Published online Apr 7, 2014. doi: 10.3748/wjg.v20.i13.3620
Revised: December 1, 2013
Accepted: January 3, 2014
Published online: April 7, 2014
Processing time: 182 Days and 19.6 Hours
AIM: To evaluate the safety and diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in a cohort of pancreatic cancer patients.
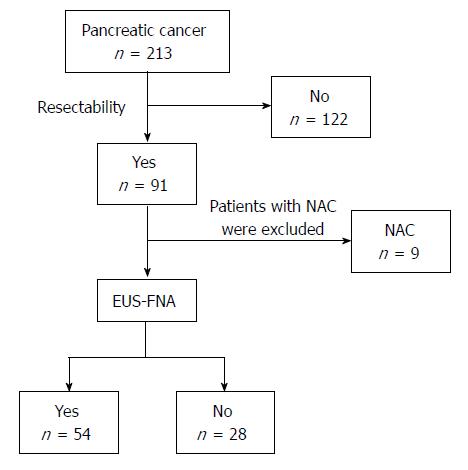
METHODS: Of 213 patients with pancreatic cancer evaluated between April 2007 and August 2011, 82 were thought to have resectable pancreatic cancer on the basis of cross-sectional imaging findings. Of these, 54 underwent EUS-FNA before surgery (FNA+ group) and 28 underwent surgery without preoperative EUS-FNA (FNA- group).
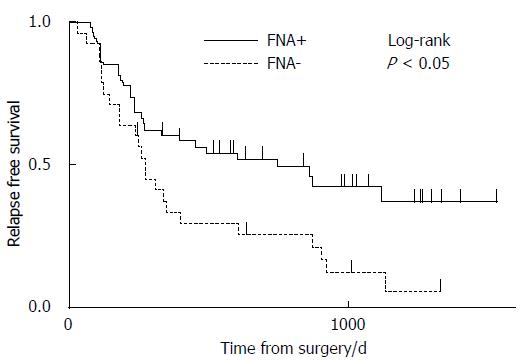
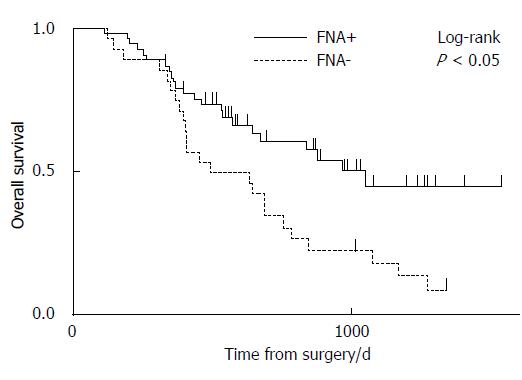
RESULTS: All 54 lesions were visible on EUS, and all 54 attempts at FNA were technically successful. The diagnostic accuracy according to cytology and histology findings was 98.1% (53/54) and 77.8% (42/54), respectively, and the total accuracy was 98.1% (53/54). One patient developed mild pancreatitis after EUS-FNA but was successfully treated by conservative therapy. No severe complications occurred after EUS-FNA. In the FNA+ and FNA- groups, the median relapse-free survival (RFS) was 742 and 265 d, respectively (P = 0.0099), and the median overall survival (OS) was 1042 and 557 d, respectively (P = 0.0071). RFS and OS were therefore not inferior in the FNA+ group. These data indicate that the use of EUS-FNA did not influence RFS or OS, nor did it increase the risk of peritoneal recurrence.
CONCLUSION: In patients with resectable pancreatic cancer, preoperative EUS-FNA is a safe and accurate diagnostic method.
Core tip: Whether preoperative endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is safe and effective for resectable pancreatic cancer has not yet been established. In the present study, patients who underwent EUS-FNA had better relapse-free survival and overall survival than did those who did not, although it should be noted that more patients in the FNA before surgery group received adjuvant chemotherapy. Our findings suggest that preoperative EUS-FNA does not adversely affect surgery or prognosis in patients with resectable pancreatic cancer. EUS-FNA can also potentially reduce the inappropriate performance of pancreatic surgery by facilitating an accurate diagnosis. These findings are important because the use of preoperative EUS-FNA is becoming more widespread.
- Citation: Kudo T, Kawakami H, Kuwatani M, Eto K, Kawahata S, Abe Y, Onodera M, Ehira N, Yamato H, Haba S, Kawakubo K, Sakamoto N. Influence of the safety and diagnostic accuracy of preoperative endoscopic ultrasound-guided fine-needle aspiration for resectable pancreatic cancer on clinical performance. World J Gastroenterol 2014; 20(13): 3620-3627
- URL: https://www.wjgnet.com/1007-9327/full/v20/i13/3620.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i13.3620
Pancreatic cancer is the fourth and fifth leading cause of cancer-related deaths in the United States and Japan, respectively, with 227000 deaths per year worldwide[1,2]. Patients with unresectable pancreatic cancer have a much worse prognosis than do those with resectable disease[2], making a sensitive screening examination and early diagnosis essential.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was first reported by Vilmann et al[3] in 1992 and has been increasingly used worldwide to diagnose pancreatic tumors, because it can be difficult to distinguish between benign and malignant tumors using conventional imaging modalities. EUS-FNA can be used to make a pathological diagnosis of pancreatic tumors and has several advantages over computed tomography (CT)- or ultrasound (US)-guided biopsy with respect to its success rate and safety[1]. However, whether the use of preoperative EUS-FNA for diagnosing pancreatic tumors is safe, given the risk of complications such as bleeding, perforations, pancreatitis, and tumor seeding, is still a matter of debate[4-13]. Previous studies have found that EUS-FNA used for pancreatic cancer is associated with only a very low risk of complications[13] and that there was no significant increase in pancreatic adenocarcinoma seeding, suggesting that the risk associated with EUS-FNA is outweighed by the likely benefit of making an accurate and early pathological diagnosis[6,8,11,12].
The utility and safety of EUS-FNA for the diagnosis of cancer in the body and tail of the pancreas has also been reported recently[14]. However, the safety and efficacy of preoperative EUS-FNA in diagnosing pancreatic cancer and the long-term prognoses of patients who have undergone preoperative EUS-FNA have not yet been reported[4].
The need for a more accurate diagnostic test is emphasized by cases in which benign pancreatic disease has been misdiagnosed as cancer and resected, increasing the associated risk of morbidity and mortality. Preoperative EUS-FNA may also reduce the misdiagnosis of benign pancreatic diseases[15]. The purpose of this study was to evaluate the efficacy and safety of preoperative EUS-FNA for diagnosing pancreatic cancer and the long-term prognosis of patients after surgery.
We evaluated 213 consecutive patients with pancreatic cancer between April 2007 and August 2011. Among them, 91 patients were diagnosed with resectable pancreatic cancer, 9 of whom underwent neoadjuvant chemotherapy or chemoradiotherapy to treat local invasion. After excluding these 9 cases, 82 patients were enrolled: 54 patients underwent EUS-FNA before surgery (FNA+ group) and 28 patients underwent surgery without preoperative EUS-FNA (FNA- group) (Figure 1). We performed EUS-FNA when requested by the surgeon or if patients were hospitalized at our department. The preoperative levels of tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), SPan-1, and DU-PAN- 2, were examined in all cases. US or CT was performed in all cases.
Preoperative EUS-FNA was performed by a single experienced endoscopist (H.K.) using a curvilinear echoendoscope (GF-UCT240-AL5; Olympus Medical Systems Co., Tokyo, Japan) and 19, 22 and 25-gauge needles (Echotip® ultra; Cook Japan, Tokyo, Japan) under conscious sedation. Briefly, the lesions were visualized by EUS, after which, the needle was advanced into the lesion through the gastric or duodenal wall. The central stylet was removed, and a syringe was attached to the needle hub to apply negative suction pressure. The needle was then moved back and forth within the lesion at least 10 times, it was removed from the lesion through the scope, and the stylet was inserted back into the needle. The specimen obtained by aspiration was placed on a slide, air-dried, alcohol-fixed, and used to prepare smears. These were then stained using the rapid Romanowsky technique allowing them to be quickly interpreted and assessed for sample adequacy (Diff-Quik stain; Kokusai Shiyaku, Kobe, Japan). Diff-Quik staining was performed on all specimens by a cytotechnologist. Cytological and histological diagnoses were made for the specimens obtained by EUS-FNA.
The characteristics of the patients, operative procedures, pathological stage according to the Union Internationale Contre le Cancer (UICC) classification, microscopic margin, the use of adjuvant chemotherapy, and the diagnostic accuracy and complications of EUS-FNA were investigated. An EUS-FNA diagnosis was considered to be accurate if it matched the pathological diagnosis of the corresponding resected specimens.
Diagnostic accuracy was assessed by comparing biopsy results with those of the final pathological diagnosis. Complications arising from the use of EUS-FNA (as described by Eloubeidi et al[5]) were monitored until surgery was performed. Pancreatitis and its severity were defined according to the criteria proposed by Cotton et al[16]. We referred to the Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy workshop[17] for the definition of other complications.
All the procedures were performed on an inpatient basis. Our institute’s review board approved the study. All patients provided written, informed consent.
Statistical analyses were performed using JMP software version 8 (SAS Institute, Cary, NC, United States). Patient characteristics were compared using the Fisher’s exact test and chi-square test. The median relapse-free survival (RFS) and overall survival (OS) time were calculated in October 2011 and were estimated using the Kaplan-Meier method and the log-rank test. The Cox proportional hazard model was used to analyze the prognostic factors for OS, including age (≥ 65 years vs < 65 years), serum CEA and CA19-9 levels prior to surgery, tumor size (> 20 mm vs≤ 20 mm), portal vein invasion (yes vs no), pathological stage according to the UICC classification (IIB-4 vs 0-IIA), microscopic margin (positive vs negative), the use of adjuvant chemotherapy (yes vs no), and EUS-FNA before surgery (yes vs no). CEA and CA19-9 were categorized into two groups according to the median value of the total study population. All reported P values are the results of two-sided tests, with P < 0.05 considered statistically significant.
Patient characteristics and the locations of the lesions are shown in Table 1. Patient characteristics did not differ significantly between the FNA+ and FNA- groups. The preoperative levels of tumor markers such as CEA, CA19-9, SPan-1, and DU-PAN-2, did not differ significantly between the 2 groups (Table 1).
| FNA+ | FNA- | ||
| Number of patients | 54 | 28 | |
| Median age (range), yr | 68 (43-82) | 70 (45-84) | NS1 |
| Gender (M/F) | 34/20 | 16/12 | NS2 |
| Location (Ph/Pb/Pt) | 33/17/4 | 20/6/2 | NS2 |
| Median CEA (95%CI), ng/mL | 4.89 (3.6-5.3) | 5.18 (-0.6-45.1) | NS1 |
| Median CA19-9 (95%CI), U/mL | 46.1 (71.5-248.9) | 96.7 (-158.9-1,661.9) | NS1 |
| Median SPan-1 (95%CI), U/mL | 33.65 (40.3-191.0) | 64.5 (-58.3-945.3) | NS1 |
| Median DU-PAN-2 (95%CI), U/mL | 129 (215-726) | 303 (211-630) | NS1 |
All lesions could be visualized using EUS, and all 54 procedures to puncture the lesions were successful. Among them, 25 procedures were performed via the gastric wall and 29 procedures were performed via the duodenal wall. The mean number of needle passes was 2.6 (range, 1-5). We used a 22-gauge, 25-gauge, and 19-gauge needle in 43, 9, and 5 procedures, respectively (in 4 cases, we used both a 22-gauge and a 25-gauge needle). The mean duration from EUS-FNA to surgery was 22.3 d (range, 5-57 d).
All procedures yielded specimens for diagnosis by cytology or histology. The accuracy of diagnoses based on cytology and histology findings was 98.1% (53/54) and 77.8% (42/54), respectively (Table 2), and the overall accuracy was 98.1% (53/54). One patient developed mild pancreatitis after EUS-FNA, but this was successfully treated by conservative therapy. In that particular case, a 22-mm lesion was found in the head of the pancreas. This was assessed using EUS-FNA with a 22-gauge needle and by making 2 punctures through the duodenal wall.
| Puncture position | Stomach/duodenum | 25/29 |
| Needle size | 19-gauge | 5 |
| 22-gauge | 43 | |
| 25-gauge | 9 | |
| Puncture number, range (mean) | 1-5 (2.6) | |
| Mean duration from EUS-FNA to surgery (d) | 22.3 | |
| Accuracy of cytology diagnosis | 98.1% (53/54) | |
| Accuracy of histology diagnosis | 77.8% (42/54) | |
All patients underwent curative surgical resection. One patient in the FNA+ group was found to have malignant cells in a peritoneal lavage cytology sample. However, there was no sign of peritoneal dissemination, for example, omental inflammation or a nodule in the peritoneum. Table 3 summarizes the operative methods, surgical outcome, tumor size, histological type of the resected specimen, UICC stage of resected specimens, and the use of adjuvant chemotherapy. We pathologically checked for lymph node metastasis, perineural and lymphovascular invasion, histological type of the lesion, and transfusion rates. No significant differences were found with respect to any of these factors between the 2 groups (data not shown). However, adjuvant chemotherapy was significantly more common among patients in the FNA+ group than among those in the FNA- group (P < 0.05).
| FNA+ | FNA- | ||
| Number of patients | 54 | 28 | |
| Operative method (%) | |||
| PD | 59.3 (32/54) | 75 (21/28) | NS1 |
| DP | 38.9 (21/54) | 17.9 (5/28) | |
| TP | 1.9 (1/54) | 3.6 (1/28) | |
| Partial resection | 1.9 (1/54) | 3.6 (1/28) | |
| Outcome (%) | |||
| R0 | 96.3 (52/54) | 96.4 (27/28) | NS1 |
| R1 | 3.7 (2/54) | 3.6 (1/28) | |
| R2 | 0 (0/54) | 0 (0/28) | |
| Tumor size (mm) | 30.0 ± 1.9 SD | 29.5 ± 2.5 SD | NS1 |
| Histological type | |||
| Adenocarcinoma | 53 | 26 | NS1 |
| Adenosquamous carcinoma | 1 | 0 | |
| IPMC | 0 | 2 | |
| UICC (%) | |||
| 0 | 0 (0/54) | 3.6 (1/28) | NS1 |
| IA | 3.7 (2/54) | 7.1 (2/28) | |
| IB | 1.9 (1/54) | 0 (0/28) | |
| IIA | 48.1 (26/54) | 21.4 (6/28) | |
| IIB | 38.9 (21/54) | 53.6 (15/28) | |
| III | 3.7 (2/54) | 3.6 (1/28) | |
| IV | 3.7 (2/54) | 10.7 (3/28) | |
| AC administration (%) | 74.1 (40/54) | 50 (14/28) | P < 0.051 |
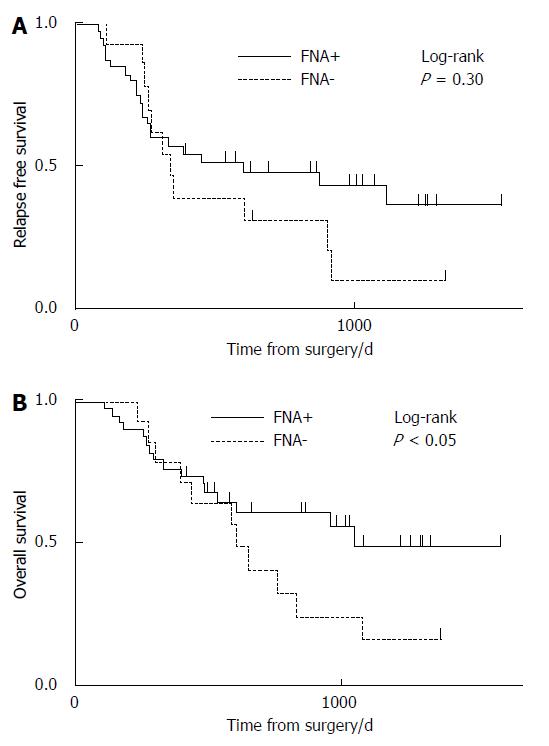
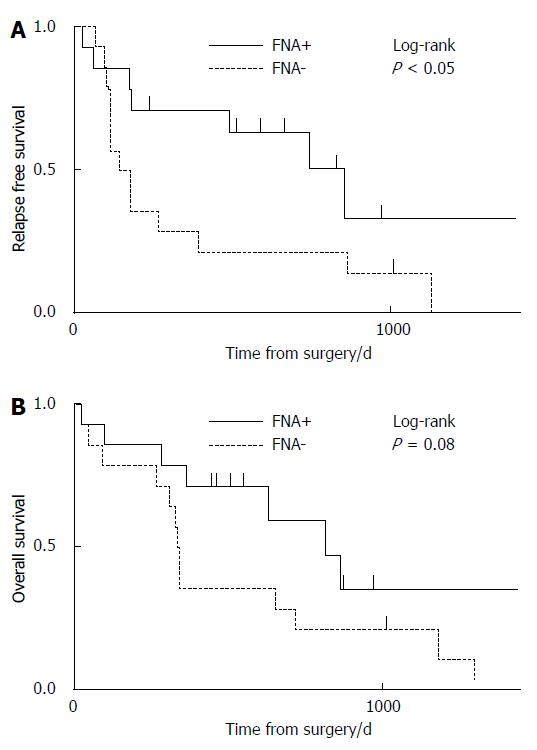
The median RFS times in the FNA+ and FNA- groups were 742 d (range, 69-1528 d) and 265 d (range, 24-1330 d), respectively (P < 0.05) (Figure 2). The median OS times in the FNA+ and FNA- groups were 1042 d (range, 114-1528 d) and 557 d (range, 119-1337 d), respectively (P < 0.05) (Figure 3). Recurrent lesions occurred in the liver (14 in the FNA+ group and 11 in the FNA- group), peripancreatic soft tissue (7 in the FNA+ group and 6 in the FNA- group), peritoneum (7 in the FNA+ and 5 in the FNA- group), lymph nodes, lungs, bone, and adrenal body. RFS and OS were also analyzed according to the administration of adjuvant chemotherapy. The RFS of patients in the FNA+ (n = 40) and FNA- (n = 14) groups who were treated with adjuvant chemotherapy was 596 d and 332 d, respectively (P = 0.30, log-rank test) (Figure 4A). The median OS of patients treated with adjuvant chemotherapy in the FNA+ and FNA- groups was 1042 d and 636 d, respectively (P < 0.05, log-rank test) (Figure 4B). For patients who did not receive adjuvant chemotherapy the RFS of the FNA+ (n = 14) and FNA- (n = 14) groups was 852 d and 158 d, respectively (P = 0.04, log-rank test) (Figure 5A). However, the median OS of patients not treated with adjuvant chemotherapy in the FNA+ and FNA- groups was 829 and 400 d, respectively (P = 0.08, log-rank test) (Figure 5B). In addition, we performed univariate and multivariate analyses for OS (Table 4) and RFS (Table 5). The hazard ratios of EUS-FNA for OS and RFS were 0.46 (P < 0.05) and 0.46 (P = 0.060), respectively, indicating that EUS-FNA was not an adverse prognostic factor for pancreatic surgery. These data indicate that the use of EUS-FNA did not influence RFS and OS, nor did it increase the risk of peritoneal recurrence.
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (over 65 years old) | 0.95 | 0.8640 | 0.98 | 0.9460 |
| (0.53-1.73) | (0.48-2.01) | |||
| CEA ≥ 3.85 ng/mL | 1.72 | 0.0827 | 1.49 | 0.2650 |
| (0.93-3.28) | (0.74-3.07) | |||
| CA19-9 ≥ 56.8 U/mL | 2.61 | 0.0030 | 1.98 | 0.0798 |
| (1.38-5.15) | (0.92-4.36) | |||
| Tumor size (> 20 mm) | 1.46 | 0.2420 | 3.27 | 0.0283 |
| (0.78-2.88) | (1.13-10.1) | |||
| Portal vein invasion | 1.88 | 0.0487 | 0.67 | 0.3640 |
| (1.00-3.49) | (0.28-1.59) | |||
| UICC ≥ IIB | 1.57 | 0.1210 | 0.78 | 0.5030 |
| (0.89-2.88) | (0.37-1.62) | |||
| R1/R0 | 2.59 | 0.2590 | 2.29 | 0.3460 |
| (0.41-8.96) | (0.36-9.38) | |||
| Adjuvant chemotherapy | 0.68 | 0.2040 | 0.46 | 0.0312 |
| (0.38-1.24) | (0.23-0.93) | |||
| EUS-FNA | 0.46 | 0.0093 | 0.44 | 0.0365 |
| (0.26-0.83) | (0.20-0.95) | |||
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (over 65 years old) | 0.81 | 0.8640 | 0.84 | 0.6300 |
| (0.47-1.43) | (0.42-1.68) | |||
| CEA ≥ 3.85 ng/mL | 1.28 | 0.0827 | 1.12 | 0.7360 |
| (0.72-2.28) | (0.57-2.19) | |||
| CA19-9 ≥ 56.8 U/mL | 2.58 | 0.0030 | 2.34 | 0.0798 |
| (1.43-4.82) | (1.14-4.92) | |||
| Tumor size (> 20 mm) | 1.67 | 0.2420 | 3.31 | 0.0254 |
| (0.92-2.28) | (1.15-10.3) | |||
| Portal vein invasion | 2.12 | 0.0123 | 0.61 | 0.2752 |
| (1.18-3.78) | (0.24-1.47) | |||
| UICC ≥ IIB | 2.44 | 0.0016 | 1.97 | 0.0588 |
| (1.39-4.42) | (0.98-4.16) | |||
| R1/R0 | 1.98 | 0.2590 | 1.96 | 0.4266 |
| (0.32-6.54) | (0.29-7.60) | |||
| Adjuvant chemotherapy | 0.78 | 0.2040 | 0.43 | 0.0168 |
| (0.44-1.40) | (0.22-0.85) | |||
| EUS-FNA | 0.5 | 0.0150 | 0.46 | 0.0606 |
| (0.29-0.87) | (0.20-1.03) | |||
The sensitivity and specificity of diagnostic tests for pancreatic neoplasms are gradually improving with the technological progress of imaging modalities. However, the diagnostic accuracy of pancreatic neoplasms based on imaging studies alone remains unsatisfactory. Approximately 10% of resected specimens that are preoperatively diagnosed as malignant pancreatic neoplasms[18] are subsequently found to be benign lesions, including focal autoimmune pancreatitis or chronic pancreatitis[19-21]. The overall mortality rate after pancreatic surgery generally ranges from 0% to 10%[22,23]. Pancreatoduodenectomy is associated with relatively high mortality and morbidity rates, ranging from 0% to 7.1% and 20.8% to 59%, respectively[24], as is distal pancreatectomy, with mortality and morbidity rates ranging from 0% to 6% and 10% to 47%, respectively[25]. Thus, surgery for patients with benign pancreatic lesions must be avoided.
EUS-FNA provides an accurate preoperative diagnosis of pancreatic solid tumors, compared to other imaging modalities, with a diagnostic accuracy[14] of 75%-95%[26-30]. The performance of EUS-FNA depends to a large extent on the ability of the endoscopist, and indeed, the diagnostic accuracy of EUS-FNA for adenocarcinoma of the pancreas has been shown to increase with operator experience[31]. It is noteworthy that the reported specificity of EUS-FNA for pancreatic solid neoplasms is almost 100%[32] and that the associated complication rate is < 1%[13].
Major complications of EUS-FNA are rare, but can include pancreatitis, bleeding, and post-procedural pain[13], although tumor seeding following EUS-FNA has been reported in 7 cases, 4 of which involved adenocarcinoma[6-12]. Preoperative EUS-FNA is avoided by some surgeons and physicians because of the risk of these complications, and it remains controversial as to whether preoperative EUS-FNA for pancreatic solid masses is always necessary[33]. Therefore, we reviewed the prognosis of postsurgical patients with pancreatic cancer and examined whether EUS-FNA adversely affected survival after pancreatic surgery. The results revealed no significant differences in complications or sites of recurrent lesions between patients who underwent FNA before surgery and those who did not.
In our study, patients who underwent EUS-FNA had better RFS and OS than did those who did not, although it should be noted that more patients in the FNA+ group underwent adjuvant chemotherapy. Multivariate analysis revealed that tumor size and adjuvant chemotherapy were both prognostic factors for OS and RFS in this study. EUS-FNA, however, was not a prognostic factor for RFS. Thus, it is possible that patients in the FNA+ group benefited from the chemotherapy administered immediately after surgery. Furthermore, neither RFS nor OS were significantly different between the 2 groups when the administration of adjuvant chemotherapy was accounted for. These data indicate that preoperative EUS-FNA does not adversely affect surgery or prognosis in patients with resectable pancreatic cancer. EUS-FNA can also potentially improve the outcomes of pancreatic surgery by providing a more accurate diagnosis. These findings are important because the use of preoperative EUS-FNA is becoming more widespread.
This study had some limitations. First, it was conducted in a single center with a small sample size, and a population bias is possible because our institute is a pancreatobiliary cancer referral center. Second, this was a retrospective study and some selection bias was observed between the 2 groups as described above. A randomized controlled trial in a multicenter setting is needed to confirm our results.
We thank Mr. Katsuji Marukawa, CT IAC and Mr. Jun Moriya, CT IAC of the Department of Surgical Pathology, Hokkaido University Hospital for their technical assistance. We also thank Satoshi Hirano, MD, PhD and Eiichi Tanaka, MD, PhD of the Department of Gastroenterological Surgery II, Hokkaido University Graduate School of Medicine for their assistance with surgical treatment.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has been developed as a practical method for obtaining specimens for the definitive diagnosis of pancreatic lesions, with a low risk of adverse events. However, it is not yet fully established whether preoperative EUS-FNA is safe and effective for resectable pancreatic cancer, and there have been very few studies to address this.
As it is now possible to resect some pancreatic cancers, the key research question is whether preoperative EUS-FNA is associated with an increased risk of adverse surgical events and whether preoperative EUS-FNA affects relapse-free survival (RFS) and overall survival (OS).
The diagnostic accuracy of EUS-FNA based on cytology and histology findings was 98.1% (53/54) and 77.8% (42/54), respectively, and the overall accuracy was 98.1% (53/54). No severe complications occurred after EUS-FNA. In the EUS-FNA and non-EUS-FNA groups, the median RFS was 742 and 265 d, respectively (P = 0.0099), and the median OS was 1042 and 557 d, respectively (P = 0.0071).
The study results suggest that preoperative EUS-FNA does not adversely affect surgery or prognosis in patients with resectable pancreatic lesions.
Relapse-free survival: In cancer cases, the length of time after the end of primary treatment for a cancer for which the patient survives without any signs or symptoms of that cancer. In a clinical trial, measuring relapse-free survival is one way to determine the efficacy of a new treatment.
This is a good descriptive study in which preoperative EUS-FNA is shown to be a practical and safe technique for acquiring pancreatic specimens. These results are interesting and suggest that preoperative EUS-FNA does not adversely affect surgery or prognosis in patients with resectable pancreatic lesions.
P- Reviewers: Shah OJ, Swan Michael, Teoh AYB S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 458] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 2. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2115] [Article Influence: 151.1] [Reference Citation Analysis (3)] |
| 3. | Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 410] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 4. | Mizuno N, Hara K, Hijioka S, Bhatia V, Shimizu Y, Yatabe Y, Yamao K. Current concept of endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer. Pancreatology. 2011;11 Suppl 2:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Eloubeidi MA, Gress FG, Savides TJ, Wiersema MJ, Kochman ML, Ahmad NA, Ginsberg GG, Erickson RA, Dewitt J, Van Dam J. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Ahmed K, Sussman JJ, Wang J, Schmulewitz N. A case of EUS-guided FNA-related pancreatic cancer metastasis to the stomach. Gastrointest Endosc. 2011;74:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Hirooka Y, Goto H, Itoh A, Hashimoto S, Niwa K, Ishikawa H, Okada N, Itoh T, Kawashima H. Case of intraductal papillary mucinous tumor in which endosonography-guided fine-needle aspiration biopsy caused dissemination. J Gastroenterol Hepatol. 2003;18:1323-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Paquin SC, Gariépy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Shah JN, Fraker D, Guerry D, Feldman M, Kochman ML. Melanoma seeding of an EUS-guided fine needle track. Gastrointest Endosc. 2004;59:923-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Doi S, Yasuda I, Iwashita T, Ibuka T, Fukushima H, Araki H, Hirose Y, Moriwaki H. Needle tract implantation on the esophageal wall after EUS-guided FNA of metastatic mediastinal lymphadenopathy. Gastrointest Endosc. 2008;67:988-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Katanuma A, Maguchi H, Hashigo S, Kaneko M, Kin T, Yane K, Kato R, Kato S, Harada R, Osanai M. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy. 2012;44 Suppl 2 UCTN:E160-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Chong A, Venugopal K, Segarajasingam D, Lisewski D. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Beane JD, House MG, Coté GA, DeWitt JM, Al-Haddad M, LeBlanc JK, McHenry L, Sherman S, Schmidt CM, Zyromski NJ. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery. 2011;150:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Klapman JB, Chang KJ, Lee JG, Nguyen P. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol. 2005;100:2658-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2036] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 17. | Davila RE, Rajan E, Adler DG, Egan J, Hirota WK, Leighton JA, Qureshi W, Zuckerman MJ, Fanelli R, Wheeler-Harbaugh J. ASGE Guideline: the role of endoscopy in the patient with lower-GI bleeding. Gastrointest Endosc. 2005;62:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Wolfson D, Barkin JS, Chari ST, Clain JE, Bell RH, Alexakis N, Neoptolemos JP. Management of pancreatic masses. Pancreas. 2005;31:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Smith CD, Behrns KE, van Heerden JA, Sarr MG. Radical pancreatoduodenectomy for misdiagnosed pancreatic mass. Br J Surg. 1994;81:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, Hruban RH. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all ‘chronic pancreatitis’? Am J Surg Pathol. 2003;27:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | van Gulik TM, Reeders JW, Bosma A, Moojen TM, Smits NJ, Allema JH, Rauws EA, Offerhaus GJ, Obertop H, Gouma DJ. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997;46:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 23. | Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda Y. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 24. | Diener MK, Knaebel HP, Heukaufer C, Antes G, Büchler MW, Seiler CM. A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg. 2007;245:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Knaebel HP, Diener MK, Wente MN, Büchler MW, Seiler CM. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 26. | Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, Büchler MW, Werner J. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 27. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 736] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 28. | Yamao K, Ohashi K, Mizutani S, Furukawa T, Watanabe Y, Nakamura T, Suzuki T, Takeda K. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) for the diagnosis of digestive diseases. Endoscopy. 1998;30 Suppl 1:A176-A178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Itoi T, Sofuni A, Itokawa F, Irisawa A, Khor CJ, Rerknimitr R. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011;23 Suppl 1:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Haba S, Yamao K, Bhatia V, Mizuno N, Hara K, Hijioka S, Imaoka H, Niwa Y, Tajika M, Kondo S. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 33. | Lachter J, Rosenthal Y, Kluger Y. A multidisciplinary survey on controversies in the use of EUS-guided FNA: assessing perspectives of surgeons, oncologists and gastroenterologists. BMC Gastroenterol. 2011;11:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |