 9 mo after surgery. The other patient with adenocarcinoma survived and showed no recurrence at the 18-mo follow-up. For the 11 patients with GISTs, although only one showed tumor progression (high-risk potential), 2 patients ultimately died: one 16 mo postoperatively from postoperative obstruction, and the other 52 mo postoperatively from tumor progression. Overall survival and disease-free survival rates are shown in Figure 5. The median overall survival rate was 84.6 mo, and the median disease-free survival rate was 94.5 mo.
The surgical procedures included segmental jejunal resection in 11 patients and multivisceral resection in one patient. The multivisceral resection comprised a segmental jejunal resection combined with segmental colon resection because of tumor infiltration into the middle colic artery. One of the patients who underwent segmental jejunal resection was treated with secondary descending duodenum jejunal side-to-side anastomosis 10 d after the initial operation because of postoperative obstruction by greater omental adhesion, which constricted the fourth part of the duodenum. The patient died 16 mo later of intractable intestinal obstruction.
Three of the 11 GIST patients received imatinib after surgery, including one patient with a medium risk of progression and 2 high-risk patients. None of these patients had any evidence of relapse. The other 8 patients did not receive adjuvant imatinib therapy. One high-risk patient (patient 7) had a synchronous carcinoma of the sigmoid colon (pT3N0M0), which was resected simultaneously, and received systemic chemotherapy for 6 cycles (FOLFOX4 regimen). After 46 mo, the patient developed liver metastasis and died 6 mo later. One of the two adenocarcinoma patients (patient 12) did not receive any adjuvant therapy and remained alive at the 18-mo follow-up. Another patient (patient 13) received neoadjuvant chemotherapy (oxaliplatin + capecitabine) for 3 cycles, but the response was mild, and the tumor did not shrink. After palliative surgery, the patient underwent subsequent radiotherapy (2 Gy, 25 times, 5 wk). However, the disease progressed, and the patient died 9 mo after resection.
Tumors of the angle of Treitz are difficult to diagnose because of their rarity and nonspecific symptoms[9]. In this group, 6 patients had no presentation and were diagnosed incidentally (by clinical examination); the average tumor size was 8.1 cm. Interestingly, these patients all had GISTs. In symptomatic patients, only those with acute gastrointestinal bleeding or vomiting were diagnosed within 2 weeks, whereas the diagnosis of patients with vague abdominal pain was delayed beyond 2 mo (range, 2 mo to 5 years). Similar to our findings, the study of Cunningham et al[10] showed that the mean time from symptom onset to diagnosis was approximately 4 mo. Therefore, the treatment of these tumors often occurs late.
According to the literature[11], barium meal radiography of the digestive tract is useful for detecting endoluminal diseases. However, the optimal examination method is capsule endoscopy or endoscopy using a tube capable of reaching the angle of Treitz[12]. Moreover, under exophytic growth conditions, as in most GISTs, CT may be useful for localizing and assessing resectability[13]. Before the use of capsule endoscopy and modern helical CT, an upper intestinal barium study was the most frequently used radiographic diagnostic method. This approach has a diagnostic rate as low as 22%[14]. Recent studies[15] have shown that CT enteroclysis using spiral and multidetector-row CT with an enteral contrast agent has become the radiographic diagnostic tool of choice for suspected small bowel neoplasms because the sensitivity and specificity are 100% and 95%, respectively.
Regarding the resection margin and lymphadenectomy of GISTs, investigators have reached a consensus that R0 resection is sufficient and that there is no need for lymphadenectomy[16]. However, the surgical procedure for adenocarcinoma of the angle of Treitz remains controversial. The particular vascularization of this area is technically challenging for surgeons. To increase the resection margin, some investigators[17] recommend resection with anastomosis on the right side of the mesenteric vessels even if there is a risk of damaging the papilla. Conversely, other investigators[5] suggest a segmental resection on the left side of the mesenteric vessels to protect the blood supply of the duodenal stump. These investigators identified radical resection of this disease as having a proximal resection margin of > 2 cm instead of > 5 cm. Additionally, the extent of lymphadenectomy is also disputed. Although extended lymphadenectomy for adenocarcinoma is recommended[18], total removal of all cancer cells for tumors in this area makes lymphatic drainage difficult[19]. From a technical perspective, we believe that only lymph nodes visible to the naked eye or involved in the area of resection should be removed[20]. In this patient group, we performed resections only on the left side of the mesenteric vessels, and all patients recovered uneventfully.
The prognoses and disease-free survival rates of GIST patients depend on tumor biology, which is determined by tumor size, localization, rupture, and mitotic index (using the NIH scale). This finding has been affirmed by Miettinen et al[21] for duodenal GISTs. This result was also verified by our patient who developed liver metastasis. In our series, 10 cases (90.9%) had a mitotic count of no more than 5 mitoses per 50 HPFs. This result is consistent with the findings of Winfield et al[22]. The use of adjuvant imatinib after radical resection is widely accepted and is recommended at a dose of 400 mg/d for 1 year for medium-risk patients and for 3 years for high-risk patients[23]. In our group, only 3 of the 6 patients who were potentially at medium or high risk received adjuvant therapy for economic reasons. None of these patients developed recurrence.
Early studies showed that the 5-year survival rate of patients with carcinomas confined to duodenal segments D3, D4, and the angle of Treitz was approximately 75%[19]. However, recent studies revealed a 5-year survival rate of only 23%-30%[10,14], which might be attributed to the different disease stages among the patients who underwent resection. The factors influencing long-term survival include R1 or palliative resection, a locally advanced tumor, positive regional lymph nodes, and poor response to adjuvant chemotherapy[18]. Consistent with the results of previous studies[14,18], two adenocarcinoma patients in our series had markedly different outcomes. One patient (with a relatively early-stage tumor) who underwent radical resection survived 18 mo and showed no tumor progression. The other patient with a locally advanced tumor underwent palliative resection but died 9 mo after surgery despite undergoing neoadjuvant chemotherapy and adjuvant radiotherapy. Several studies have shown that adjuvant therapy has no benefit after radical resection[24].
In conclusion, the overall survival of patients with tumors of the angle of Treitz was encouraging even though the tumor size was often relatively large. Segmental resection on the left side of the mesenteric vessels is considered to be a reliable and curative option for tumors of the angle of Treitz. For GIST patients, the prognosis depends on the risk scale. The use of adjuvant imatinib is recommended for patients at medium or high risk. Surgery remains the treatment of choice for adenocarcinoma patients. The role of neoadjuvant or adjuvant chemotherapy requires further investigation.
It is known that tumors of the small intestine rarely occur, and tumors of the angle of Treitz are even rarer. The majority of the published literature consists of case reports. Patients usually present with abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, or vomiting caused by obstruction. Tumors of the angle of Treitz may also be incidental findings during a clinical examination. The unique vascularization of this area is technically challenging for surgeons. Although segmental resection is the treatment of choice, this is not apparent from the literature.
Some authors recommend resection with anastomosis on the right side of the mesenteric vessels, whereas others suggest segmental resection on the left side. Surgeons debate over the best approach.
Most previous studies involved case reports of adenocarcinoma of the angle of Treitz. According to our study, tumors of the angle of Treitz include more gastrointestinal stromal tumor cases than adenocarcinoma cases. As previously reported, patients usually present with abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, or vomiting caused by obstruction. Tumors of the angle of Treitz may also be incidental findings during a clinical examination. Based on experiences in our center, we performed all resections on the left side of the mesenteric vessels.
The results of our study suggest that resection with anastomosis on the left side of the mesenteric vessels is a safe and effective treatment for tumors of the angle of Treitz.
The angle of Treitz is also known as the ligament of Treitz and is the junction of the duodenum and jejunum adjacent to the mesenteric vessels.
In this manuscript, the authors describe clinicopathologic characteristics and surgical outcomes of tumors of the angle of Treitz, which is a rare condition, experienced at a single center. The results are of great value for further clinical study.
P- Reviewers: Bar H, Takahashi T S- Editor: Qi Y L- Editor: Cant MR E- Editor: Wang CH
| 1. | Moglia A, Menciassi A, Dario P, Cuschieri A. Clinical update: endoscopy for small-bowel tumours. Lancet. 2007;370:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Maglio R, Valabrega S, Ramacciato G. Duodenal carcinoma at ligament of Treitz. Case report and review of the literature. G Chir. 2012;33:179-181. [PubMed] |
| 3. | Kalogerinis PT, Poulos JE, Morfesis A, Daniels A, Georgakila S, Daignualt T, Georgakilas AG. Duodenal carcinoma at the ligament of Treitz. A molecular and clinical perspective. BMC Gastroenterol. 2010;10:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Nabeshima K, Machimura T, Wasada M, Takayasu H, Ogoshi K, Makuuchi H. A case of primary jejunal cancer diagnosed by preoperative small intestinal endoscopy. Tokai J Exp Clin Med. 2008;33:42-45. [PubMed] |
| 5. | Fronticelli CM, Borghi F, Gattolin A, Ferrero A, Delsedime L. Primary adenocarcinoma of the angle of Treitz. Case report and review of the literature. Arch Surg. 1996;131:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Chang HK, Yu E, Kim J, Bae YK, Jang KT, Jung ES, Yoon GS, Kim JM, Oh YH, Bae HI. Adenocarcinoma of the small intestine: a multi-institutional study of 197 surgically resected cases. Hum Pathol. 2010;41:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Chen WG, Shan GD, Zhang H, Li L, Yue M, Xiang Z, Cheng Y, Wu CJ, Fang Y, Chen LH. Double-balloon enteroscopy in small bowel tumors: a Chinese single-center study. World J Gastroenterol. 2013;19:3665-3671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 865] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 9. | Han SL, Cheng J, Zhou HZ, Guo SC, Jia ZR, Wang PF. Surgically treated primary malignant tumor of small bowel: a clinical analysis. World J Gastroenterol. 2010;16:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (2)] |
| 10. | Cunningham JD, Aleali R, Aleali M, Brower ST, Aufses AH. Malignant small bowel neoplasms: histopathologic determinants of recurrence and survival. Ann Surg. 1997;225:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Zhan J, Xia ZS, Zhong YQ, Zhang SN, Wang LY, Shu H, Zhu ZH. Clinical analysis of primary small intestinal disease: A report of 309 cases. World J Gastroenterol. 2004;10:2585-2587. [PubMed] |
| 12. | He Q, Bai Y, Zhi FC, Gong W, Gu HX, Xu ZM, Cai JQ, Pan DS, Jiang B. Double-balloon enteroscopy for mesenchymal tumors of small bowel: nine years’ experience. World J Gastroenterol. 2013;19:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Landi B. Gastrointestinal stromal tumors: clinical features and diagnosis. Bull Acad Natl Med. 2012;196:845-852; discussion 852-853. [PubMed] |
| 14. | Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 15. | Schmidt S, Felley C, Meuwly JY, Schnyder P, Denys A. CT enteroclysis: technique and clinical applications. Eur Radiol. 2006;16:648-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Siu J, Lim M, Fischer J, Dobbs B, Wakeman C, Ing A, Frizelle F. Ten-year review of gastrointestinal stromal tumours at a tertiary referral hospital in New Zealand. ANZ J Surg. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Sabiani P, Le Treut YP, Maillet B, Bozon-Verduraz E, Bricot R. Adenocarcinoma of the duodenojejunal angle. Diagnostic and therapeutic problems. J Chir (Paris). 1987;124:30-34. [PubMed] |
| 18. | Agrawal S, McCarron EC, Gibbs JF, Nava HR, Wilding GE, Rajput A. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol. 2007;14:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Lowell JA, Rossi RL, Munson JL, Braasch JW. Primary adenocarcinoma of third and fourth portions of duodenum. Favorable prognosis after resection. Arch Surg. 1992;127:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Barnes G, Romero L, Hess KR, Curley SA. Primary adenocarcinoma of the duodenum: management and survival in 67 patients. Ann Surg Oncol. 1994;1:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Winfield RD, Hochwald SN, Vogel SB, Hemming AW, Liu C, Cance WG, Grobmyer SR. Presentation and management of gastrointestinal stromal tumors of the duodenum. Am Surg. 2006;72:719-722; discussion 722-723. [PubMed] |
| 23. | Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 971] [Article Influence: 60.7] [Reference Citation Analysis (1)] |
| 24. | Scott-Coombes DM, Williamson RC. Surgical treatment of primary duodenal carcinoma: a personal series. Br J Surg. 1994;81:1472-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |